Abstract
Inheritance of the ε4 allele of ApoE is the only confirmed and consistently replicated risk factor for late onset AD. ApoE is also a key ligand for LRP, a major neuronal LDL receptor. Despite the considerable converging evidence that implicates ApoE and LRP in the pathogenesis of AD, the precise mechanism by which ApoE and LRP modulate the risk for AD remains elusive. Moreover, studies investigating expression of ApoE and LRP in AD brain have reported variable and contradictory results. To overcome these inconsistencies, we studied the mRNA expression of ApoE and LRP in the postmortem brain of persons who died at different stages of dementia and AD-associated neuropathology relative to controls by qPCR and Western blotting. Clinical dementia rating scores were used as a measure of dementia severity, whereas, Braak neuropathological staging and neuritic plaque density were used as indices of the neuropathological progression of AD. ApoE and LRP mRNA expression was significantly elevated in the postmortem inferior temporal gyrus (area 20) and the hippocampus from individuals with dementia compared to those with intact cognition. In addition to their strong association with the progression of cognitive dysfunction, LRP and ApoE mRNA levels were also positively correlated with increasing neuropathological hallmarks of AD. Additionally, Western blot analysis of ApoE protein expression in the hippocampus showed that the differential expression observed at the transcriptional level is also reflected at the protein level. Given the critical role played by LRP and ApoE in Aβ and cholesterol trafficking, increased expression of LRP and ApoE may not only disrupt cholesterol homeostasis but may also contribute to some of the neurobiological features of AD, including plaque deposition.
1. Introduction
In humans, there are three common alleles of the apolipoprotein E (ApoE) gene, ε2, ε3 and ε4. Other than age, the ε4 allele of ApoE is the strongest risk factor for late onset AD (LOAD) (Corder et al., 1993; Strittmatter et al., 1993a). ApoE ε4 allele increases the risk for developing AD by three times in heterozygotes and by 12 times in homozygotes (Bertram, 2009; Roses, 1996). The effects of ε4 allele on AD risk are maximal between 60 and 70 years of age (Blacker, 1997). ApoE ε4 is also associated with an earlier age of AD onset (Gomez-Isla, 1996; Roses, 1996) relative to persons with the ε2/ε3 genotype (Corder, 1994; Corder et al., 1993). Human ApoE is a 34 kDa glycoprotein, with the highest expression in the liver and brain. In the brain, ApoE is predominantly synthesized by astrocytes and to some extent by microglia (Grehan et al., 2001; Pitas et al., 1987) while neurons preferentially express the receptors for ApoE, the low-density lipoprotein (LDL) receptor family (Beffert et al., 2004). In the central nervous system, ApoE is the principal cholesterol carrier protein and after binding to LDL receptor family members on neuronal cell surfaces, lipidated ApoE facilitates synaptogenesis and modulates neurite outgrowth in an isoform-specific manner, with ε4 inhibiting and ε3 stimulating neurite outgrowth (Mauch et al., 2001; Nathan et al., 1994). Following receptor-mediated endocytosis, ApoE may be either degraded or recycled back to the cell surface (Rensen et al., 2000). ApoE also avidly binds amyloid beta (Aβ) peptide and has been found to codeposit with amyloid plaques in AD brains. Complete absence of fibrillar Aβ in apoE-null AD transgenic mice (Bales et al., 1997; Holtzman et al., 2000) strongly suggests that ApoE is a key participant in in vivo Aβ fibrillization.
Converging evidence also implicates the LDL receptor-related protein (LRP), a key metabolic ApoE receptor, in the pathogenesis of AD. LRP is synthesized as a single glycosylated protein of (~600 kDa) and then cleaved by furin in the trans-Golgi network to generate a 515 kDa extracellular subunit and an 85 kDa transmembrane subunit, which remain covalently associated with one another. LRP is one of the largest endocytic receptors identified to date (Herz et al., 1988; Krieger and Herz, 1994; Oleinikov et al., 2000) that is highly expressed in neuronal cell bodies and dendritic processes (Bu et al., 1994; Moestrup et al., 1992). LRP undergoes rapid endocytosis (t1/2 < 30 s) (Li et al., 2001) to transport its ligands including those associated with AD (ApoE, Aβ and α2-macroglobulin [α2M]), from the cell surface to intracellular compartments. LRP has also been shown to interact with amyloid precursor protein (APP) and modulate its endocytic trafficking and processing (Kounnas et al., 1995; Ulery et al., 2000). Finally, the finding that LRP is a prominent component of compact senile plaques and colocalizes exclusively with ApoE, and that several of its ligands are present in senile plaques (Arelin et al., 2002; Rebeck et al., 1995) underscores the importance of LRP mediated endocytosis in AD. Although the precise mechanism by which ApoE and LRP modulate the risk for AD is not understood, prevailing data suggest that by inducing LRP expression, ApoE can influence not only receptor mediated trafficking but also Aβ metabolism, trafficking and aggregation, which in turn contributes to some of the many neurobiological features of AD, including the deposition of neuritic plaques (NPs).
Given the strong association of ApoE with the risk of AD, numerous studies have investigated whether the levels of ApoE are altered in AD. Previous studies of ApoE levels in cerebrospinal fluid (Landen et al., 1996; Lefranc et al., 1996; Sihlbom et al., 2008) and brain parenchyma (Beffert et al., 1999; Bertrand et al., 1995; Bray et al., 2004; Harr et al., 1996; Hesse et al., 1999; Lambert et al., 1997; Pirttila et al., 1996a; Pirttila et al., 1996b) of individuals with AD have yielded conflicting results. Likewise, studies investigating expression of LRP in AD brain have reported variable and contradictory observations. One study reported that LRP levels were reduced in mid frontal regions of AD brains. It was proposed that impaired LRP-mediated Aβ clearance could be a causative factor in AD pathogenesis (Kang et al., 2000). That study also reported that a genetic polymorphism (C766T) in exon 3 of the LRP gene is under-represented in AD and is associated with increased soluble Aβ levels and amyloid deposition. In contrast, Causevic et al. (2003) determined LRP protein levels in the frontal cortex and found no significant relationship between the levels of LRP, the status of the LRP exon 3 polymorphisms and progressive dementia. Another discordant study reported dramatic increase in LRP levels in AD frontal cortex along with increased levels of LRP ligands, ApoE and α2M (Qiu et al., 2001). Because LRP ligands increased LRP expression in vitro, it was proposed that elevated levels of LRP ligands could induce the increased expression of LRP in AD brain.
Many of the discordant results reviewed above could have arisen from differences in the specimens and brain regions studied, the degree of neuropathology, the presence or absence of non-AD neuropathology, levels of cognitive compromise, and the clinical and neuropathological diagnostic systems used. More specifically, protein quantification by Western blotting is characterized by inherently lower reproducibility and higher variability relative to qPCR. Interestingly, results of studies of ApoE mRNA levels have been more consistent and have shown ApoE mRNA levels to be elevated in AD brain (Ishii et al., 1997; Wolf et al., 1998; Zarow and Victoroff, 1998). To overcome these potential sources of variability, we studied the gene expression of ApoE and LRP in multiple brain regions of a relatively large cohort of cognitively, clinically and neuropathologically well-characterized cases. Additionally, Western blot analysis of ApoE protein expression in the hippocampus was performed to determine whether they were convergent with the mRNA expression. These protein-based assays were performed to determine whether in addition to mRNA expression protein expression levels were altered as well. They were not intended to recapitulate the larger gene expression studies. The use of detailed quantitative cognitive compromise measures and neuropathological lesion density data provided a unique opportunity to relate LRP levels with its ligand ApoE, along a continuum of cognitive and neuropathological changes/disease progression. Further examination of ApoE and LRP mRNA levels in the inferior temporal gyrus (area 20) and hippocampus enabled us to extend the previous findings to brain regions that show the earliest (Haroutunian et al., 2009) and most severe pathologic changes (Berg et al., 1998; Braak and Braak, 1991; Giannakopoulos et al., 1995; Mitchell et al., 2002) during the course of disease.
