Abstract
The majority of patients with multiple myeloma develop bone osteolytic lesions, which may lead to severe complications, including pain and fractures. The pathogenesis of bone disease depends on uncoupled bone remodeling, characterized by increased bone resorption due to upregulation of osteoclast activity and decreased bone formation due to osteoblast inhibition. In myeloma, impaired osteoblast differentiation and increased apoptosis have been described. Responsible for these effects are integrin-mediated adhesion to tumor cells and soluble factors, including WNT antagonists, BMP2 inhibitors and numerous cytokines. Based on the evidence of osteoblast suppression in myeloma, bone anabolic agents have been developed and are currently undergoing clinical evaluation. Due to bidirectional inhibitory effects characterizing tumor cells and osteoblasts interactions, agents targeting osteoblasts are expected to reduce tumor burden along with improvement of bone health. This review summarizes the current knowledge on osteoblast inhibition in myeloma and provides an overview on the clinical grade agents with bone anabolic properties, which represent new promising therapeutic strategies in myeloma.
Keywords: Myeloma, Bone osteolytic disease, Osteoblast, Osteoclast
Introduction
More than 60000 people in the US live with multiple myeloma (MM), a plasma cell malignancy characterized by monoclonal paraprotein production and bone involvement. Nearly 80% of MM patients develop bone lesions, which often lead to severe complications including pain, pathologic bone fractures and hypercalcemia [1]. Patients experiencing bone disease have a decreased quality of life; in addition, development of fractures is associated with shorter survival [2, 3]. Bone lesions in MM patients are the result of an uncoupled bone remodeling due to the interactions between tumor cells and the bone marrow (BM) microenvironment.
Cellular and extracellular elements form the BM milieu. Bone marrow stromal cells (BMSC), osteoblasts (OB), osteoclasts (OC), as well as endothelial and immune cells regulate each others function by direct cell-to-cell contact, cytokine secretion and extracellular matrix protein deposition. The balanced interactions within the BM niche are responsible for effective immune response, normal hematopoiesis and coupled bone remodeling. Bone remodeling, in particular, depends on the concerted activity of OCs, resorbing bone and OBs, forming new bone. Uncoupled bone remodeling derives from the unbalanced activity of OC and OB, as observed in MM. Malignant plasma cells home to the BM, disrupt its balance and upregulate bone resorption. Despite the generalized osteoclast activation, OB function in MM is impaired, with decreased bone formation and calcification rate [4–6]. Due to the suppressed OB activity, bone resorption is not compensated leading to osteolytic lesions. In addition, tumor cells stimulate angiogenesis, and alter the cytokine profile in the BM milieu, favoring the release of chemotactic and growth cytokines as well as OC activating factors [7–9]. Importantly, the interactions within the BM milieu are bidirectional, since OCs, BMSCs and endothelial cells support tumor cell proliferation and mediate chemoresistance [1]. Conversely, OBs and immune cells have an overall inhibitory effect on tumor cell proliferation [10, 11].
Therefore, malignant plasma cells shape the BM microenvironment in a niche permissive to cancer propagation and therapies targeting the tumor milieu may restore bone remodeling and also reduce tumor burden. Indeed, the introduction of new therapeutic agents, such as the anti-MM and bone-anabolic agent bortezomib, significantly prolonged patients’ survival. Nonetheless, there is still no evidence of cure and more than 10,000 patients die each year in the US from MM related complications [12]. In addition, treatment strategies for patients with bone disease are limited and largely palliative, since they aim at alleviating pain and reducing the incidence of complications. Ongoing studies therefore attempt to unravel the pathogenesis of bone lesions in MM with the goal of identifying novel therapeutically relevant targets. The data supporting the key role of OB inhibition in the pathogenesis of bone disease have lead to the development of anabolic agents. This review will provide an overview of the mechanisms of OB suppression in MM and discuss the bone anabolic agents in clinical development as well as those with promising preclinical data.
Pathogenesis of Osteoblast Inhibition in MM
OB originate from mesenchymal progenitor cells along with adipocytes, chondrocytes and myocytes. Together with OCs, they are responsible for bone remodeling. Active and inactive forms of OBs line the bone surface to regulate new bone formation. Once bone matrix is deposited, they remain trapped within the bone and form osteocytes, which function as mechanical receptors directing the process of bone remodeling according to stress forces [13, 14]. In addition to maintaining bone structure, OBs negatively affect MM cell survival. Coculture data showed a diminished tumor cell proliferation in the presence of OBs compared to OCs or BMSCs [10]. Increased OB differentiation, via up-regulation of the osteogenic signaling β-catenin, results in tumor growth inhibition in murine models of myeloma bone disease [15]. Although the mechanism of inhibition remains unclear, small leucine-rich proteoglycans may be involved. Decorin, in particular, is an OB-derived extracellular matrix component inducing MM cell apoptosis via p21 activation and inhibiting angiogenesis and osteoclastogenesis [16]. In addition, OBs affect tumor cell growth indirectly via their regulatory effects on OCs. OBs secrete receptor activator of NF-kB ligand (RANKL), a critical growth factor for OCs, and osteoprotegerin (OPG), a soluble RANKL inhibitor. The balance between RANKL and OPG modulates osteoclastogenesis and MM cells typically increase the RANKL/OPG ratio [17, 18] promoting OC development.
Systemic and local factors like parathyroid hormone (PTH), fibroblast growth factor (FGF), Wnt and BMP (bone morphogenetic proteins) [19] regulate osteogenesis by activating signaling pathways and transcription factors, including β-catenin, SMAD, runt-related transcription factor (RUNX)2, DLX5 and osterix. MM cells deregulate these osteogenic signaling by means of cellular interactions and cytokine secretion, resulting in inhibition of OB differentiation and function. In addition, tumor cells induce OB and osteocytes apoptosis [20, 21], the latter is followed by release of pro-osteoclastogenic cytokines such as CCL3 further contributing to the OB/OC uncoupling.
Direct cell-to-cell contact between MM and OB progenitor cells downregulates RUNX2 activity, a critical osteogenic transcription factor [22]. RUNX2 mediates progenitor cell commitment to the OB lineage and modulates the expression of several bone matrix protein genes [23]. RUNX2 knockout mice have impaired OB differentiation and consequently lack bone formation [24]. RUNX2 effects on early OB differentiation are at least partially mediated by osterix. Osterix null mice have endochondral skeleton in the absence of bone formation [25]. Osterix is also critical to OB function, since postnatal gene knockout reduces bone formation rate and mineralization without affecting OB proliferation or differentiation [26]. Responsible for MM inhibitory effect on RUNX2 is the interaction between the integrin very late antigen (VLA)-4 on MM cells and vascular cell adhesion molecule (VCAM)-1 on BMSCs, since neutralizing antibodies against VLA4 restore RUNX2 expression and OB differentiation in MM-OB co-culture [22].
