Abstract
Membrane transporters are major determinants of the absorption, distribution and elimination of many of the most commonly used drugs. In the past decade, the field of membrane transporter pharmacogenomics has undergone enormous growth. In particular, functional genomic and clinical studies have provided new information regarding the contribution of coding variants in transporters to drug disposition and response. With continuing advances in sequencing technologies and large-scale human variation studies, over the next decade, knowledge in the field will be transformed. In particular, functional variants in noncoding regions of transporters will be discovered, and large clinical studies will result in the identification of variants in multiple genes, including transporter genes, which contribute to variation in clinical drug response.
Keywords: Pharmacogenomics, membrane transporters, genomewide association study, coding variant, noncoding variant, polymorphisms
Pharmacogenomics of Membrane Transporters Before 2000
The first draft of the human genome sequence, published in back to back issues of Nature and Science in 2001, ushered in a new era of research in pharmacogenetics. Prior to the Human Genome Project, the field of pharmacogenetics had focused largely on genetic variants in drug-metabolizing enzymes (DME), which were associated primarily with drug toxicities. In the 1990s and early 2000s, membrane transporter proteins began to be recognized as important determinants of systemic and tissue-specific drug levels. During this period, the molecular identities of many transporters were revealed. Numerous studies suggested that transporters work in concert with DME to mediate drug absorption and disposition, and ultimately, are major determinants of therapeutic and adverse drug response. With the recognition that transporters played key roles in drug response, questions began to be raised regarding the role of transporter polymorphisms in variation in drug response. Against this backdrop, the field of membrane transporter pharmacogenomics emerged.
Pharmacogenomics of Membrane Transporters (2000–2009)
Functional Genomic Studies
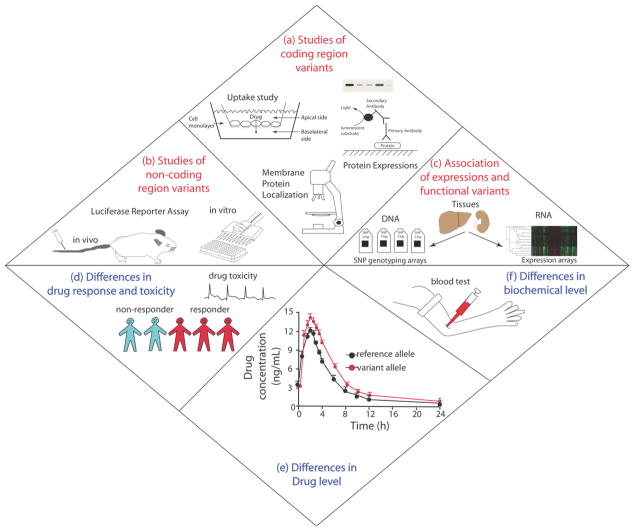
The field of pharmacogenomics of membrane transporters progressed rapidly and with a different trajectory from the classical field of pharmacogenetics. That is, classical pharmacogenetic studies typically started with an observed profound phenotype in a small group of patients on a drug. In this group, a causal polymorphism, typically in a DME, was identified as being associated with the phenotype and then characterized in in vitro assays (Figure 1A). By contrast, largely as a result of the Human Genome Project, great advances in molecular biology and sequencing methods, genetic variants in the transporters could be identified by the sequencing of DNA samples in healthy populations, functionally characterized (Figure 1A & 1B) and associated with various drug-response phenotypes (Figure 1D–1F). The availability of genome-wide technologies facilitated the discovery of genetic variants across the entire genome, including coding and noncoding regions of multiple transporter genes.
Figure 1. Functional genomic and clinical studies of membrane transporter variants.
Experiments are conducted to characterize and identify functional variants in membrane transporters in the (a) coding region and (b) noncoding region including (c) variants that associate with expression levels of transporters in various tissues. The relevant functional transporter variants are translated clinically or by candidate gene studies to demonstrate differences in (d) drug response and toxicity, (e) drug level and (f) levels of various biomarkers found in serum. In recent years, genomewide and candidate gene studies have identified many membrane transporter variants that are associated with various phenotypes (d to f).