2. Materials and Methods
2.1. Study Cohort
The cohort included in this study was part of a larger clinical and neuropsychological investigation of early AD. These individuals were extensively evaluated for their cognitive function. Their cognitive status during the 6 months proximal to death was used to define the absence, presence and extent of dementia at the time of death, as previously described (Davis et al., 1999; Haroutunian et al., 1998; Haroutunian et al., 1999). Cases were selected from a pool of over 600 donors with either no discernable neuropathology or only those neuropathological lesions associated with AD alone (e.g., exclusion of cases with vascular lesions, Lewy body inclusions, normal pressure hydrocephalus). Because postmortem intervals (PMI) (Barton et al., 1993; Johnson et al., 1986) and tissue pH (a proxy measure for agonal state) (Lipska et al., 2006; Vawter et al., 2006) are important issues for consistency and reproducibility of quantitative gene and protein expression studies, brain samples were obtained from cases who met the following criteria: postmortem delay of less than 24 hrs, brain tissue pH of 6.3 or greater no perimortem coma longer than 6 hrs, no evidence of seizures in the 3 months preceding death. Controls were derived from persons who, on extensive medical record review and/or neuropsychological examination and caregiver interview, showed no evidence of neurological or neuropsychiatric diseases, died of natural causes (myocardial infarction, various non-brain non-hepatic cancers, and congestive heart failure) and had no discernable neuropathology (Purohit et al., 1998). None of the subjects had a history of licit or illicit drug abuse (tobacco use excepted). All diagnostic and cognitive assessment procedures were approved by the Mount Sinai Medical Center (New York, NY)/J. J. Peters Veterans Administration Medical Center (Bronx, NY) Institutional Review Boards, and postmortem consent for autopsy and research use of tissue was obtained from the next of kin or a legally authorized official.
2.1.1. Classification of Subjects into Dementia Severity Groups
In order to perform post-assay analyses based on a clinical index of disease severity, the subjects were classified with respect to the clinical dementia rating (CDR) score at the time of death (Burke et al., 1988; Dooneief, 1996; Hughes et al., 1982; Morris, 1993) (Table 1). The assessments, on which these classifications were based, were performed blind to clinical or neuropathological disease diagnosis. Table 2 describes the sample size, sex, age at the time of death, pH and PMI of the study cohort when grouped on the basis of CDR.
Table 1.
Group classifications for gene expression analyses.
| Gene Expression Analysis
| |||
|---|---|---|---|
| CDR Groups | Dementia Severity | Number of individuals
|
|
| Hippocampus | Area 20 | ||
| 0 | No dementia | 18 | 18 |
| 0.5 | Questionable dementia | 13 | 13 |
| 1 | Mild dementia | 9 | 8 |
| 2 | Moderate dementia | 9 | 13 |
| 3 | Severe dementia | 12 | 18 |
| 4–5 | Very severe/terminal dementia | 12 | 18 |
|
| |||
| Braak Groups | Braak stages | ||
|
| |||
| 0 | None | 7 | 9 |
| I | Mild transentorhinal | 8 | 9 |
| II | Severe transentorhinal | 15 | 16 |
| III | Limbic | 10 | 12 |
| IV | Limbic/Hippocampal CA1 | 8 | 8 |
| V | Isocortical | 12 | 15 |
| VI | Isocortical/Primary sensory areas | 13 | 19 |
|
| |||
| NP Density Groups | Plaques (number/mm2) | ||
|
| |||
| 0 | 0 | 24 | 26 |
| 1 | 1–5 | 10 | 13 |
| 2 | 6–10 | 20 | 23 |
| 3 | 11–20 | 14* | 14 |
| 4 | 21 and more | 5* | 12 |
NP density groups 4 and 5 were pooled for gene expression analyses in hippocampus.
Table 2.
Demographic details of study cohort stratified with respect to CDR (Clinical Dementia Rating) groups.
| Characteristics | Area | CDR 0 | CDR 0.5 | CDR 1 | CDR 2 | CDR 3 | CDR4-5 |
|---|---|---|---|---|---|---|---|
| Total subjects | Hipp | 18 | 13 | 9 | 9 | 12 | 12 |
| Area 20 | 18 | 13 | 8 | 13 | 18 | 18 | |
| Gender (men/women) | Hipp | 7/11 | 6/7 | 3/6 | 0/9 | 3/9 | 3/9 |
| Area 20 | 6/12 | 7/6 | 3/5 | 1/12 | 7/11 | 8/10 | |
| Age (years) | Hipp | 75.2 ± 3.5 | 85.4 ± 2.7 | 83.4 ± 3.4 | 87.9 ± 2.0 | 88.8 ± 1.7 | 85.0 ± 1.9 |
| Area 20 | 77.0 ± 3.9 | 85.5 ± 2.8 | 85.6 ± 3.8 | 87.6 ± 2.0 | 86.2 ± 8.5 | 84.2 ± 2.5 | |
| Brain pH | Hipp | 6.43 ± 0.04 | 6.43 ± 0.07 | 6.31 ± 0.1 | 6.38 ± 0.09 | 6.34 ± 0.05 | 6.39 ± 0.07 |
| Area 20 | 6.42 ± 0.05 | 6.42 ± 0.07 | 6.35 ± 0.11 | 6.39 ± 0.08 | 6.43 ± 0.05 | 6.36 ± 0.05 | |
| RNA integrity number (RIN) | Hipp | 6.6 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.2 | 6.2 ± 0.2 | 6.1 ± 0.1 | 6.2 ± 0.1 |
| Area 20 | 6.9 ± 0.1 | 6.9 ± 0.1 | 6.7 ± 0.2 | 6.3 ± 0.2 | 6.9 ± 0.2 | 6.3 ± 0.1 | |
| Postmortem interval (minutes) | Hipp | 713 ± 137 | 393 ± 85 | 264 ± 39 | 336 ± 66 | 276 ± 40 | 332 ± 80 |
| Area 20 | 574 ± 109 | 381 ± 91 | 325 ± 52 | 358 ± 64 | 244 ± 29 | 310 ± 73 |
2.1.2. Neuropathological Assessment
The neuropathological assessment procedures used have been previously described in detail (Haroutunian et al., 1998; Haroutunian et al., 1999). Neuropathological assessments were performed on the right hemisphere and consisted of microscopic assessment of paraffin embedded blocks from multiple brain regions using hematoxylin and eosin, modified Bielschowski, modified thioflavin S, and anti-amyloid, anti-tau immunohistochemistry. All neuropathology data regarding the extent and distribution of neuropathologic lesions were collected blind to the subject’s dementia status. Specimens for this study were dissected from the frozen, never-thawed, left hemisphere, using previously described procedures (Haroutunian, 2007).
For pathologic staging of AD, neurofibrillary tangle density was assessed using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (Mirra et al., 1987; Mirra et al., 1991) criteria. Each case was assigned a Braak neuropathological score for progression of neurofibrillary using the criteria by Braak and Braak (Braak and Braak, 1991) (Table 1). NPs were identified as the dystrophic neurites arranged radially and forming a discrete spherical lesion about 30 mm in diameter with amyloid cores. A composite score of NPs counts in 5 cortical regions was used for the stratification of individuals into groups based on the severity of NP neuropathology (Table 1). Previous studies have indicated that this composite measure of NP density corresponds closely to the average NP densities in most cortical regions examined (Haroutunian et al., 1998).