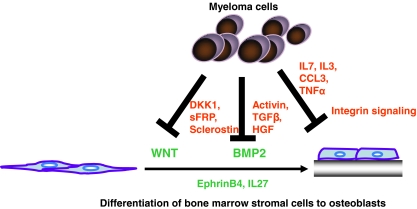
In addition to cellular interactions, several soluble factors contribute to OB suppression in MM, including WNT signaling antagonists, transforming growth factor (TGF)-β family members, chemokines and several interleukins (Fig. 1).
Fig. 1.
Mechanisms of OB inhibition by MM cells. MM cells inhibit OB differentiation by deregulating the WNT and BMP2 signaling pathway via the secretion of inhibitory cytokines, such as DKK1 or Activin. In addition, integrin-mediated cell interactions are responsible for impaired osteoblastogenesis. Inhibition of OB function and induction of apoptosis play also a role in MM-mediated OB impairment
WNT Signaling Antagonists By binding to surface receptors, low-density lipoprotein receptor-related protein (LRP)5/6 and Frizzled, WNT and other ligands modulate β-catenin- or calcium-dependent intracellular signaling pathways, which ultimately converge to RUNX2 and osterix transcription factors. WNT3a promotes OB differentiation and function, conversely dickkopf (DKK)1, soluble frizzled receptor-like proteins (sFRP)3 and sclerostin inhibit osteogenesis [27–29]. BM serum of MM patients is often characterized by high levels of WNT inhibitors, which are considered promising therapeutic targets.MM cells secrete DKK1, whose BM serum levels in patients correlate with the presence of osteolytic lesions [30]. DKK1 inhibits osteoblastogenesis by sequestering LRP5/6 from binding WNT, thereby downregulating RUNX2 activity. Most likely, the effects are mediated by the non-canonical Wnt signaling pathway since DKK1 levels are independent from β-catenin activation status [31, 32]. In addition, MM-derived DKK1 inhibits OPG expression in OBs and upregulates RANKL secretion [18], therefore stimulating osteoclastogenesis. Neutralizing antibodies against DKK1 promote OB differentiation [30] and limit MM cell proliferation when co-cultured with BMSC [33]. In several in-vivo models of MM bone disease, DKK1 inhibition prevents tumor-induced bone destruction, increases trabecular bone formation in both bones bearing MM cells and uninvolved bones, and reduces tumor burden [31, 33, 34].Genetic studies in family with sclerosteosis, a rare genetic disease characterized by high bone mass due to loss of SOST (sclerostin) gene expression, identified sclerostin as an osteocyte-derived WNT and BMP inhibitor [19, 35, 36]. Compared to MGUS patients and healthy controls, MM patients have higher BM plasma levels of sclerostin, which positively correlate with β2-microglobulin and advanced international staging system (ISS) stage, and negatively with bone alkaline phosphatase (bALP), a marker of OB function. In addition, survival of patients with high amounts of sclerostin is shorter, 27 vs. 98 months [37]. Sclerostin has also been detected in MM cells, both cell lines and primary cells. MM-derived sclerostin inhibits osteoblastogenesis presumably via suppression of β-catenin signaling, since neutralizing antibodies lead to intranuclear β-catenin accumulation and ultimately restore OB differentiation in the presence of MM cells. In addition, sclerostin deregulates the RANKL/OPG balance [38]. Sclerostin may therefore play an important role in MM-mediated OB inhibition.In addition to DKK1 and sclerostin, levels of sFRP2 and 3 are also upregulated in BM plasma of MM patients with bone lesions. Secreted by MM cells as well, they inhibit WNT signaling and OB differentiation. Neutralizing antibodies targeting sFRP2 partially restore OB differentiation in the presence of MM cell conditioned media, thereby sFRP may contribute to OB inhibition [32, 39].
Activin A and TGFβ In addition to the WNT/β-catenin signaling pathway, OB differentiation is modulated by several TGFβ family members, including bone morphogenic proteins (BMPs), activin and TGFβ itself. These soluble factors activate dimeric receptors and primarily SMAD signaling pathways. BMP2 in particular stimulates OB differentiation via SMAD1 and distal-less homeobox (DLX)5 upregulation [40]. Opposite effects are instead exerted by other members, such as activin A and TGFβ.Activin A is a dimeric protein binding to the activin receptor 2A (ACVR2A), whose intracellular moiety recruits and activates type I receptors (in particular, ALK4). The type I receptor activates SMAD2, that induces the nuclear translocation of SMAD4 resulting in gene modulation, such as DLX5 inhibition [41, 42]. DLX5 contributes to the regulation of osterix expression [43]. Lack of DLX5 gene expression induces abnormal osteogenesis. DLX5 is also a common target of the β-catenin signaling pathway [44, 45], so that differential effects on DLX5 transcription account for the opposing effects of Wnt10b, BMP2, TGFβ and activin on OB differentiation [45–47]. High activin A levels correlate with advanced ISS stage, extensive bone disease and decreased survival in patients at diagnosis and in the relapse setting. Interestingly, increase in activin A corresponds to increased bone resorption marker, while no association with parameters of bone formation have been observed [48]. Tumor cells do not directly produce activin but stimulate its secretion by BMSC; OCs represent also a source for the cytokine. Activin-mediated upregulation of SMAD2 signaling pathway results in DLX5 inhibition and impairment of osteoblastogenesis. DLX5 downregulation in BM biopsies of MM patients correlates with bone disease and high BM plasma activin A levels [47]. In addition, activin exerts a pro-OC effect, stimulating OC differentiation and function [47, 49]. Other roles of activin A include regulation of craniofacial development, gonadal function via modulation of FSH secretion and erythropoiesis [50, 51]. Promising preclinical data using inhibitors of activin A signaling have been obtained in breast cancer and MM mouse models [47, 52].Known to stimulate bone resorption and tumor growth in breast cancer, TGFβ also inhibits differentiation of mesenchymal stem cells and preosteoblastic cell lines into OBs, at least partially via downregulation of RUNX2 and DLX5 expression [46, 53]. In addition, it impairs also adipogenesis, thus suggesting effects on a common progenitor cell [54]. TGFβ inhibition has potent anabolic effects [55] and restores osteogenesis in the presence of MM cell-conditioned media and BM plasma from patients. It also suppresses MM cell growth. In vivo these effects translated in tumor burden reduction and prevention of lytic lesions in murine models of MM [54].Finally, MM-derived HGF has also been described as negative regulator of BMP-induced osteogenesis. HGF blocks SMAD nuclear translocation, thus suppressing both RUNX2 and osterix transcription factor and maintaining mesenchymal stem cells in a proliferative, undifferentiated state. HGF levels in sera of MM patients negatively correlate with bALP levels, further supporting its role as OB inhibitor [56].
