Abstract
Bone serves one of the most congenial metastatic microenvironments for multiple types of solid tumors, but its role in this process remains under-explored. Among many cell populations constituting the bone and bone marrow microenvironment, osteoblasts (originated from mesenchymal stem cells) and osteoclasts (originated from hematopoietic stem cells) have been the main research focus for pro-tumorigenic roles. Recently, increasing evidence further elucidates that hematopoietic lineage cells as well as stromal cells in the bone marrow mediate distinct but critical functions in tumor growth, metastasis, angiogenesis and apoptosis in the bone microenvironment. This review article summarizes the key evidence describing differential roles of bone marrow cells, including hematopoietic stem cells (HSCs), megakaryocytes, macrophages and myeloid-derived suppressor cells in the development of metastatic bone lesions. HSCs promote tumor growth by switching on angiogenesis, but at the same time compete with metastatic tumor cells for occupancy of osteoblastic niche. Megakaryocytes negatively regulate the extravasating tumor cells by inducing apoptosis and suppressing proliferation. Macrophages and myeloid cells have pro-tumorigenic roles in general, suggesting a similar effect in the bone marrow. Hematopoietic and stromal cell populations in the bone marrow, previously considered as simple by-standers in the context of tumor metastasis, have distinct and active roles in promoting or suppressing tumor growth and metastasis in bone. Further investigation on the extended roles of bone marrow cells will help formulate better approaches to treatment through improved understanding of the metastatic bone microenvironment.
Keywords: Bone marrow, Metastasis, Hematopoietic stem cells, Megakaryocytes, Macrophages, Myeloid-derived suppressor cells
Introduction
The majority of cancer patients ultimately develop metastatic lesions, contributing to excessive morbidity and mortality, even though metastasis is a very selective and extremely inefficient process, with less than 0.1% of the intravasated tumor cells surviving cascades of events to form metastatic lesions in distant sites [1, 2]. More importantly, tumor metastasis is determined not by locoregional anatomy of draining vasculature (i.e. hemodynamic factors), but by highly specific interactions between disseminating tumor cells (“seed”) and the microenvironment of the target organ (“soil”) [3]. This seminal concept of “seed and soil” was originally proposed by Stephen Paget in the 19th century [4], but soon challenged by Ewing and many others proposing that mechanical forces and hemodynamic factors determine the metastatic patterns [1, 5, 6]. Later, the “seed and soil” hypothesis was revisited by central evidence that the primary tumor is comprised of biologically heterogeneous cell populations (i.e. subpopulations of different metastatic potentials), and also that metastases selectively develop in congenial microenvironments regardless of hemodynamic trafficking [7, 8]. In addition, Tarin et al. provided clinical evidence that the specific organ microenvironment is a critical determinant in metastasis, independent of vascular anatomy, rate of blood flow and the number of tumor cells delivered to the organ [9, 10]. Indeed, the current cancer statistics clearly show that the primary tumors of individual organs have strong preference for their metastatic sites [11, 12]. For example, colon and pancreatic tumors preferentially metastasize to liver; and renal cell carcinoma and bladder cancer frequently spread to lungs. Therefore, tumor metastasis occurs in a predictable manner, tightly regulated by the microenvironment of the recipient organ.
Interestingly, bone is the predominant metastatic soil for a number of human cancers, including prostate, breast and lung cancers as well as multiple myeloma [11, 13, 14]. Skeletal metastasis is the major cause of mortality and morbidity of afflicted patients. For example, approximately 90% of advanced stage prostate cancer patients develop bone lesions, resulting in morbidities such as severe bone pain, immobility, hematopoietic complications and spinal cord compression [12, 15]. Current treatment modalities for bone metastatic lesions are not curative, and the average time from the surgery (for bone lesions, such as pathologic fracture) to death is only 1.5 ± 1.9 years for prostate cancer patients. To overcome this urgent clinical problem, better understanding of the metastatic bone microenvironment is critically important. Bone is an intriguing microenvironment for tumor biology, and still remains largely unexplored [16]. This uniquely complex milieu is due not only to the calcified matrix but also to multiple types of constituting cells, including bone cells (osteocytes, osteoclasts and osteoblasts), hematopoietic cells, immune cells, stromal cells and endothelial cells [17]. Considerable research efforts have been devoted to characterizing this complex microenvironment and also to elucidating differential roles of individual cell types in their contribution to tumor growth and metastasis in bone [13]. Notably, Mundy and colleagues proposed a ‘vicious cycle’ theory which involves bi-directional interactions between disseminated tumor cells and osteoclasts (as well as osteoblasts) leading to osteolysis and, in turn, tumor growth [13, 18, 19]. For example, parathyroid hormone-related peptide (PTHrP) derived from breast cancer cells promotes osteolytic bone lesions (mediated by activation of osteoclasts), leading to release of transforming growth factor-beta (TGF-β) from the bone matrix to further aggravate tumor growth in the bone [18, 19]. Later, prostate cancer cells were shown to express PTHrP in order to upregulate expression of tumorigenic factors (such as C-C chemokine ligand 2 [CCL2]) in osteoblasts, resulting in destructive cascades in the bone as well as osteoblastic lesions [20–22]. However, the majority of experimental results are from murine models, and the vicious cycle in human breast/prostate cancer skeletal metastases is lacking yet difficult to discern.
Collectively, the current data demonstrate a positive feedback loop of tumor cell interaction with the hard tissue compartment of the bone microenvironment (i.e. osteoclasts, osteoblasts and calcified bone matrix). Additionally, however, current evidence suggests that different hematopoietic lineage cell populations in the bone marrow, previously considered as simple bystanders in the metastatic process, provide distinctive contributions for promoting or suppressing tumor growth and/or metastasis [23, 24]. This review paper will examine the current literature regarding cells in the bone microenvironment, with particular focus on hematopoietic lineage cells in the bone marrow, and their roles in skeletal metastasis.