2.2.1. RNA Isolation
Total RNA was isolated from 50 mg of microdissected pulverized frozen brain samples from inferior temporal gyrus and the hippocampus with the guanidinium isothiocyanate method (Chomczynski and Sacchi, 1987) using ToTALLY RNA kits (Ambion, Austin, TX) according to the manufacturer’s protocol as described previously (Katsel et al., 2009). To remove genomic DNA contamination, isolated RNA samples were treated with 40 units of DNase I (Ambion, Austin, TX) in the presence of 120 units of RNaseOUT (Gibco BRL, Grand Island, NY) for 1 hour at 37°C. The quality of the isolated total RNA for each case was assessed using a combination of 260 nm/280 nm ratio obtained spectrophotometrically (Beckman Instruments, Fullerton, CA) and by Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA) before proceeding with cDNA synthesis. Only specimens with an RNA integrity number (RIN) ≥ 5.5 were included in the analyses.
2.2.2. Reverse Transcriptase Reaction
cDNA synthesis was performed with iScript cDNA Synthesis kit (BioRad Laboratories, Hercules, CA) which uses both random and poly-dT priming for the reverse transcription (RT) reaction. Total RNA (1 μg) was employed for each 20 μl reaction. The resulting cDNA was diluted 25 times for qPCR.
2.2.3. qPCR
LRP and ApoE mRNA expression was measured by quantitative polymerase chain reaction (qPCR) using an ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA) and gene-specific fluorogenic TaqMan® probes (Applied Biosystems). Each 20 μl PCR reaction contained 5 μl of the relevant cDNA, 20X TaqMan® assay (used at a final concentration of 0.5X), and 10 μl of TaqMan® Universal PCR Reaction Mix which contains ROX as a passive internal reference (Applied Biosystems). The thermal cycling program consisted of 2 min at 50 °C, 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The reactions were quantified by selecting the amplification cycle when the PCR product of interest was first detected (threshold cycle, Ct). Tests of primers and probes sensitivity and assay linearity were conducted for all real-time PCR assays by amplification of mRNA in 10-fold serial dilutions of pooled cDNA as previously described (Dracheva et al., 2001). Each reaction was performed in triplicate and the average Ct value was used in all analyses.
The relative gene expression level was calculated using the Relative Standard Curve Method (see Guide to Performing Relative Quantitation of Gene Expression Using Real-time Quantitative PCR, Applied Biosystems) which accounts for differences in the efficiencies of the target and control amplifications, thereby, producing accurate, quantitative results. Standard curves were generated for target assay and for each endogenous control assay by the association between the Ct values and different quantities (5 serial dilution steps) of a “calibrator” cDNA. The “calibrator” was prepared by mixing small quantities of all experimental samples. Expression values of the target and the control genes were extrapolated from their respective standard curves. Relative expression of target genes was computed as the ratio of the target mRNA levels to the geometric mean of the four endogenous controls: β-glucuronidase (GUSB), cyclophilin A (PP1A), β2-microglobulin (β2M), and ribosomal protein, large, P0 (RPLP0) which were picked for their stability using geNorm (Byne et al., 2008; Vandesompele et al., 2002). Samples with Ct values > 33 were considered outside the range of sensitivity of the assay and were not included in the analyses.
2.3. Protein Quantitation
Protein expression studies were carried out to determine whether different levels of ApoE gene expression were reflected in the expression level of ApoE protein. Because of the inherently lower reproducibility and higher variability characteristic of Westerns in postmortem tissue relative to qPCR and because we wished to determine whether mRNA expression was faithfully reflected in protein expression, we restricted ApoE protein analyses to cases with robust changes in gene expression. Therefore, a subset of the hippocampal samples (N = 30) studied for mRNA expression was analyzed by Western blotting to reflect broad variations in gene expression. As fewer protein analyses were performed than gene analyses, adjacent categories for all three indices of disease severity for gene expression analyses were combined to achieve sufficiently large sample sizes for reliable comparisons (Table 3).
Table 3.
Group classifications for protein expression analysis.
| Protein Expression Analysis in the hippocampus
| ||
|---|---|---|
| CDR Groups | Dementia Severity | Number of individuals |
| 0 | No dementia | 8 |
| 0.5–5 | Questionable dementia/terminal dementia | 22 |
|
| ||
| Braak Groups | Braak stages | |
|
| ||
| 0–I | None/Mild transentorhinal | 8 |
| II–VI | Severe transentorhinal/Isocortical | 22 |
|
| ||
| NP Density Groups | Plaques (number/mm2) | |
|
| ||
| 1 | 0 | 12 |
| 2–5 | 1 and more | 18 |
2.3.1. Tissue Lysate Preparation
Total tissue lysates were prepared from frozen hippocampal specimens from sister aliquots of the same brain samples as those used for qPCR analysis as described previously (Akram et al., 2010). Total protein concentration of the lysate was determined using a CBQCA Quantitation Kit (Molecular Probes, Eugene, OR) with fluorescence measured on a SpectraMAX Gemini XS spectrofluorometer (Molecular Devices, Sunnyvale, CA).
2.3.2. Western Blot Analysis
For gel electrophoresis, 10 μg of total protein was mixed with loading buffer and loaded onto pre-cast 10–20% Tris-glycine gels (Bio-Rad Laboratories, Hercules, CA), and run at 150 V for 1 hr. Each gel was loaded with three experimental samples in triplicate and “standard tissue homogenate” (the mix of small aliquots of tissue from all samples), run in quadruplicates. Separated proteins were transferred to polyvinylidene difluoride membranes at 100 V for 1 hour and probed with anti ApoE antibody (Abcam, Cambridge, MA) diluted 1:20,000 in 3 % non-fat dry milk in TBS overnight at 4°C with gentle shaking. To ensure equal protein loading between individual samples, membranes were also incubated with an anti-valosin containing protein (VCP) antibody. VCP, a 97 kDa protein, has been previously validated as reliable internal standard (Akram et al., 2010; Bauer et al., 2009). Following 1 hour incubation with the secondary HRP conjugated antibodies, blots were developed using SuperSignal® West Femto Maximum Sensitivity Substrate (Pierce Biotechnology, Rockford, IL). Images made on Hyperfilm ECL (GE Healthcare, Piscataway, NJ) were digitized with Alpha ChemImager™ 5500 Imaging System and quantitated with AlphaEaseFC software version 4.0 (Alpha Innotech, San Leandro, CA). The average digital signal per band was measured after subtraction of the appropriate background. Optical density for each ApoE band was first normalized to the corresponding average signal for the standard tissue homogenate and then for the VCP band from the same sample. The linearity of the dose responses for the antibodies used was established in preliminary experiments.
2.4. Statistical Analyses
We performed a logarithmic transformation of ApoE and LRP gene expression to eliminate heterogeneity, and used the transformed gene expression values for all subsequent statistical analyses. A preliminary analysis assessed linear associations with sex, pH, PMI and RIN to evaluate their use as covariates. In addition, age, the most significant risk factor for dementia, was used as a covariate in all analyses regardless of its association with the dependent variable.
We determined the linear association of ApoE and LRP gene expression with CDR, Braak stages and NP density by partial correlation analyses, controlling for potential covariates if preliminary analyses showed significant correlation with the expression level of the gene under analysis. Because the associations of each of these interrelated scales with gene expression is at least partly mediated through the associations with the other two scales, additional partial correlation analyses assessed each scale controlling also for the other two scales.
In order to determine non-linear association of CDR Braak stages, and NP density with ApoE and LRP gene expression, each of these disease severity indices was classified as a categorical variable. ANCOVA was performed for each categorical variable controlling for age and any other potential covariates. Another ANCOVA for each categorical variable controlled also for other two variables as scales, similar to the partial correlation analyses.
For each categorical variable, some individuals were classified as controls: CDR = 0, Braak stages = 0 and I, and NP Density = 1. For each categorical variable, ANCOVA compared controls with individuals with varying degree of dementia (CDR ≥ 0.5) and AD associated neuropathology (Braak stage ≥ II, NP density ≥ 2). Analyses for protein expression were the same as for gene expression. All analyses were performed with SPSS 17.0 (SPSS, Chicago, IL).