Chemokines and Interleukins There are evidences for the involvement of several cytokines in MM bone disease, which play a dual role as OC stimulators and OB inhibitors, including CCL3, tumor necrosis factor (TNF)-α, interleukin (IL)-3 and IL-7.The chemokine CCL3 has a critical role in the pathogenesis of bone disease for its pro-OC effects. It strongly correlates with osteolytic burden in MM [57–59]. Indeed, CCL3 by binding two receptors, CCR1 and CCR5, potently stimulates OC differentiation mediating the fusion of precursor cells into active mature OCs and upregulating RANKL expression by OBs [60–62]. CCL3 is also responsible for increased angiogenesis, tumor cell migration to the BM and tumor cell growth [7, 63]. Recent studies suggest an additional inhibitory effect on OB function. CCL3 suppresses OB mineralization activity by impairing osterix expression and osteocalcin secretion via ERK signaling activation. These effects are at least partly mediated by CCR1, since a trend for increased osteocalcin levels is observed with CCR1 antagonists both in vitro and in vivo [64].Along with CCL3 other inflammatory cytokines, namely TWEAK and TNFα, show OB inhibitory properties. TNFα impairs pre-OB proliferation and induces mature OB apoptosis; mediators of these effects are RUNX2 suppression via Gfi-1 upregulation and induction of sclerostin [65–68]. In addition, TNFα upregulates IL-6 secretion by BMSC, thus promoting tumor cell proliferation [69].Secreted by both malignant plasma cells and T lymphocytes, interleukin (IL)-3 synergies with RANKL to induce OC differentiation and stimulates MM cell growth. Furthermore, IL-3 inhibits BMP2-induced OB differentiation indirectly via stimulation of CD45+ monocytic-macrophagic cell population [70–72]. IL-7 is also a MM-derived cytokine, which induces RANKL production by T lymphocytes [73] and mediates MM-induced OB inhibition via downregulation of RUNX2 transcriptional activity [22]. Interestingly, IL-27 exerts opposing effects in comparison to IL-3 and IL-7. This cytokine has anti-tumor, anti-angiogenic and anabolic effect in MM, and therefore may represent a promising therapeutic agent [74].
Ephrin Signaling Along with these mechanisms, a deregulation of the ephrin signaling pathway also contributes to OB suppression in MM. The Ephrin/Eph pathway mediates bidirectional signaling coupling between OBs and OCs. Osteoblastogenesis is promoted by stimulation of the receptor EphB4 on OB surface via the membrane-bound ligand ephrinB2 expressed by OCs. In turn, reverse signaling via ephrinB2 inhibits OC differentiation [75], thus regulating bone remodeling. MM cells downregulate expression levels of both ligand and receptor in BMSC. In vivo treatment with chimeric ephrinB2-Fc stimulates angiogenesis, osteoblastogenesis, and bone formation. In addition to these effects, treatment with EphB4 inhibits tumor growth, osteoclastogenesis, and angiogenesis [76].In addition to restoring bone health in MM, stimulating OB differentiation may inhibit MM cell growth directly and indirectly via reduced OC differentiation. Therefore, OB inhibitors identified in MM are considered optimal targets to develop new anti-MM strategies.
Treatment of MM Bone Disease with Bone-Anabolic Agents
Without treatment patients with bone disease experience more than 2 skeletal related events (SRE) per year, defined as pathologic fractures, vertebral body compression fractures, hypercalcemia, pain, need for radiation or surgery [77]. Despite the occurrence of such complications, which reduce performance status and increase mortality rate, treatment of bone disease remains mainly palliative [2, 3]. The only FDA-approved bone-targeted agents in MM-related are Bisphosphonates (eg, zoledronic acid), which prevent bone lesions and significantly reduce SRE, but have purely anti-catabolic properties without anabolic effects. Nearly one SRE per year still occurs under bisphosphonates therapy and bone lesions do not heal [77]. The strong evidences on the critical role played by OB suppression in the pathogenesis of osteolytic lesions in MM suggest that bone anabolic agents may have a positive impact on bone remodeling in MM. In addition, a balanced bone homeostasis may inhibit tumor growth. Several agents with bone anabolic properties are currently undergoing clinical evaluation, including bortezomib, sotatercept and BHQ880, other clinical grade compounds have shown promising pre-clinical data, such as CCR1 antagonists, sclerostin inhibitors and teraparamide (Table 1).
Table 1.
Novel clinical grade bone-anabolic agents
| Agent | Mechanism of action | In-vivo preclinical data in MM | Clinical results in MM |
|---|---|---|---|
| Bortezomib [85, 90] | Proteasome and NF-kB signaling pathway inhibitor | Increases BMD and OBn | Increases bone formation markers in responding patients. Decreases bone resorption markers. |
| Decreases OCn and tumor burden | Ongoing clinical trials are assessing effects on BMD | ||
| Sotatercept [47, 52, 96] | Decoy receptor neutralizing activin A | Increases bone volume and OBn | Increases bone formation markers, decreases pain. |
| Decreases tumor burden | |||
| BHQ880 [33] | Neutralizing anti-DKK1 antibody | Increases bone volume and OBn | NA |
| Decreases tumor burden | |||
| MLN3897 [64] | Small molecule CCR1 antagonists | Trend for increase in osteocalcin expression | NA |
| Decreases OCn and tumor burden | |||
| Teriparatide [109] | Recombinant parathyroid hormone | Increases BMD | NA |
| Decreases tumor burden | |||
| AMG775 | Neutralizing anti-sclerostin antibody | NA | NA |
BMD bone mineral density; OBn osteoblast number; OCn osteoclast number; NA not available
Bortezomib Bortezomib is a potent anti-tumor agent, whose mechanisms of action range from plasma cell-directed cytotoxicity to modulation of the MM microenvironment. Inhibition of IkBα degradation, an inhibitor of NF-kB, limits NFkB signal transduction and leads to cell apoptosis. Bortezomib is also a reversible inhibitor of the proteasome, a scavenger pathway degradating immunoglobulin and other proteins in excess. The proteasome plays a key role in plasma cells and its inhibition further contributes to cell death [78]. In addition, Bortezomib impairs MM-BMSC interactions and modulates the OC/OB balance in the BM niche. It suppresses OC differentiation by p38 inhibition, impairment of NF-kB signaling and AP1 [79]. Importantly, it has a strong anabolic activity, which relies on proteasome inhibition and partly on DKK1 downregulation [80]. The proteasome regulates RUNX2 levels via its degradation, thereby proteasome inhibition results in RUNX2 and osterix upregulation [81–83]. Independently from the presence of MM cells, in-vivo treatment with bortezomib stimulates MSC towards osteogenic differentiation, at the expense of adipogenesis [82]. In several murine models of MM bone disease, treatment with bortezomib resulted in anti-tumor and bone anabolic effects [84, 85]. The diffusion of bortezomib in the clinical praxis, along with the immunomodulators thalidomide and lenalidomide, translated in longer patients’ survival [86]. Treatment with bortezomib upregulates parathyroid hormone (PTH) and parameters of OB activation, including bone alkaline phosphatase (bALP). In addition it downregulates bone-resorption markers. These effects are particularly evident in treatment responders [87–89]. A post-hoc analysis of the VISTA trial (melphalan-prednisone (MP) vs bortezomib plus MP in untreated MM patients) suggests that patients receiving bortezomib developed less bone lesions as opposed to MP-treated patients. Importantly, a subgroup of responding patients experienced healing of bone lesions. While MP alone upregulates DKK1 levels, treatment with bortezomib decreases them. Importantly, nearly 80% of the patients in each arm have been concomitantly treated with bisphosphonates [90]. Notably, combination with thalidomide (VMTD) diminishes benefits of bortezomib on OB differentiation [91]. Therefore, addition of bortezomib to standard regimens like MP has positive effects on osteogenesis independently from bisphosphonate treatment, whereas combination with thalidomide has a negative impact. With the aim of assessing effects on bone mineral density, a trial (NCT00972959) combining bortezomib, zoledronic acid and dexamethasone in MM patients with relapsed disease is currently recruiting patients. Preliminary results suggest that a subset of patients with osteoporosis and limited osteolytic burden may benefit from the combination in terms of increased bone mineral density (BMD) [92]. Additional studies have been designed to assess the bone effects of bortezomib. Low dose bortezomib (0.7 mg/m2) will be administered to patients with smoldering (NCT00983346) or relapsed MM (NCT01062230) to evaluate markers of OB activation. A third study (NCT01286077) will assess BMD in MM patients treated with bortezomib as consolidation therapy after autologous transplant.