Studies addressing questions regarding the contribution of genetic variation in the membrane transporters to drug levels or response typically began with the identification of naturally occurring genetic variants (or polymorphisms) in DNA samples from healthy populations. Between 2000 and 2005, many coding region variants in membrane transporters were discovered and characterized in functional genomic studies. The two major superfamilies of transporters, ATP-Binding Cassette (ABC) and Solute Carrier (SLC), were shown to harbor many naturally occurring genetic variants in the coding region. Nonsynonymous variants in many transporters (e.g., P-glycoprotein [ABCB1], ABCC transporters [ABCC2 and ABCC4], ABCG transporters [ABCG2], organic cation transporters 1 and 2 [OCT1 and OCT2], organic anion transporters 1 and 3 [OAT1 and OAT3], organic anion transporting polypeptides [OATP1B1, OATP1B3, OATP2B1 and OATP1A2] and multidrug and toxin extrusion transporters 1 and 2 [MATE1 and MATE2K]) were all functionally characterized. Many laboratories, including ours, contributed to a vast and growing literature centered on functional genomic studies of membrane transporters, with a particular focus on nonsynonymous variants. From these studies, the following general observations can be made regarding nonsynonymous polymorphisms in membrane transporters [1–4]:
Nonsynonymous SNPs that alter function may affect the interactions of substrates and inhibitors with the transporter, but generally appear to affect the expression level of the transporter on the plasma membrane through changes in protein stability, subcellular localization or membrane trafficking;
Some nonsynonymous SNPs may result in substrate-dependent changes in transporter function;
Rare nonsynonymous variants (minor allele frequency <1%) are more likely to exhibit reduced function than common nonsynonymous variants;
Multiple variants in a single transporter may result in reduced function.
Furthermore, a synonymous polymorphism, c.3435C>T, in ABCB1 P-glycoprotein, received considerable attention in the transporter field and beyond [5,6]. Although controversial, the variant has been found to be associated with various drug-response phenotypes. The polymorphism reportedly alters the structure and function of the transporter owing to changes in the kinetics of translation of the protein [6]. Collectively, the functional genomic studies of coding region variants in membrane transporters have resulted in a wealth of information on the effects of transporter polymorphisms on drug uptake and ehfflux. The most interesting coding region variants were further studied for clinical effects on drug levels and response (see later).
Since 2006, functional genomic studies of membrane transporters have focused on noncoding region variants. In comparison to nonsynonymous variants, noncoding region variants are more abundant and minor allele frequencies are often higher owing to lower selective pressures on these gene regions [7]. Numerous reduced and gain-of-function variants in the proximal promoter region of membrane transporters have been identified and characterized [7–10]. In contrast to nonsynonymous variants, for which a single polymorphism may cause a total loss of function, the effects of variants in the proximal promoter region of transporter genes are generally more modest and are often highly dependent upon the haplotype [8–10]. On average, a greater variation has been observed in the proximal promoter of SLC transporters than of ABC transporters, and in transporters highly expressed in the liver in comparison to transporters that are highly expressed in the kidney [7]. To date, there have been few studies of genetic variants in the 5′- and 3′-UTRs of transporter transcripts. Variants in these regions may affect regulation of transcript levels or translation [11,12]. Available data indicate that intronic regions harbor elements that affect transcript levels through modulation of transcription rate as enhancers, repressors or promoters. Although many intronic variants have been associated with clinical phenotypes [13], in general, the functional effect of the variants in this region have not been studied.
Clinical Studies
The current technological waves of SNP genotyping methodologies, gene-expression arrays and next-generation sequencing have accelerated clinical discoveries associating the SNPs discovered and functionally characterized in human variation projects with various clinical phenotypes. The National Human Genome Research Institute (NHGRI) Catalog of Genome-Wide Association Studies [101] reveals numerous genome-wide studies that describe significant associations of SNPs within or in close proximity to membrane transporter genes with phenotypes related to biochemistry level [14], cancer risk [15], disease risk [16], drug response and drug toxicities [17]. Furthermore, many studies have demonstrated an association of nonsynonymous SNPs in membrane transporter genes, for example, OATP1B1, ABCB1, ABCG2 and OCT1, with variation in plasma drug levels (Figure 1E) [18–20]. Table 1 summarizes the results of many studies associating the effects of various polymorphisms in membrane transporters on phenotypes related to therapeutic or adverse drug response. In addition to associations with drug-response phenotypes, increasing numbers of genomewide studies associating gene-expression levels with genotypes have been performed in various human tissues, for example, lymphoblastoid cells, liver, adipose tissue and cortical cells, and have identified important quantitative trait loci that inform clinically relevant phenotypes [21]. These expression SNPs, termed eSNPs, need to be functionally characterized to identify functional SNPs that may result in variation in transporter expression levels and, consequently, variation in drug response.