3. Results
3.1. qPCR analysis of LRP mRNA expression in area 20
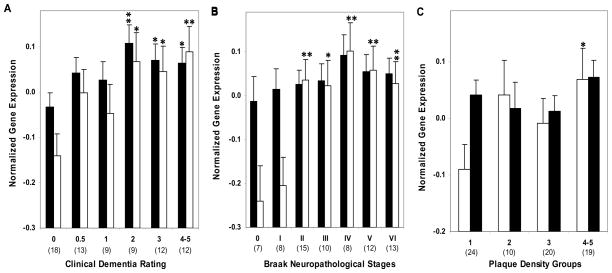
Comparison of individuals with and without dementia by the CDR criterion showed higher levels of LRP gene expression (F1,85 = 25.40, p < 0.0005) in individuals with varying dementia severity (Figure 1). The partial correlation analysis of LRP mRNA expression controlling for age demonstrated significant associations with CDR (r = 0.344, df = 85, p = 0.001), Braak neuropathological stages (r = 0.227, df = 85, p = 0.035) and NP density (r = 0.232, df = 85, p = 0.031). In ANCOVAs controlling for age, the levels of LRP varied significantly as a function of dementia severity (F5,81 = 4.97, p = 0.001) and NP density (F4,82 = 4.29, p = 0.003). Although, the association of Braak score with LRP gene expression was not significant (F6,80 = 1.33, p = 0.255), polynomial contrast revealed a significant linear association with gene expression (p = 0.001). The association of LRP gene expression with CDR, controlling also for Braak neuropathological score and NP density, remained strong (F5,79 = 3.63, p = 0.005). Figure 2 presents the estimated means and standard error of mean (SEM) from the ANCOVAs, adjusting for the covariates.
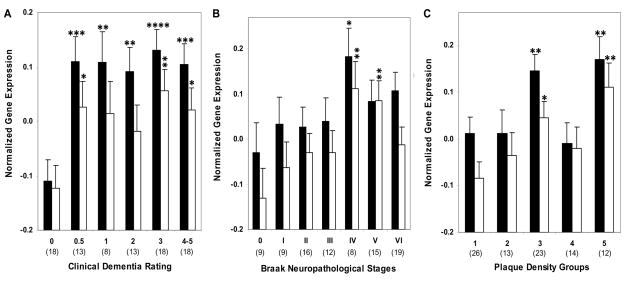
Figure 1.
LRP (Black bars) and ApoE mRNA (white bars) expression in individuals with and without dementia or AD-associated neuropathology in area 20. Mean values ± standard error of the mean (SEM) are shown. *, p < 0.05; **, p < 0.01; ****, p < 0.0001. Number within the parentheses indicates the individuals within each group.
Figure 2.
Normalized LRP (Black bars) and ApoE mRNA (white bars) expression in area 20 plotted against CDR scores, Braak neuropathological stages and NP density groups. ANCOVA was used to compare gene expression in individuals with varying degree of dementia (CDR 0.5–5) and AD associated neuropathology (Braak stage I-VI, NP density 2–5) relative to the control group. Mean values ± SEM are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. Number within the parentheses indicates the individuals within each group.
3.2. qPCR analysis of ApoE mRNA expression in area 20
Gene expression analysis in cognitively intact elderly controls and persons with dementia (CDR 0.5–5) showed higher levels of ApoE gene expression in individuals with dementia (F1,84 = 10.31, p = 0.002; Figure 1A). Comparisons of controls and individuals with AD-associated neuropathology also showed more ApoE gene expression as a function of increasing NP density (F1,84 = 8.02, p = 0.006) and Braak neuropathological staging (F1,84 = 3.95, p = 0.050; Figures 1B and C). The partial correlation analysis of ApoE mRNA expression controlling for age and RIN demonstrated significant associations with CDR (r = 0.254, df = 84, p = 0.018), Braak neuropathological stages (r = 0.229, df = 84, p = 0.034) and NP density (r = 0.319, df = 84, p = 0.003). In ANCOVAs controlling for age and RIN, CDR (F5,80 = 2.27, p = 0.055) and Braak score (F6,79 = 2.06, p = 0.067) showed trend level associations with ApoE mRNA expression. Polynomial contrast revealed a significant linear trend for CDR (p = 0.038) and Braak scores (p = 0.008). The association of NP density (F4,81 = 3.38, p = 0.013) with ApoE gene expression was significant and also showed strong linear association with gene expression (p = 0.004) in the polynomial contrast. Figure 1 presents the estimated means and SEM from the ANCOVAs, adjusting for the covariates.
3.3. qPCR analysis of LRP mRNA expression in the hippocampus
Comparison of gene expression in individuals with and without dementia showed significantly increase in LRP gene expression (F1,69 = 6.35, p = 0.014) in individuals with varying dementia severity (Figure 3). The partial correlation analysis of LRP mRNA expression controlling for age and pH demonstrated significant associations with CDR (r = 0.254, df = 69, p = 0.032) and NP density (r = 0.236, df = 69, p = 0.048) but not with Braak neuropathological stages (r = 0.133, df = 69, p = 0.267). However, in ANCOVAs controlling for age and pH, when CDR (F5,65 = 1.73, p = 0.141), Braak score (F6,64 = 0.41, p = 0.867) and NP density (F3,67 = 0.82, p = 0.488) were treated as categories rather than as scales, the associations were not significant (Figure 4).
Figure 3.
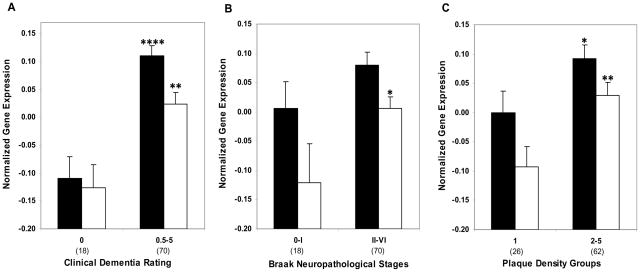
LRP (Black bars) and ApoE mRNA (white bars) expression in individuals with and without dementia or AD associated neuropathology in the hippocampus. Mean values ± SEM are shown. *, p < 0.05;**, p < 0.01; ***, p < 0.001; ****, p < 0.0001. Number within the parentheses indicates the individuals within each group.
Figure 4.
Normalized LRP (black bars) and ApoE mRNA (white bars) expression in the hippocampus plotted against CDR scores, Braak neuropathological stages and NP density groups. ANCOVA was used to compare gene expression in individuals with varying degree of dementia (CDR 0.5–5) and AD associated neuropathology (Braak stage I–VI, NP density 2–5) relative to the control group in the hippocampus. Mean values ± SEM are shown. *, p < 0.05; **, p < 0.01. Number within the parentheses indicates the individuals within each disease severity group.
3.4. qPCR analysis of ApoE mRNA expression in hippocampus
Gene expression analysis in individuals with and without dementia or AD-associated neuropathology showed higher levels of ApoE gene expression by the CDR (F1,70 = 8.91, p = 0.004), the Braak neuropathological staging (F1,70 = 8.25, p = 0.005) and NP density (F1,70 = 4.17, p = 0.045; Figure 3). The partial correlation analysis of ApoE mRNA expression controlling for age demonstrated significant associations with CDR (r = 0.350, df = 70, p = 0.003) Braak neuropathological stages (r = 0.304, df = 70, p = 0.009) and NP density (r = 0.251, df = 70, p = 0.034). In ANCOVAs controlling for age, when CDR (F5,66 = 2.45, p = 0.043) and Braak score (F6,65 = 3.30, p = 0.007) were treated as categories, the associations were similarly evident. However, the association of NP density (F3,68 = 2.11, p = 0.107) with ApoE gene expression was not significant. Figure 4 presents the estimated means and SEM from the ANCOVAs, adjusting for the covariates.