Sotatercept Sotatercept (ACE-011, Acceleron Pharma) is a chimeric protein derived from the fusion of the extracellular component of the ACVR2A to the Fc domain of human IgG1. It sequesters activin A from binding to its membrane bound receptor, thus promoting osteogenesis. The murine counterpart of sotatercept, referred to as RAP-011, has been extensively preclinically evaluated in mouse models of cancer- and osteoporosis-related bone loss [47, 52, 93]. In vitro inhibition of activin A increases OB differentiation overcoming the inhibitory effect of MM cells. This effect may be partly due to attenuation of MM-cell induced SMAD2 phosphorylation in OB and rescue of the downregulation of DLX5 expression. In addition, RAP-011 decreases RANKL-mediated OC development. No direct effects on tumor cell survival or proliferation have been reported [47]. In the SCID-hu mouse model, a humanized model of MM generated by subcutaneously implanting fetal bone chips that are then injected with human MM cells [94], twice weekly treatment with RAP-011 increased bone volume and OB number. Indirect anti-tumor effect has been observed with a decline in the levels of soluble human IL-6 receptor secretion (a marker of tumor burden) and tumor cell infiltration in the implanted bones [47]. Similar results have been obtained with the 5T2MM mouse model. Compared with vehicle, RAP-011 increased OB number, bone surface occupied by OB and mineralization. Again RAP-011 yielded a 41% reduction in serum paraprotein levels and a 37% reduction in the tumor burden of bone [52]. Interestingly, neither of these models showed a significant difference in OC number. The anabolic effects of RAP-011 have also been confirmed in mouse models of breast cancer associated-bone disease and osteoporosis [52, 93]. A phase I dose-escalation trial in healthy post-menopausal women showed dose-dependent increase in serum levels of bALP, persisting up to 120 days after a single intravenous injection of 3-mg/kg. Increase in serum levels of procollagen type I N-terminal propeptide (PINP, a marker of collagen deposition) was also observed, in contrast serum cross-linked C-telopeptide of type I collagen (CTX, a marker of bone resorption) levels were reduced by sotatercept [95]. Adverse events were mild and transient consisting of headache, toothache, infusion site reaction and infusion site hemorrhage. Progressive and persistent hypertension was also observed in one patient due to a rapid and significant increase in hemoglobin levels at 1 week after administration of the second dose of sotatercept [95]. Given these promising results, the bone anabolic effects of sotatercept (ACE-011) have been assessed in 30 stage II/III MM patients with osteolytic lesions receiving standard chemotherapy, consisting of melphalan, prednisone and thalidomide (NCT00747123). Nearly half of the patients also received bisphosphonate treatment [96]. In this phase II randomized, double-blind, placebo-controlled, multidose (0.1, 0.3 and 0.5 mg/kg sc, q4w for 4 months) study, Sotatercept was associated with a trend towards improvement in ostelolyic lesions (as indicated by skeletal X-rays). In 20% of the patients a persistent reduction in pain after the first dose has been observed. In accordance with the improvement in lesions, in bisphosphonate-naïve patients, sotatercept correlated with an increase in bALP levels and a slight reduction in serum CTX levels. Of 22 evaluable patients who received sotatercept, seven (32%) experienced either complete remission or very good partial remission. Of note 75% of the patients treated with 0.5 mg/kg of Sotatercept experienced an increase in Hb level of 1 g/mL at day 29 compared to 33% in the placebo. As a consequence, ongoing studies are evaluating sotatercept effects on tumor-induced anemia.
BHQ880 and DKK1 Antagonists BHQ880 is a clinical grade neutralizing antibody against DKK1 (Novartis), which promotes OB differentiation by reversing the negative effects of MM cells on OB formation and inhibits IL-6 production by BMSC, thereby blocking tumor cells proliferation [33]. In vivo studies using both murine and humanized models of MM-bone disease confirmed the bone-anabolic properties of DKK1 antagonists, with increased bone formation, increased OB number and improvement of osteolytic lesions [31, 33, 34]. Importantly, two studies showed a significant reduction in tumor burden, mainly as an indirect effect via modification of the tumor microenvironment by DKK1 inhibition [31, 33]. A clinical trial (NCT00741377) has been opened in 2008 to assess safety and tolerability as well as efficacy of the DKK1 inhibitor BHQ880 in combination with standard chemotherapy with or without bisphosphonates in relapsed or refractory MM patients. Another Novartis-sponsored study (NCT01302886) will evaluate the effects of monthly infusions of BHQ880 in high risk smoldering MM patients on tumor burden and bone metabolism.
Sclerostin Antagonists Sclerostin inhibitors stimulate generalized bone formation in ovariectomized rats, thus not only preventing development of osteoporosis, but also increasing bone mass in comparison to control animals [97]. Similarly, sclerostin inhibition in cynomolgus monkeys increases bone mineral density up to 30% [98]. In MM, neutralizing antibodies against sclerostin overcome MM-induced OB inhibition via accumulation of nuclear β-catenin [38]. AMG785 (Amgen) is an anti-sclerostin antibody assessed in a phase I randomized, double-blind, placebo-controlled, ascending, single-dose trial in 72 healthy men and postmenopausal women. Compared to placebo, treatment with AMG785 increases dose-dependently P1NP, bALP and osteocalcin, along with a decrease in the CTX levels. In addition, BMD at the lumbar spine and total hip were significantly upregulated by AMG785 at 3 months [99]. A phase 2 clinical trial (NCT00896532) is currently evaluating AMG785 in osteoporotic post-menopausal women in comparison to placebo, alendronate and teriparatide. No studies in tumor-induced bone disease have been opened until now, due to concerns regarding potential tumorigenic effects, since tumor cell growth may be stimulated by WNT signaling [100]. Further studies to exclude any stimulatory effects on tumor growth are required for all anabolic agents acting on the WNT/β-catenin pathway.