Table 1.
Widely studied genetic variants in membrane transporter genes and associated clinical phenotypes
| Polymorphisms in membrane transporter genes | Affected drug |
|---|---|
| The common reduced function variant in OATP1B1 (SLCO1B1*5, Val174Ala, c.521T>C) is associated with individual susceptibility to drug induced adverse events and with increases in drug plasma levels. | atorvastatin, pravastatin, pitavastatin, rosuvastatin, simvastatin, repaglinide, fexofenadine, methotrexate |
| The allele frequency of the functional variant in ABCG2 (rs2231142, Gln141Lys, c.421C>A) is associated with higher drug levels and with increased incidence of drug-induced toxicity. | atorvastatin, rosuvastatin, gefitinib, sulfasalazine, diflomotecan |
| The reduced function variants in OCT1 (R61C, P160L, G401S, 420del and G465R) and in OCT2 (A270S) have been widely studied for their effects on plasma levels of drugs, renal drug clearance and pharmacological effects. | metformin, cisplatin, imatinib |
| Many common variants in ABCB1 (c.3435C>T, 2677G/T/A) and/or ABCC2 (−24C>T, c.1249G>T, c.3972C>T, c.4544C>T) transporters have been widely studied with positive associations in retrospective clinical studies related to therapeutic and adverse response to anticancer, anti-viral and/or anti-epileptic drugs. | irinotecan, mycophenalic acid, tenofovir, docetaxel, various antiepileptic drugs |
| The insertion/deletion polymorphism in the promoter region of the serotonin transporter gene, SLC6A4 (HTTLPR), has been widely studied for various associations with diseases and behavioral illnesses. The polymorphism in this transporter has been studied for response to drug therapy related to depression. | citalopram, respiridone, paroxetine, fluoxetine |
Future Studies of Pharmacogenomics of Membrane Transporters
In the next decade, great advances in the field of membrane transporter pharmacogenomics are anticipated. In particular, it is envisioned that a functional map of the ‘transporter genome’ will be developed. The map will include annotations of gene regions that harbor functional elements. For example, enhancer regions upstream, downstream and in the intronic regions of transporter genes will be identified. With the 1000 Genomes Project, human genetic variation in multiple ethnic populations will be superimposed onto the mapped regions of transporter genes. Further functional genomic experimental studies will reveal whether variants are likely to be neutral or to affect transcription rates and levels of mRNA transcripts. A detailed molecular understanding of the functional effects of variants in regulatory regions of transporters will be obtained. Such variants may associate with changes in transcription factor binding (promoter region) [8,10], methylation of promoter regions, RNA-binding protein interactions (UTR), miRNA binding (3′-UTR) [11,12], or with precursor mRNA splicing or enhancer binding (intron). Currently, only a few structures of membrane transporters are available. In the next decade, it is likely that many more structures will be solved, and that refined structural models of human transporters will be developed. Computational models predicting the functional effects of nonsynonymous SNPs, which will use structure and include docking of substrates and inhibitors to binding sites in transporters, will facilitate understanding of the effects of coding region variants on transporter function. Finally, all of these studies will be used to inform clinical studies of therapeutic and adverse drug reactions. Current clinical studies have focused on the association of single genetic variants with drug response. It is anticipated that gene–gene interactions will be identified and will reveal epistatic interactions among transporter genes, or transporter genes and genes encoding enzymes or other proteins that interact with transporters. Collectively, such interactions may contribute to the vast amount of variation in drug response that remains unexplained.
In summary, the past decade has ushered in a new field focused on genetic variation in membrane transporters. Because transporters are involved in the pharmacokinetics and pharmacodynamics of most clinically used drugs, it is anticipated that the field of pharmacogenomics of membrane transporters will contribute enormously to understanding the determinants of variation in drug response. Transporters and their genetic variants can be characterized according to their interaction with drugs; thus, studies of functional genomics of membrane transporters will provide mechanistic support for clinical studies of drug-response phenotypes. Ultimately, it is anticipated that a detailed functional and mechanistic understanding of the variants in coding and noncoding regions of transporter genes that contribute to variation in drug response will be revealed.