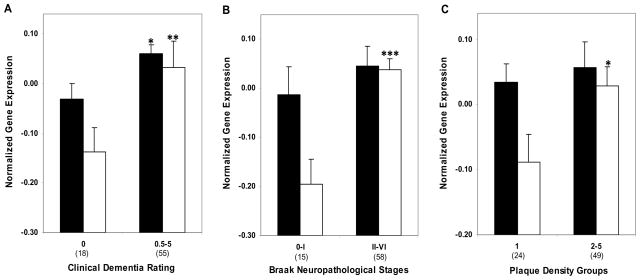
3.5. ApoE protein expression
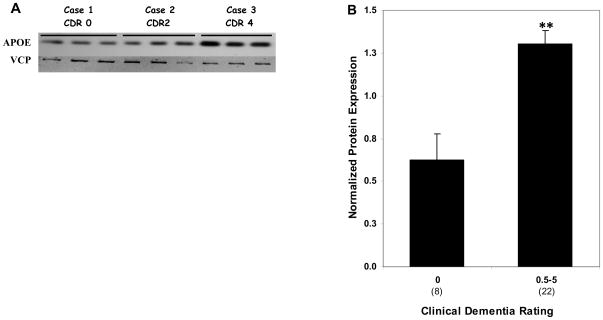
Western blot analysis revealed robust ApoE protein expression in hippocampal tissue homogenates (Fig. 5A). ApoE protein expression was highly correlated with mRNA levels of ApoE (r = 0.71, p < 0.0001). These findings suggest coordinated transcription and translation modulation of ApoE during the course of dementia and AD-associated neuropathology. Comparison of controls and individuals with dementia or varying degree of AD-associated neuropathology showed higher levels of ApoE protein by the CDR criterion (F1,26 = 14.38, p = 0.001; Fig 5B) and Braak neuropathological staging (F1,26 = 4.37, p = 0.047). The partial correlation analysis of ApoE protein expression controlling for age were comparable to those of gene expression for CDR (r = 0.541, df = 26, p = 0.003) but not significant for Braak score (r = 0.276, df = 26, p = 0.155) and NP density (r = 0.118, df = 26, p = 0.549). Even after controlling also for Braak score and NP density, ApoE protein expression was still significantly correlated with CDR (r = 0.440, df = 24, p = 0.031).
Figure 5.
Western blot analysis of ApoE in the hippocampus of cognitively intact controls and individuals with varying severity of dementia. A, Representative immunoblots of ApoE protein expression are shown. Total tissue homogenates were separated by reducing SDS-PAGE and probed with mouse anti-ApoE and mouse anti-VCP antibodies. Tissue lysate from each individual were loaded in triplicate and pooled tissue lysate (last 4 lanes) were run in quadruplicates. B, Protein quantification was done by assessing the ratio of APOE and VCP signal. Mean values ± SEM are shown. *, p < 0.05; **, p < 0.01. Number within the parentheses indicates the individuals within each group.
3.6. Association between LRP and ApoE gene expression
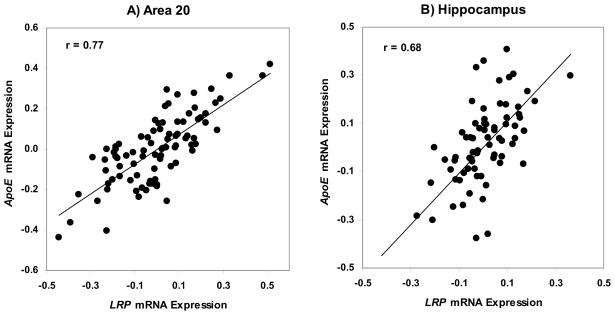
The partial correlation analysis of LRP and ApoE gene expression controlling for age and RIN in area 20, indicated strong association (r = 0.768, df = 84, p < 0.0001; Figure 6A). Similarly, strong association between LRP and ApoE gene expression controlling for age and pH was observed in the hippocampus (r = 0.681, df = 69, p < 0.0001; Figure 6B). All data were used for comparison of LRP and ApoE mRNA expression.
Figure 6.
Association between ApoE and LRP gene expression. A. Partial correlation of LRP and ApoE gene expression controlling for age and RIN in area 20 is (r = 0.768, df = 84, p < 0.0001). B. Partial correlation of LRP and ApoE gene expression controlling for age and pH in hippocampus is (r = 0.681, df = 69, p < 0.0001).
4. Discussion
The studies described provide evidence that ApoE and LRP gene expression is upregulated in the inferior temporal gyrus (area 20) and in the hippocampus of persons with varying severity of dementia and AD-associated neuropathology. In order to determine whether ApoE and LRP expression was upregulated early in the course of AD pathogenesis, individuals were grouped based on dementia severity (CDR score) at the time of death, progression of NFT pathology (Braak neuropathological staging) and severity of NP pathology. Subsequent analyses indicated that the expression of ApoE and LRP was significantly correlated with these indices of disease progression. Specifically, alteration in gene expression was strongly correlated with cognitive impairment. Additionally, highly coordinated upregulation of ApoE mRNA and protein were observed in AD hippocampus.
The observed changes in ApoE and LRP gene expression in hippocampus and area 20 are particularly intriguing in light of the susceptibility of these regions at early stages of disease. Hippocampus shows most severe and early pathogenic changes during the course of AD (Berg et al., 1998; Braak and Braak, 1991; Giannakopoulos et al., 1995; Mitchell et al., 2002). Similarly, area 20 undergoes transcriptional dysregulation at the onset of dementia (CDR 0.5) (Haroutunian et al., 2009). Moreover, longitudinal studies examining rate of atrophy in cases with mild cognitive impairment who progressed to AD have consistently found atrophy in the inferior temporal gyrus (Chetelat et al., 2005; Desikan et al., 2008; McDonald et al., 2009). Therefore, differential expression of genes that have critical roles in pathogenesis of AD may underlie early or increased vulnerability of these regions. Furthermore, mild to moderate cognitive deficits identified by the CDR scale represent some of the earliest manifestations of the disease process (Bennett, 2005; Morris, 2001) and this is borne out in our data.
As mentioned earlier, several studies have addressed the expression of LRP protein in AD brain albeit with conflicting results (Kang et al., 2000; Qiu et al., 2001; Causevic et al., 2003; Donahue et al., 2006; Matsui et al., 2007). The results presented here are consistent with those of Matsui et al. (2007) and Qiu et al. (2001) who reported an increase in ApoE and LRP mRNA in temporal neocortex and frontal cortex, respectively of individuals with AD compared to nondemented controls brains (Qiu et al., 2001). Based on increased expression of LRP by its ligands in vitro, it was proposed that elevated levels of LRP ligands could induce the increased expression of LRP in AD brain. Because increased levels of LRP and of its ligands α2M and ApoE were observed in the brains from cases with severe AD pathology, Qiu et al. (2001) concluded that the changes in LRP levels occur during late stages of AD. Using a carefully characterized cohort of individuals presenting a continuum of cognitive and neuropathological changes the present findings show that LRP and ApoE gene and protein expression changes occur very early in the course of disease. In addition, examination of ApoE and LRP mRNA levels in hippocampus enabled us to extend these previous findings to one of the most vulnerable brain regions to AD pathology. The current results show that upregulation of LRP and ApoE gene expression is coordinated such that their levels are highly correlated with each other when measured in the same samples. Interestingly, very preliminary analyses suggest that the dysregulation of LRP levels in individuals with dementia is accentuated among homozygous ApoE4 allele carriers (data not shown). However, the sample size was too small for confident conclusions (N =3). Although LRP protein levels were not evaluated in this study, elevated levels of LRP ligands have been shown to induce expression of LRP in vitro (Qiu et al., 2001).