CCR1 Inhibitors Neutralizing antibodies against CCL3 and small molecule CCR1 antagonists consistently reverse bone loss in murine models of MM [63, 64, 101–103]. Orally available, specific, small molecule CCR1 antagonists include CCX721 (ChemoCentryx), BX471 (Berlex) [104] and MLN3897 (Millennium Pharmaceuticals). CCX721 decreases osteolytic and tumor burden in vivo, resulting as potent as zoledronic acid [103]. In the 5T2MM murine model of MM, animals treated daily with BX471 showed a 40% reduction in bone lesions, a 2-fold increase in trabecular bone area, a 20% reduction in tumor load, and almost complete inhibition of microvessel density. Of note, the CCR5 inhibitor, TAK779 lead to a 20% decrease in lytic lesions, with no effects on tumor burden [63]. Finally, daily administration of MLN3897 for 4 weeks reduced tumor burden assessed by soluble IL-6 receptors, as well as tumor infiltration in the bone specimens in SCID-hu mice. In addition, OC number per bone area was reduced and trabecular bone area increased. Notably, for the first time this study suggests a positive effect of CCR1 inhibition on OB function, by showing an upregulation of osteocalcin expression [64]. Several CCR1 antagonists have been clinically assessed in the context of inflammatory diseases without significant effects, presumably due to redundancy of signaling in the chemokine family and suboptimal pharmacokinetic properties of the inhibitors used [105]. Novel agents with improved potency and physical/chemical properties are currently under evaluation [106, 107].
Teriparatide Parathyroid hormone (PTH) is critical to bone health for its effects on calcium metabolism. In addition, PTH regulates bone remodeling. By binding its receptor on OB, PTH stimulates RANKL secretion, thus promoting OC differentiation, but stimulates also osteogenesis. Daily recombinant parathyroid hormone, teriparamide, injections decreased the risk of vertebral and nonvertebral fractures in post menopausal women with a history of vertebral fractures and increased total-body bone mineral density [108]. In vivo studies in murine model of MM (SCID-rab and SCID-hu mouse models) suggested that regular PTH administration increases BMD and reduces tumor burden. The bone effect was observed in murine as well as human bones independently from MM cell grafts. The increased BMD was mainly related to increased OB formation, while OC have not been affected [109]. Teriparatide is the only FDA- approved anabolic agent for the treatment of osteoporosis. However, its side effects, including hypercalcemia and stimulation of bone resorption, and reports on increased incidence of osteosarcoma in animal models limit its use [108]. Further studies are therefore needed to assess its safety profile in the oncological setting.
Conclusion
In the past few years our knowledge on the pathogenesis of bone lesions in MM has greatly improved. Strong evidence for suppressed bone formation in MM-induced osteolysis lead to the identification of novel agents with bone anabolic properties. The compounds in most advanced clinical development, bortezomib and sotatercept, may increase BMD and improve lytic lesions, at least in a subgroup of patients. Since bone disease results from the imbalanced OC/OB axis, combination treatments targeting both components are under investigation. Ongoing studies are evaluating bisphosphonates with DKK1 inhibitors and bortezomib. Similarly, lenalidomide, known to inhibit OCs, may synergize with anabolic agents such as ACE-011 [110]. In conclusion, balanced OB activity is important not only to restore bone homeostasis but is also critical to tumor cell inhibition. Strategies to rescue OB function in MM will soon become a relevant part in the treatment of MM.
Contributor Information
Sonia Vallet, Email: sonia.vallet@med.uni-heidelberg.de.
Noopur Raje, Phone: +1-617-7260711, FAX: +1-617-7246801, Email: nraje@partners.org.
References
- 1.Roodman GD. New potential targets for treating myeloma bone disease. Clin Cancer Res. 2006;12(20 Pt 2):6270s–6273s. doi: 10.1158/1078-0432.CCR-06-0845. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–1594. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110(8):1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 4.Valentin-Opran A, Charhon SA, Meunier PJ, Edouard CM, Arlot ME. Quantitative histology of myeloma-induced bone changes. Br J Haematol. 1982;52(4):601–610. doi: 10.1111/j.1365-2141.1982.tb03936.x. [DOI] [PubMed] [Google Scholar]
- 5.Bataille R, Chappard D, Marcelli C, Dessauw P, Sany J, Baldet P, Alexandre C. Mechanisms of bone destruction in multiple myeloma: the importance of an unbalanced process in determining the severity of lytic bone disease. J Clin Oncol. 1989;7(12):1909–1914. doi: 10.1200/JCO.1989.7.12.1909. [DOI] [PubMed] [Google Scholar]
- 6.Taube T, Beneton MN, McCloskey EV, Rogers S, Greaves M, Kanis JA. Abnormal bone remodelling in patients with myelomatosis and normal biochemical indices of bone resorption. Eur J Haematol. 1992;49(4):192–198. doi: 10.1111/j.1600-0609.1992.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 7.Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101(9):3568–3573. doi: 10.1182/blood-2002-08-2383. [DOI] [PubMed] [Google Scholar]
- 8.Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105(4):1383–1395. doi: 10.1182/blood-2004-07-2909. [DOI] [PubMed] [Google Scholar]
- 9.Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, Spencer JA, Kimlinger T, Ghobrial JM, Jia X, Lu G, Timm M, Kumar A, Cote D, Veilleux I, Hedin KE, Roodman GD, Witzig TE, Kung AL, Hideshima T, Anderson KC, Lin CP, Ghobrial IM. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109(7):2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaccoby S, Wezeman MJ, Zangari M, Walker R, Cottler-Fox M, Gaddy D, Ling W, Saha R, Barlogie B, Tricot G, Epstein J. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91(2):192–199. [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006;108(13):3992–3996. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Xu J, Ward E Cancer statistics, 2010. CA: a cancer journal for clinicians 60 (5):277–300 [DOI] [PubMed]
- 13.Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235(1):176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- 14.Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99(5):1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 15.Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111(5):2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112(1):159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98(13):3527–3533. doi: 10.1182/blood.V98.13.3527. [DOI] [PubMed] [Google Scholar]