Contributor Information
Sook Wah Yee, Schools of Pharmacy & Medicine, University of California San, Francisco, CA, USA.
Ligong Chen, Schools of Pharmacy & Medicine, University of California San Francisco, CA, USA.
Kathleen M Giacomini, Schools of Pharmacy & Medicine, University of California San Francisco, CA, USA.
References
- 1.Gradhand U, Kim RB. Pharmacogenomics of MRP transporters (ABCC1–5) and BCRP (ABCG2) Drug Metab Rev. 2008;40(2):317–354. doi: 10.1080/03602530801952617. [DOI] [PubMed] [Google Scholar]
- 2.Leabman MK, Huang CC, DeYoung J, et al. Natural variation in human membrane transporter genes reveals evolutionary and functional constraints. Proc Natl Acad Sci U S A. 2003;100(10):5896–5901. doi: 10.1073/pnas.0730857100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban TJ, Sebro R, Hurowitz EH, et al. Functional genomics of membrane transporters in human populations. Genome Res. 2006;16(2):223–230. doi: 10.1101/gr.4356206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda K, Sugiyama Y. Impact of genetic polymorphisms of transporters on the pharmacokinetic, pharmacodynamic and toxicological properties of anionic drugs. Drug Metab Pharmacokinet. 2008;23(4):223–235. doi: 10.2133/dmpk.23.223. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 7.Hesselson SE, Matsson P, Shima JE, et al. Genetic variation in the proximal promoter of ABC and SLC superfamilies: liver and kidney specific expression and promoter activity predict variation. PLoS One. 2009;4(9):e6942. doi: 10.1371/journal.pone.0006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JH, Yee SW, Kim MJ, et al. Identification and characterization of novel polymorphisms in the basal promoter of the human transporter, MATE1. Pharmacogenet Genomics. 2009;19(10):770–780. doi: 10.1097/FPC.0b013e328330eeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahara H, Yee SW, Urban TJ, et al. Functional genetic variation in the basal promoter of the organic cation/carnitine transporters OCTN1 (SLC22A4) and OCTN2 (SLC22A5) J Pharmacol Exp Ther. 2009;329(1):262–271. doi: 10.1124/jpet.108.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yee SW, Shima JE, Hesselson S, et al. Identification and characterization of proximal promoter polymorphisms in the human concentrative nucleoside transporter 2 (SLC28A2) J Pharmacol Exp Ther. 2009;328(3):699–707. doi: 10.1124/jpet.108.147207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3′ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol. 2008;28(17):5147–5161. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009;75(6):1374–1379. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009;58(3):745–749. doi: 10.2337/db08-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanna S, Busonero F, Maschio A, et al. Common variants in the SLCO1B3 locus are associated with bilirubin levels and unconjugated hyperbilirubinemia. Hum Mol Genet. 2009;18(14):2711–2718. doi: 10.1093/hmg/ddp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murabito JM, Rosenberg CL, Finger D, et al. A genome-wide association study of breast and prostate cancer in the NHLBI’s Framingham Heart Study. BMC Med Genet. 2007;8 (Suppl 1):S6. doi: 10.1186/1471-2350-8-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tregouet DA, Konig IR, Erdmann J, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41(3):283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- 17.Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359(8):789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 18.Shu Y, Brown C, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83(2):273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keskitalo JE, Pasanen MK, Neuvonen PJ, Niemi M. Different effects of the ABCG2 c.421C>A SNP on the pharmacokinetics of fluvastatin, pravastatin and simvastatin. Pharmacogenomics. 2009;10(10):1617–1624. doi: 10.2217/pgs.09.85. [DOI] [PubMed] [Google Scholar]
- 20.Ho RH, Choi L, Lee W, et al. Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics. 2007;17(8):647–656. doi: 10.1097/FPC.0b013e3280ef698f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schadt EE, Molony C, Chudin E, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6(5):e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroetz DL, Yee SW, Giacomini KM. The pharmacogenomics of membrane transporters project: research at the interface of genomics and transporter pharmacology. Clin Pharmacol Ther. 2010;87(1):109–116. doi: 10.1038/clpt.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]