There is considerable converging evidence that implicates LRP and ApoE in the pathogenesis of AD. The ε4 allele of ApoE is a strong genetic risk factor for LOAD (Corder et al., 1993; Strittmatter et al., 1993a). ApoE is a key ligand for LRP (Rebeck et al., 1995; Strittmatter et al., 1993a) and LRP is a major neuronal receptor for ApoE. In addition, LRP binds and internalizes several molecules associated with AD such as α2M, APP and Aβ (Herz and Strickland, 2001). Furthermore, several genetic studies have reported an association between polymorphisms within the LRP gene and AD (Kang et al., 1997; Lendon et al., 1997). The genetic associations of ApoE and LRP to LOAD are particularly interesting, considering the fact that ApoE is the best characterized Aβ chaperone (Jordan et al., 1998). Upon forming a stable complex with Aβ, ApoE promotes LRP-mediated internalization of Aβ (Beffert et al., 1999; Jordan et al., 1998; Winkler et al., 1999). Receptor mediated Aβ endocytosis has been hypothesized to be an efficient way of reducing brain Aβ load (Kang et al., 1997; LaDu et al., 1994; Rebeck et al., 1995). In particular, LRP-mediated transport of Aβ/ApoE complex across the blood brain barrier (BBB) is a major pathway of Aβ clearance from the brain to the periphery (Shibata et al., 2000; Tanzi et al., 2004).
We have previously reported significant increases in ATP-binding cassette, sub- family A, member 1 (ABCA1) expression in the hippocampus of persons with varying severity of AD (Akram et al., 2010). ABCA1 is an integral membrane protein that mediates the efflux of cellular cholesterol and phospholipids to lipid-deficient apolipoprotein acceptors such as ApoE (Bodzioch et al., 1999; Brooks-Wilson et al., 1999; Repa and Mangelsdorf, 2002). Increased expression of ABCA1 and ApoE, together with the critical role played by ABCA1 in ApoE lipidation, suggest that lipidation of ApoE is increased in subjects with dementia. Interestingly, ApoE lipidation status has also been shown to have a pronounced effect on Aβ transport and metabolism because lipidated ApoE interacts with Aβ with a significantly higher affinity than lipid-poor ApoE (LaDu et al., 1995; Strittmatter et al., 1993b; Tokuda et al., 2000). Accumulating recent evidence suggests that lipidation of ApoE strongly disrupts Aβ clearance across the BBB (Bell et al., 2007; Deane et al., 2008). These observations suggest that increased lipidation of ApoE-Aβ complex may reduce the fraction of Aβ that is cleared via LRP. Concomitant retention of ApoE-Aβ complexes in the brain is likely to exacerbate the extracellular Aβ load (DeMattos et al., 2004; Fryer et al., 2005).
It is noteworthy that the involvement of LRP and ApoE in Aβ metabolism is not limited to facilitating the clearance of Aβ. Aged PDAPP and Tg2576 APP transgenic mice with considerable amyloid deposits, when crossed onto a mouse with an ApoE knockout background, showed a significant decrease in Aβ deposits and an almost complete lack of true amyloid plaques and neuritic dystrophy (Bales et al., 1997; Holtzman et al., 1999; Holtzman et al., 2000; Irizarry et al., 2000). Later studies with APP transgenic mice carrying human ApoE isoforms show an isoform specific effect on Aβ accumulation (murine apoE>ApoE4>ApoE3>ApoE2), and supported the hypothesis that ApoE is critical for the development of fibrillar, compact amyloid plaques. LRP has also been shown to interact directly with the extracellular domain of APP through its Kunitz-type serine protease inhibitor (KPI) domain and also indirectly with the cytoplasmic tail of APP through a common adaptor protein FE65 (Kinoshita et al., 2001; Knauer et al., 1996; Kounnas et al., 1995; Pietrzik et al., 2004; Trommsdorff et al., 1998). Interestingly, an increase in KPI containing isoforms of APP protein levels has been reported in AD (Harrison et al., 1996; Moir et al., 1998; Preece et al., 2004). Furthermore, it has been shown that LRP facilitates APP endocytic trafficking and amyloidogenic processing by bringing both APP and β-secretase into close proximity (Pietrzik et al., 2002; Ulery et al., 2000). The slightly acidic pH of the endosomes also favors β-secretase mediated amyloidogenic processing (Vassar and Citron, 2000). On the other hand, retention of APP at the cell surface favors the non-amyloidogenic processing of APP (Koo et al., 1996; Koo and Squazzo, 1994; Perez et al., 1999). Although Aβ is produced intracellularly, once secreted, it can be internalized via receptor mediated endocytosis and targeted for degradation. It has been shown that a fraction of Aβ that is internalized by neurons can lead to intraneuronal Aβ accumulation (Zerbinatti et al., 2006) which is toxic (Billings et al., 2005). Recent evidence suggests that a large portion of intracellular Aβ accumulates because of the interaction between Aβ, ApoE and LRP (LaFerla et al., 2007). The accumulation of Aβ inside neurons has also been observed in both AD patients and mice with brain amyloid deposition (Chui et al., 1999; Gouras et al., 2000; Mochizuki et al., 2000; Oddo et al., 2003; Shie et al., 2003). Intraneuronal Aβ accumulation appears to occur prior to extracellular amyloid deposition and is a prominent neuropathological feature in brain regions that are vulnerable in AD, such as hippocampus. Specifically, intraneuronal Aβ accumulation is believed to be toxic (Billings et al., 2005) and to contribute to early cognitive deficits in transgenic AD mouse model (Oddo et al., 2003; Zerbinatti et al., 2004). These in vitro and animal model studies are completely consistent with those of the current analyses. Therefore, upregulated expression of LRP (reported here) is likely to facilitate increased amyloidogenic processing of APP, and the resulting intraneuronal accumulation of Aβ may contribute to cognitive decline in individuals harboring dysregulated LRP expression. However, it is important to note that postmortem and homogenate nature of the current study permits only the inference of progression and not a cause and effect measurement. As such it is possible that the observed transcriptional changes represented “compensatory” upregulation to overcome Aβ neurotoxicity presumably through the modulation of membrane cholesterol efflux and increased ApoE lipidation. The validation of these two critically important alternative hypotheses must rest with future studies of physiologically relevant and appropriate animal models.
In summary, the present study has shown that LRP and ApoE expression is upregulated in individuals with varying severity of dementia. This association is present even after controlling for various potential confounding factors such as age, pH and RIN. In addition, the relationship between LRP and ApoE dysregulation and AD extends not only to cognitive dysfunction but to its neuropathological hallmarks. In light of the critical role played by LRP and ApoE in APP processing, Aβ and cholesterol trafficking, these data suggest that cholesterol homeostasis is altered at the earliest recognizable stage of cognitive impairment.
Acknowledgments
This research is supported by following grant: P01-AG02219.