- 18.Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, Barlogie B, Shaughnessy JD., Jr Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112(1):196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sims NA, Gooi JH. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19(5):444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Silvestris F, Cafforio P, Calvani N, Dammacco F. Impaired osteoblastogenesis in myeloma bone disease: role of upregulated apoptosis by cytokines and malignant plasma cells. Br J Haematol. 2004;126(4):475–486. doi: 10.1111/j.1365-2141.2004.05084.x. [DOI] [PubMed] [Google Scholar]
- 21.Giuliani N, Storti P, Abeltino M, Bolzoni M, Ferretti M, Lazzaretti M, Dalla Palma B, Todoerti K, Martella E, Agnelli L, Neri A, Rizzoli V, Palumbo C (2010) In vitro and in vivo evidences of osteocyte involvement in myeloma-induced osteolysis. Blood:Abstract 131
- 22.Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106(7):2472–2483. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 23.Komori T Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell and tissue research 339(1):189–195 [DOI] [PubMed]
- 24.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 26.Baek WY, de Crombrugghe B, Kim JE Postnatally induced inactivation of Osterix in osteoblasts results in the reduction of bone formation and maintenance. Bone 46 (4):920–928 [DOI] [PMC free article] [PubMed]
- 27.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 28.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9(11):1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 29.Giuliani N, Mangoni M, Rizzoli V. Osteogenic differentiation of mesenchymal stem cells in multiple myeloma: identification of potential therapeutic targets. Exp Hematol. 2009;37(8):879–886. doi: 10.1016/j.exphem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 31.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109(5):2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, Bonomini S, Sala R, Mangoni M, Rizzoli V. Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res. 2007;67(16):7665–7674. doi: 10.1158/0008-5472.CAN-06-4666. [DOI] [PubMed] [Google Scholar]
- 33.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD, Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI (2009) Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res 24(3):425–436 [DOI] [PubMed]
- 35.Balemans W, Ebeling M, Patel N, Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 36.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Lindner C, Harmelin S, Hawa G, Boutsikas G, Migkou M, Gavriatopoulou M, Michalis E, Pouli C, Kastritis E, K Z, Dimopoulos MA (2009) High serum sclerostin correlates with advanced stage, increased bone resorption, reduced osteoblast function, and poor survival in newly-diagnosed patients with multiple myeloma Blood: Abstract 425
- 38.Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Liso V, Curci P, Miccolis R, Rinaldi E, Specchia G, Passeri G, Zallone A, Rizzi R, Grano M (2010) Myeloma cells induce osteoblast suppression through sclerostin secretion. Blood:Abstract 2961 [DOI] [PMC free article] [PubMed]
- 39.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106(9):3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 40.Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366(1):51–57. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Woodruff TK. Regulation of cellular and system function by activin. Biochem Pharmacol. 1998;55(7):953–963. doi: 10.1016/S0006-2952(97)00477-2. [DOI] [PubMed] [Google Scholar]
- 42.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 43.Samee N, Geoffroy V, Marty C, Schiltz C, Vieux-Rochas M, Levi G, Vernejoul MC. Dlx5, a positive regulator of osteoblastogenesis, is essential for osteoblast-osteoclast coupling. Am J Pathol. 2008;173(3):773–780. doi: 10.2353/ajpath.2008.080243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holleville N, Quilhac A, Bontoux M, Monsoro-Burq AH. BMP signals regulate Dlx5 during early avian skull development. Dev Biol. 2003;257(1):177–189. doi: 10.1016/S0012-1606(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 45.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, Sung JH, Wozney JM, Kim HJ, Ryoo HM. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278(36):34387–34394. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- 47.Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S, Santo L, Cirstea D, Patel K, Sohani R, Guimaraes A, Xie W, Chauhan D, Schoonmaker J, Attar E, Churchill M, Weller E, Munshi N, Seehra J, Weissleder R, Anderson K, Scadden D, Raje N (2010) Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci U S A 107(11):5124–5129 [DOI] [PMC free article] [PubMed]
- 48.Terpos E, Christoulas D, Kastritis E, Gkotzamanidou M, Gavriatopoulou M, Eleutherakis-Papaiakovou E, Migkou M, Roussou M, Papatheodorou A, Dimopoulos MA (2010) Elevated levels of circulating activin-A correlate with features of advanced disease, extensive bone involvement and inferior survival in patients with multiple myeloma. Blood:Abstract 2967
- 49.Fuller K, Bayley KE, Chambers TJ. Activin A is an essential cofactor for osteoclast induction. Biochem Biophys Res Commun. 2000;268(1):2–7. doi: 10.1006/bbrc.2000.2075. [DOI] [PubMed] [Google Scholar]
- 50.Shiozaki M, Sakai R, Tabuchi M, Nakamura T, Sugino K, Sugino H, Eto Y. Evidence for the participation of endogenous activin A/erythroid differentiation factor in the regulation of erythropoiesis. Proc Natl Acad Sci U S A. 1992;89(5):1553–1556. doi: 10.1073/pnas.89.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374(6520):356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- 52.Chantry AD, Heath D, Mulivor AW, Pearsall S, Baud’huin M, Coulton L, Evans H, Abdul N, Werner ED, Bouxsein ML, Key ML, Seehra J, Arnett TR, Vanderkerken K, Croucher P (2010) Inhibiting activin-A signaling stimulates bone formation and prevents cancer induced bone destruction in vivo. J Bone Miner Res 25(12):2633–2646 [DOI] [PubMed]
- 53.Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20(9):2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeuchi K, Abe M, Hiasa M, Oda A, Amou H, Kido S, Harada T, Tanaka O, Miki H, Nakamura S, Nakano A, Kagawa K, Yata K, Ozaki S, Matsumoto T Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth. PloS one 5 (3):e9870 [DOI] [PMC free article] [PubMed]
- 55.Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SS, Bracey JW, Hogue WR, Wong DH, Ritchie RO, Suva LJ, Derynck R, Guise TA, Alliston T. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PloS one. 2009;4(4):e5275. doi: 10.1371/journal.pone.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Standal T, Abildgaard N, Fagerli UM, Stordal B, Hjertner O, Borset M, Sundan A. HGF inhibits BMP-induced osteoblastogenesis: possible implications for the bone disease of multiple myeloma. Blood. 2007;109(7):3024–3030. doi: 10.1182/blood-2006-07-034884. [DOI] [PubMed] [Google Scholar]