Footnotes
Disclosure Statement
There are no actual or potential conflicts of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akram A, Schmeidler J, Katsel P, Hof PR, Haroutunian V. Increased expression of cholesterol transporter ABCA1 is highly correlated with severity of dementia in AD hippocampus. Br Res. 2010;1318:167–177. doi: 10.1016/j.brainres.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT. LRP and senile plaques in Alzheimer’s disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104:38–46. doi: 10.1016/s0169-328x(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid beta- peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Barton AJ, Pearson RC, Najlerahim A, Harrison PJ. Pre- and postmortem influences on brain RNA. J Neurochem. 1993;61:1–11. doi: 10.1111/j.1471-4159.1993.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116:487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Cohn JS, Petit-Turcotte C, Tremblay M, Aumont N, Ramassamy C, Davignon J, Poirier J. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer’s disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843:87–94. doi: 10.1016/s0006-8993(99)01894-6. [DOI] [PubMed] [Google Scholar]
- Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J Lipid Res. 2004;45:403–409. doi: 10.1194/jlr.R300017-JLR200. [DOI] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Bertram L. Alzheimer’s disease genetics current status and future perspectives. Int Rev Neurobiol. 2009;84:167–184. doi: 10.1016/S0074-7742(09)00409-7. [DOI] [PubMed] [Google Scholar]
- Bertrand P, Poirier J, Oda T, Finch C, Pasinetti G. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Mol Brain Res. 1995;33:174–178. doi: 10.1016/0169-328x(95)00097-c. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Blacker D, Haines JL, Rodes L, Terwedow H, Go RC, Harrell LE, Perry RT, Bassett SS, Chase G, Meyers D, Albert MS, Tanzi R. ApoE-4 and age at onset of Alzheimer’s disease: the NIMH genetics initiative. Neurology. 1997;148:139–147. doi: 10.1212/wnl.48.1.139. [DOI] [PubMed] [Google Scholar]
- Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Jehu L, Moskvina V, Buxbaum JD, Dracheva S, Haroutunian V, Williams J, Buckland PR, Owen MJ, O’Donovan MC. Allelic expression of APOE in human brain: effects of epsilon status and promoter haplotypes. Human Molecular Genetics. 2004;13:2885–2892. doi: 10.1093/hmg/ddh299. [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- Bu G, Maksymovitch EA, Nerbonne JM, Schwartz AL. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J Biol Chem. 1994;269:18521–18528. [PubMed] [Google Scholar]
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek J, Wittels IG, Berg L. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol. 1988;45:31–32. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- Byne W, Dracheva S, Chin B, Schmeidler JM, Davis KL, Haroutunian V. Schizophrenia and Sex Associated Differences in the Expression of Neuronal and Oligodendrocyte Specific Genes in Individual Thalamic Nuclei. Schizo Res. 2008;98:118–128. doi: 10.1016/j.schres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causevic M, Ramoz N, Haroutunian V, Davis KL, Buxbaum JD. Lack of association between the levels of the low-density lipoprotein receptor-related protein (LRP) and cognitive decline or dementia. Journal of Neuropathology and Experimental Neurology. 2003;62:999–1005. doi: 10.1093/jnen/62.10.999. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de lSV, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analyt Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chui DH, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirotani K, Takahashi K, Gallyas F, Tabira T. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med. 1999;5:560–564. doi: 10.1038/8438. [DOI] [PubMed] [Google Scholar]
- Corder E, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s Disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA. 1999;281:1401–1406. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;8:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and Clusterin Cooperatively Suppress Abeta Levels and Deposition. Evidence that ApoE Regulates Extracellular Abeta Metabolism In Vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Fischl B, Cabral HJ, Kemper TL, Guttmann CR, Blacker D, Hyman BT, Albert MS, Killiany RJ. MRI measures of temporoparietal regions show differential rates of atrophy during prodromal AD. Neurology. 2008;71:819–825. doi: 10.1212/01.wnl.0000320055.57329.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- Dooneief G. The Clinical Dementia Rating scale: community-based validation of “profound’ and “terminal’ stages. 1996 doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;920:1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Fryer J, Simmons K, Parsadanian M, Bales K, Paul S, Sullivan P, Holtzman D. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. Journal of Neuroscience. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Giannakopoulos AS, Herrmann FR, Michel JP, Bouras C. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of very old patients. Arch Neurol. 1995;52:1150–1159. doi: 10.1001/archneur.1995.00540360028012. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locascio JJ, Perls TT, Lipsitz LA, Hyman BT. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer’s disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grehan S, Tse E, Taylor JM. Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J Neurosci. 2001;21:812–822. doi: 10.1523/JNEUROSCI.21-03-00812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Davies P, Vianna C, Buxbaum JD, Purohit DP. Tau protein abnormalities associated with the progression of alzheimer disease type dementia. Neurobiol Aging. 2007;28:1–7. doi: 10.1016/j.neurobiolaging.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Schmeidler J. Transcriptional vulnerability of brain regions in Alzheimer’s disease and dementia. Neurobiology of Aging. 2009;30:561–573. doi: 10.1016/j.neurobiolaging.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Purohit DP, Perl DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol. 1999;56:713–718. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ. Brain expression of apolipoproteins E, J, and A-I in Alzheimer’s disease. J Neurochem. 1996;66:2429–2435. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Wighton-Benn WH, Heffernan JM, Sanders MW, Pearson RC. Amyloid precursor protein mRNAs in Alzheimer’s disease. Neurodegeneration. 1996;5:409–415. doi: 10.1006/neur.1996.0055. [DOI] [PubMed] [Google Scholar]
- Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse C, Bogdanovic N, Davidsson P, Blennow K. A quantitative and immunohistochemical study on apolipoprotein E in brain tissue in Alzheimer’s disease. Dement Geriatr Cogn Disord. 1999;10:452–459. doi: 10.1159/000017189. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Fagan AM, Chang LK, Sun Y, Paul SM. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer’s disease. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000;47:739–747. [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Rebeck GW, Cheung B, Bales K, Paul SM, Holzman D, Hyman BT. Modulation of A beta deposition in APP transgenic mice by an apolipoprotein E null background. Ann N Y Acad Sci. 2000;920:171–178. doi: 10.1111/j.1749-6632.2000.tb06919.x. [DOI] [PubMed] [Google Scholar]
- Ishii K, Tamaoka A, Mizusawa H, Shoji S, Ohtake T, Fraser PE, Takahashi H, Tsuji S, Gearing M, Mizutani T, Yamada S, Kato M, St George-Hyslop PH, Mirra SS, Mori H. Abeta1-40 but not Abeta1-42 levels in cortex correlate with apolipoprotein E epsilon4 allele dosage in sporadic Alzheimer’s disease. Brain Res. 1997;748:250–252. doi: 10.1016/s0006-8993(96)01363-7. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Morgan DG, Finch CE. Extensive postmortem stability of RNA from rat and human brain. J Neurosci Res. 1986;16:267–280. doi: 10.1002/jnr.490160123. [DOI] [PubMed] [Google Scholar]
- Jordan J, Galindo MF, Miller RJ, Reardon CA, Getz GS, LaDu MJ. Isoform-specific effect of apolipoprotein E on cell survival and beta-amyloid-induced toxicity in rat hippocampal pyramidal neuronal cultures. J Neurosci. 1998;18:195–204. doi: 10.1523/JNEUROSCI.18-01-00195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor- related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- Katsel P, Tan W, Haroutunian V. Gain in Brain Immunity in the Oldest-Old Differentiates Cognitively Normal from Demented Individuals. PloS ONE. 2009;4:e7642. doi: 10.1371/journal.pone.0007642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Whelan CM, Smith CJ, Mikhailenko I, Rebeck GW, Strickland DK, Hyman BT. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J Neurosci. 2001;21:8354–8361. doi: 10.1523/JNEUROSCI.21-21-08354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer MF, Orlando RA, Glabe CG. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP) Brain Res. 1996;740:6–14. doi: 10.1016/s0006-8993(96)00711-1. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J Cell Sci. 1996;109(Pt 5):991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- LaDu MJ, Pederson TM, Frail DE, Reardon CA, Getz GS, Falduto MT. Purification of apolipoprotein E attenuates isoform-specific binding to beta-amyloid. J Biol Chem. 1995;270:9039–9042. doi: 10.1074/jbc.270.16.9039. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Perez-tur J, Dupire MJ, Galasko D, Mann D, Amouyel P, Hardy J, Delacourte A, Chartier-Harlin MC. Distortion of allelic expression of apolipoprotein E in Alzheimer’s disease. Hum Mol Genet. 1997;6:2151–2154. doi: 10.1093/hmg/6.12.2151. [DOI] [PubMed] [Google Scholar]
- Landen M, Hesse C, Fredman P, Regland B, Wallin A, Blennow K. Apolipoprotein E in cerebrospinal fluid from patients with Alzheimer’s disease and other forms of dementia is reduced but without any correlation to the apoE4 isoform. Dementia. 1996;7:273–278. doi: 10.1159/000106892. [DOI] [PubMed] [Google Scholar]
- Lefranc D, Vermersch P, Dallongeville J, Daems-Monpeurt C, Petit H, Delacourte A. Relevance of the quantification of apolipoprotein E in the cerebrospinal fluid in Alzheimer’s disease. Neurosci Lett. 1996;212:91–94. doi: 10.1016/0304-3940(96)12774-9. [DOI] [PubMed] [Google Scholar]
- Lendon CL, Talbot CJ, Craddock NJ, Han SW, Wragg M, Morris JC, Goate AM. Genetic association studies between dementia of the Alzheimer’s type and three receptors for apolipoprotein E in a Caucasian population. Neurosci Lett. 1997;222:187–190. doi: 10.1016/s0304-3940(97)13381-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Cam J, Bu G. Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol Neurobiol. 2001;23:53–67. doi: 10.1385/MN:23:1:53. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, Kleinman JE. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol Psychiatry. 2006;60:650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Matsui T, Ingelsson M, Fukumoto H, Ramasamy K, Kowa H, Frosch MP, Irizarry MC, Hyman BT. Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res. 2007;1161:116–123. doi: 10.1016/j.brainres.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Jr, Holland D, Koyama A, Brewer JB, Dale AM. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73:457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Vogel FS, Heyman A. CERAD. Durham, NC: 1987. Guide to the CERAD Protocol for the Neuropathological Assessment of Alzheimer’s Disease. [Google Scholar]
- Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, Han LY, Bienias JL, Lee VM, Trojanowski JQ, Bennett DA, Arnold SE. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- Mochizuki A, Tamaoka A, Shimohata A, Komatsuzaki Y, Shoji S. Abeta42-positive non-pyramidal neurons around amyloid plaques in Alzheimer’s disease. Lancet. 2000;355:42–43. doi: 10.1016/S0140-6736(99)04937-5. [DOI] [PubMed] [Google Scholar]
- Moestrup SK, Gliemann J, Pallesen G. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269:375–382. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- Moir RD, Lynch T, Bush AI, Whyte S, Henry A, Portbury S, Multhaup G, Small DH, Tanzi RE, Beyreuther K, Masters CL. Relative increase in Alzheimer’s disease of soluble forms of cerebral Abeta amyloid protein precursor containing the Kunitz protease inhibitory domain. J Biol Chem. 1998;273:5013–5019. doi: 10.1074/jbc.273.9.5013. [DOI] [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Nathan B, Bellosta S, Sanan D, Weisgraber K, Mahley R, Pitas R. Differential Effects of Apolipoproteins E3 and E4 on Neuronal Growth in Vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oleinikov AV, Zhao J, Makker SP. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem J. 2000;347(Pt 3):613–621. [PMC free article] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Pietrzik CU, Busse T, Merriam DE, Weggen S, Koo EH. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 2002;21:5691–5700. doi: 10.1093/emboj/cdf568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci. 2004;24:4259–4265. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirttila T, Lehtimaki T, Rinne J, Mattila K, Frey H, Nikkari T. The frequency of apolipoprotein E4 allele is not increased in patients with probable vascular dementia. Acta Neurol Scand. 1996a;93:352–354. doi: 10.1111/j.1600-0404.1996.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Pirttila T, Soininen H, Heinonen O, Lehtimaki T, Bogdanovic N, Paljarvi L, Kosunen O, Winblad B, Riekkinen P, Sr, Wisniewski HM, Mehta PD. Apolipoprotein E (apoE) levels in brains from Alzheimer disease patients and controls. Brain Res. 1996b;722:71–77. doi: 10.1016/0006-8993(96)00183-7. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Preece P, Virley DJ, Costandi M, Coombes R, Moss SJ, Mudge AW, Jazin E, Cairns NJ. Amyloid precursor protein mRNA levels in Alzheimer’s disease brain. Brain Res Mol Brain Res. 2004;122:1–9. doi: 10.1016/j.molbrainres.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Arch Gen Psychiatry. 1998;55:205–211. doi: 10.1001/archpsyc.55.3.205. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Strickland DK, Hyman BT, Rebeck GW. Elevation of LDL receptor-related protein levels via ligand interactions in Alzheimer disease and in vitro. J Neuropathol Exp Neurol. 2001;60:430–440. doi: 10.1093/jnen/60.5.430. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the alpha 2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann Neurol. 1995;37:211–217. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- Rensen PC, Jong MC, van Vark LC, van der Boom H, Hendriks WL, van Berkel TJ, Biessen EA, Havekes LM. Apolipoprotein E is resistant to intracellular degradation in vitro and in vivo. Evidence for retroendocytosis . J Biol Chem. 2000;275:8564–8571. doi: 10.1074/jbc.275.12.8564. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Mangelsdorf DJ. The liver X receptor gene team: potential new players in atherosclerosis. Nat Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annual Review of Medicine. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;123:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie FS, LeBoeuf RC, Jin LW. Early intraneuronal Abeta deposition in the hippocampus of APP transgenic mice. NeuroReport. 2003;14:123–129. doi: 10.1097/01.wnr.0000051151.87269.7d. [DOI] [PubMed] [Google Scholar]
- Sihlbom C, Davidsson P, Sjogren M, Wahlund LO, Nilsson CL. Structural and quantitative comparison of cerebrospinal fluid glycoproteins in Alzheimer’s disease patients and healthy individuals. Neurochem Res. 2008;33:1332–1340. doi: 10.1007/s11064-008-9588-x. [DOI] [PubMed] [Google Scholar]
- Strittmatter JM, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: High-avidity binding to B-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer’s disease. PNAS. 1993a;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993b;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;5:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, LaDu MJ, Rostagno A, Frangione B, Ghiso J. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid beta peptides. BiochemJ. 2000;348(Pt 2):359–365. [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J Biol Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034.1–0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Citron M. Abeta-generating enzymes: recent advances in beta- and gamma-secretase research. Neuron. 2000;27:419–422. doi: 10.1016/s0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Tomita H, Meng F, Bolstad B, Li J, Evans S, Choudary P, Atz M, Shao L, Neal C, Walsh DM, Burmeister M, Speed T, Myers R, Jones EG, Watson SJ, Akil H, Bunney WB. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders. Molecular Psychaitry. 2006;11:663–679. doi: 10.1038/sj.mp.4001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler K, Scharnagl H, Tisljar U, Hoschutzky H, Friedrich I, Hoffmann MM, Huttinger M, Wieland H, Marz W. Competition of Abeta amyloid peptide and apolipoprotein E for receptor-mediated endocytosis. J Lipid Res. 1999;40:447–455. [PubMed] [Google Scholar]
- Wolf H, Grunwald M, Ecke GM, Zedlick D, Bettin S, Dannenberg C, Dietrich J, Eschrich K, Arendt T, Gertz HJ. The prognosis of mild cognitive impairment in the elderly. J Neural Transm Suppl. 1998;54:31–50. doi: 10.1007/978-3-7091-7508-8_4. [DOI] [PubMed] [Google Scholar]
- Zarow C, Victoroff J. Increased apolipoprotein E mRNA in the hippocampus in Alzheimer disease and in rats after entorhinal cortex lesioning. Exp Neurol. 1998;149:79–86. doi: 10.1006/exnr.1997.6709. [DOI] [PubMed] [Google Scholar]
- Zerbinatti CV, Wahrle SE, Kim H, Cam JA, Bales K, Paul SM, Holtzman DM, Bu G. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta42 accumulation in amyloid model mice. J Biol Chem. 2006;281:36180–36186. doi: 10.1074/jbc.M604436200. [DOI] [PubMed] [Google Scholar]
- Zerbinatti CV, Wozniak DF, Cirrito J, Cam JA, Osaka H, Bales KR, Zhuo M, Paul SM, Holtzman DM, Bu G. Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A. 2004;101:1075–1080. doi: 10.1073/pnas.0305803101. [DOI] [PMC free article] [PubMed] [Google Scholar]