- 57.Uneda S, Hata H, Matsuno F, Harada N, Mitsuya Y, Kawano F, Mitsuya H. Macrophage inflammatory protein-1 alpha is produced by human multiple myeloma (MM) cells and its expression correlates with bone lesions in patients with MM. Br J Haematol. 2003;120(1):53–55. doi: 10.1046/j.1365-2141.2003.04040.x. [DOI] [PubMed] [Google Scholar]
- 58.Terpos E, Politou M, Szydlo R, Goldman JM, Apperley JF, Rahemtulla A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br J Haematol. 2003;123(1):106–109. doi: 10.1046/j.1365-2141.2003.04561.x. [DOI] [PubMed] [Google Scholar]
- 59.Roussou M, Tasidou A, Dimopoulos MA, Kastritis E, Migkou M, Christoulas D, Gavriatopoulou M, Zagouri F, Matsouka C, Anagnostou D, Terpos E. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia. 2009;23(11):2177–2181. doi: 10.1038/leu.2009.130. [DOI] [PubMed] [Google Scholar]
- 60.Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97(11):3349–3353. doi: 10.1182/blood.V97.11.3349. [DOI] [PubMed] [Google Scholar]
- 61.Tsubaki M, Kato C, Manno M, Ogaki M, Satou T, Itoh T, Kusunoki T, Tanimori Y, Fujiwara K, Matsuoka H, Nishida S. Macrophage inflammatory protein-1alpha (MIP-1alpha) enhances a receptor activator of nuclear factor kappaB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol Cell Biochem. 2007;304(1–2):53–60. doi: 10.1007/s11010-007-9485-7. [DOI] [PubMed] [Google Scholar]
- 62.Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, Pozzi S, Breitkreutz I, Kiziltepe T, Yasui H, Ocio EM, Shiraishi N, Jin J, Okawa Y, Ikeda H, Mukherjee S, Vaghela N, Cirstea D, Ladetto M, Boccadoro M, Anderson KC. MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood. 2007;110(10):3744–3752. doi: 10.1182/blood-2007-05-093294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menu E, Leenheer E, Raeve H, Coulton L, Imanishi T, Miyashita K, Valckenborgh E, Riet I, Camp B, Horuk R, Croucher P, Vanderkerken K. Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clin Exp Metastasis. 2006;23(5–6):291–300. doi: 10.1007/s10585-006-9038-6. [DOI] [PubMed] [Google Scholar]
- 64.Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti M, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden D, Anderson K, Raje N (2011) A novel role for CCL3 (MIP-1α) in Myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia 25(7):1174–1181 [DOI] [PMC free article] [PubMed]
- 65.Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/S0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 66.Vincent C, Findlay DM, Welldon KJ, Wijenayaka AR, Zheng TS, Haynes DR, Fazzalari NL, Evdokiou A, Atkins GJ. Pro-inflammatory cytokines TNF-related weak inducer of apoptosis (TWEAK) and TNFalpha induce the mitogen-activated protein kinase (MAPK)-dependent expression of sclerostin in human osteoblasts. J Bone Miner Res. 2009;24(8):1434–1449. doi: 10.1359/jbmr.090305. [DOI] [PubMed] [Google Scholar]
- 67.D’Souza S, Prete D, Esteve F, Sammut B, Yu S, Xiao G, Galson D, Roodman D. Multiple myeloma cell induction of GFI-1 in stromal cells suppresses osteoblast differentiation in patients with myeloma. Blood. 2009;114:742. doi: 10.1182/blood-2009-04-216861. [DOI] [PubMed] [Google Scholar]
- 68.Olfa G, Christophe C, Philippe L, Romain S, Khaled H, Pierre H, Odile B, Jean-Christophe D RUNX2 regulates the effects of TNFalpha on proliferation and apoptosis in SaOs-2 cells. Bone 46(4):901–910 [DOI] [PubMed]
- 69.Jourdan M, Tarte K, Legouffe E, Brochier J, Rossi JF, Klein B. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur Cytokine Netw. 1999;10(1):65–70. [PMC free article] [PubMed] [Google Scholar]
- 70.Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, Choi SJ. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103(6):2308–2315. doi: 10.1182/blood-2003-06-1992. [DOI] [PubMed] [Google Scholar]
- 71.Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S, Rizzoli V, Roodman GD, Giuliani N. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106(4):1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- 72.Giuliani N, Morandi F, Tagliaferri S, Colla S, Bonomini S, Sammarelli G, Rizzoli V. Interleukin-3 (IL-3) is overexpressed by T lymphocytes in multiple myeloma patients. Blood. 2006;107(2):841–842. doi: 10.1182/blood-2005-07-2719. [DOI] [PubMed] [Google Scholar]
- 73.Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, Monica S, Bonomini S, Hojden M, Sammarelli G, Barille S, Bataille R, Rizzoli V. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood. 2002;100(13):4615–4621. doi: 10.1182/blood-2002-04-1121. [DOI] [PubMed] [Google Scholar]
- 74.Cocco C, Giuliani N, Di Carlo E, Ognio E, Storti P, Abeltino M, Sorrentino C, Ponzoni M, Ribatti D, Airoldi I Interleukin-27 acts as multifunctional antitumor agent in multiple myeloma. Clin Cancer Res 16(16):4188–4197 [DOI] [PubMed]
- 75.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4(2):111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 76.Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD, Jr, Barlogie B, Yaccoby S. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009;114(9):1803–1812. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs MJ, Blacklock HA, Bell R, Simeone J, Reitsma DJ, Heffernan M, Seaman J, Knight RD. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 78.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 79.Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, Kaiser M, Jakob C, Sterz J, Kleeberg L, Heider U, Sezer O. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21(9):2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 80.Oyajobi BO, Garrett IR, Gupta A, Flores A, Esparza J, Munoz S, Zhao M, Mundy GR. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: implications for myeloma bone disease. Br J Haematol. 2007;139(3):434–438. doi: 10.1111/j.1365-2141.2007.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, Mancini C, Martella E, Ferrari L, Tabilio A, Rizzoli V. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110(1):334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 82.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118(2):491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Matteo M, Brunetti AE, Maiorano E, Cafforio P, Dammacco F, Silvestris F Constitutive down-regulation of Osterix in osteoblasts from myeloma patients: in vitro effect of Bortezomib and Lenalidomide. Leukemia research 34(2):243–249 [DOI] [PubMed]
- 84.Deleu S, Lemaire M, Arts J, Menu E, Valckenborgh E, Vande Broek I, Raeve H, Coulton L, Camp B, Croucher P, Vanderkerken K. Bortezomib alone or in combination with the histone deacetylase inhibitor JNJ-26481585: effect on myeloma bone disease in the 5T2MM murine model of myeloma. Cancer Res. 2009;69(13):5307–5311. doi: 10.1158/0008-5472.CAN-08-4472. [DOI] [PubMed] [Google Scholar]
- 85.Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol. 2009;84(1):6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zangari M, Esseltine D, Lee CK, Barlogie B, Elice F, Burns MJ, Kang SH, Yaccoby S, Najarian K, Richardson P, Sonneveld P, Tricot G. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131(1):71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 88.Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A, Pouli A, Katodritou E, Verrou E, Vervessou EC, Dimopoulos MA, Croucher PI. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135(5):688–692. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 89.Zangari M, Yaccoby S, Pappas L, Cavallo F, Kumar NS, Ranganathan S, Suva LJ, Gruenwald JM, Kern S, Zhan F, Esseltine D, Tricot G A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica 96(2):333–336 [DOI] [PMC free article] [PubMed]
- 90.Delforge M, Terpos E, Richardson PG, Shpilberg O, Khuageva NK, Schlag R, Dimopoulos MA, Kropff M, Spicka I, Petrucci MT, Samoilova OS, Mateos MV, Magen-Nativ H, Goldschmidt H, Esseltine DL, Ricci DS, Liu K, Deraedt W, Cakana A, van de Velde H, San Miguel JF (2011) Fewer bone disease events, improvement in bone remodelling, and evidence of bone healing with bortezomib plus melphalan-prednisone versus melphalan-prednisone, in the phase III VISTA trial in multiple myeloma. Eur J Haematol 86(5):372–384 [DOI] [PubMed]
- 91.Terpos E, Kastritis E, Roussou M, Heath D, Christoulas D, Anagnostopoulos N, Eleftherakis-Papaiakovou E, Tsionos K, Croucher P, Dimopoulos MA. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22(12):2247–2256. doi: 10.1038/leu.2008.235. [DOI] [PubMed] [Google Scholar]
- 92.Terpos E, Christoulas D, Kokkoris P, Gavriatopoulou M, Boutsikas G, Migkou M, Anargyrou K, Kastritis E, Tsionos K, Dimopoulos MA. Increased bone mineral density in a subset of patients with relapsed multiple myeloma who received the combination of bortezomib, dexamethasone and zoledronic acid. Haematologica. 2009;94(suppl.2):385. doi: 10.1093/annonc/mdq259. [DOI] [PubMed] [Google Scholar]
- 93.Pearsall RS, Canalis E, Cornwall-Brady M, Underwood KW, Haigis B, Ucran J, Kumar R, Pobre E, Grinberg A, Werner ED, Glatt V, Stadmeyer L, Smith D, Seehra J, Bouxsein ML. A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci U S A. 2008;105(19):7082–7087. doi: 10.1073/pnas.0711263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tassone P, Neri P, Carrasco DR, Burger R, Goldmacher VS, Fram R, Munshi V, Shammas MA, Catley L, Jacob GS, Venuta S, Anderson KC, Munshi NC. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood. 2005;106(2):713–716. doi: 10.1182/blood-2005-01-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruckle J, Jacobs M, Kramer W, Pearsall AE, Kumar R, Underwood KW, Seehra J, Yang Y, Condon CH, Sherman ML. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24(4):744–752. doi: 10.1359/jbmr.081208. [DOI] [PubMed] [Google Scholar]
- 96.Abdulkadyrov K, Salogub G, Khuazheva N, Woolf R, Haltom E, Borgstein N, Knight R, Renshaw G, Yang Y, Sherman M (2009) ACE-011, a soluble activin receptor type iia igg-fc fusion protein, increases hemoglobin (Hb) and improves bone lesions in multiple myeloma patients receiving myelosuppressive chemotherapy: preliminary analysis. Blood: Abstract 749
- 97.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24(4):578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 98.Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, Tipton B, Cai J, Deshpande R, Zhou L, Hale MD, Lightwood DJ, Henry AJ, Popplewell AG, Moore AR, Robinson MK, Lacey DL, Simonet WS, Paszty C Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25(5):948–959 [DOI] [PubMed]
- 99.Padhi D, Jang G, Stouch B, Fang L, Posvar E Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26(1):19–26 [DOI] [PubMed]
- 100.Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, Carrasco DE, Zheng M, He H, Tai YT, Mitsiades C, Anderson KC, Carrasco DR. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007;104(18):7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi SJ, Oba Y, Gazitt Y, Alsina M, Cruz J, Anderson J, Roodman GD. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108(12):1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, Grubbs B, Zhao M, Chen D, Sherry B, Mundy GR. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 2003;102(1):311–319. doi: 10.1182/blood-2002-12-3905. [DOI] [PubMed] [Google Scholar]
- 103.Oyajobi B, Dairaghi D, Gupta A, McCluskey B, Wang Y, Seitz L, Powers J, Miao S, Zhang P, Schall T, Jaen J (2010) CCR1 blockade by an orally-available CCR1 antagonist reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease blood. Blood: Abstract 3000 [DOI] [PMC free article] [PubMed]
- 104.Liang M, Mallari C, Rosser M, Ng HP, May K, Monahan S, Bauman JG, Islam I, Ghannam A, Buckman B, Shaw K, Wei GP, Xu W, Zhao Z, Ho E, Shen J, Oanh H, Subramanyam B, Vergona R, Taub D, Dunning L, Harvey S, Snider RM, Hesselgesser J, Morrissey MM, Perez HD. Identification and characterization of a potent, selective, and orally active antagonist of the CC chemokine receptor-1. J Biol Chem. 2000;275(25):19000–19008. doi: 10.1074/jbc.M001222200. [DOI] [PubMed] [Google Scholar]
- 105.Reuss R, Schreiber V, Klein A, Infante-Duarte C, Filippi M, Pabst W, Pohl C, Oschmann P No significant effect of orally administered chemokine receptor 1 antagonist on intercellular adhesion molecule-3 expression in relapsing--remitting multiple sclerosis patients. Multiple sclerosis (Houndmills, Basingstoke, England) 16(3):366–369 [DOI] [PubMed]
- 106.Clucas AT, Shah A, Zhang YD, Chow VF, Gladue RP. Phase I evaluation of the safety, pharmacokinetics and pharmacodynamics of CP-481,715. Clin Pharmacokinet. 2007;46(9):757–766. doi: 10.2165/00003088-200746090-00003. [DOI] [PubMed] [Google Scholar]
- 107.Merritt JR, Liu J, Quadros E, Morris ML, Liu R, Zhang R, Jacob B, Postelnek J, Hicks CM, Chen W, Kimble EF, Rogers WL, O’Brien L, White N, Desai H, Bansal S, King G, Ohlmeyer MJ, Appell KC, Webb ML. Novel pyrrolidine ureas as C-C chemokine receptor 1 (CCR1) antagonists. J Med Chem. 2009;52(5):1295–1301. doi: 10.1021/jm801416q. [DOI] [PubMed] [Google Scholar]
- 108.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 109.Pennisi A, Ling W, Li X, Khan S, Wang Y, Barlogie B, Shaughnessy JD, Jr., Yaccoby S Consequences of daily administered parathyroid hormone on myeloma growth, bone disease, and molecular profiling of whole myelomatous bone. PloS one 5(12):e15233 [DOI] [PMC free article] [PubMed]
- 110.Vallet S, Patel K, Cirstea D, Luly K, Pozzi S, Santo L, Eda H, Seehra J, Mahindra A, Scadden D, Raje N (2010) Lenalidomide in combination with the activin receptor type ii murine Fc protein RAP-011: preclinical rationale for a novel anti-myeloma strategy. Blood: Abstract 4075