Cellular Components of the Congenial Soil: Anatomy and Histology of Bone Marrow
The bone marrow is one of the largest organs in the human body, and comprises approximately 5% of body weight in humans and 3% in adult rats [25, 26]. Bone marrow is the primary hematopoietic organ and a primary lymphoid tissue, responsible for the production of the cellular components of blood [27]. It consists of hematopoietic tissue, endosteum, connective tissue and endothelium. The endosteal lining in the marrow cavity contains a single layer of cells, including osteoblasts and osteoclasts, supported by a thin layer of reticular connective tissue. Other connective tissues in the bone marrow include bony trabeculae, adipocytes, fibroblasts and nerves. Of particular note, the bone marrow is extremely well vascularized tissue, served by multiple arteries entering the marrow via nutrient canals of diverse size. Arteries branch and taper down to thin-walled arterioles and capillaries anastomosing with a plexus of venous sinuses. Venus sinuses then merge to form collecting veins and further the central venous sinus draining back via nutrient canals into the systemic circulation. Sinusoidal vessels are thin-walled, consisting of a layer of flat endothelial cells with little to no basement membrane. Bone marrow sinusoids function as an entering point for hematopoietic cells into the systemic circulation. Similarly, metastatic cancer cells are considered to extravasate via sinusoidal barrier. The bone marrow does not have a lymphatic drainage system [27–29].
The hematopoietic compartment of the bone marrow is comprised of stem cells, hematopoietic lineage cells, adventitial reticular cells, adipocytes and macrophages. Hematopoietic cells are not randomly dispersed, but are structured within the microenvironment [30]. More importantly, hematopoiesis occurs as a compartmentalized process, with erythropoiesis occurring in erythroblastic islands; granulopoiesis in less defined areas and megakaryopoiesis adjacent to the sinus endothelium. On demand, the hematopoietic cells transverse the sinusoidal barrier to enter the systemic circulation, whereas platelets are released directly from the cytoplasm of megakaryocytes into the bloodstream.
During embryonic development, hematopoiesis occurs in the liver, and shortly after birth hematopoietic stem cells (HSCs) migrate and repopulate the bone marrow. This unique feature of bone marrow biology, bone marrow homing, has been extensively exploited clinically to improve the engrafting efficiency of bone marrow transplantation, carried out by simple intravenous injection of marrow cells [31]. Molecular mechanisms of bone marrow homing have been demonstrated primarily by exploring factors inhibiting homing in various mouse models. For example, mice deficient in E- and P-selectins were found to have impaired homing, suggesting that tethering and rolling of bone marrow cells on the sinusoidal endothelium is critical for correct engraftment [32, 33]. More importantly, Peled et al. provide pivotal evidence that stromal-derived factor-1 (SDF-1, also known as CXCL12) expressed by the bone marrow stroma and endothelium interacts with its cognate ligand, CXCR-4 expressed on HSCs, is critical to human HSC engraftment and repopulation in a immune-deficient mouse model [34]. In sum, the bone marrow is structured hierarchically, containing various populations of hematopoietic cells supported by stromal cells, all of which potentially have unique function in skeletal tumor growth and/or metastasis.
Hematopoietic Stem Cells Compete with Metastatic Tumor Cells
Tumor cells frequently usurp physiological mechanisms to promote growth, angiogenesis, invasion and metastasis. For example, most of the so-called tumorigenic molecules (such as vascular endothelial growth factor [VEGF], matrix metalloproteinases [MMPs] and epidermal growth factor [EGF] among myriad others) play critical roles in normal physiology and development. As stated above, liver is the primary hematopoietic organ until birth, and subsequently HSCs migrate into the bone marrow where the microenvironment supports engraftment, repopulation and self-renewal. This phenomenon of physiological HSC homing in the bone marrow led scientists to an interesting hypothesis that bone metastatic cancer cells may mimic the established pathway of HSC homing. Müller et al. for the first time provided pivotal evidence that chemokine receptors (CXCR4 and CCR7, highly expressed by breast cancer cells) and their cognate ligands (expressed in metastatic recipient tissues) play critical roles in organ-specific breast cancer metastasis [35], in the same way that chemokine-chemokine receptor axes mediate HSC homing in the bone marrow during normal development and bone marrow transplantation (BMT). Subsequently, Taichman et al. demonstrated that CXCL12/SDF-1 (expressed by osteoblasts and endothelial cells) and its receptor (CXCR4, expressed by prostate cancer cells) regulate bone-tropism of prostate cancer cells [36]. In addition to the CXCL12/CXCR4/CXCR7 axis [37], Annexin II, expressed by osteoblasts and endothelium regulates HSC adhesion, homing and engraftment [38]. Interestingly, human prostate cancer cells isolated from the metastatic lesions (PC-3, DU145 and LNCaP) were shown to express receptors for Annexin II, contributing to prostate cancer growth and homing in the bone marrow [39]. Given that data collectively demonstrated that bone metastatic tumor cells (breast and prostate) utilize the chemokine axes of HSC homing, it is reasonable to expect that HSCs may compete with metastatic cancer cells for occupancy in the bone marrow.
Recently, crucial evidence demonstrating that hematopoietic stem cells (HSC) negatively regulate bone metastasis by competing with metastatic cancer cells to preoccupy the HSC endosteal niche came from the works of Shiozawa et al. [40] The authors demonstrated that increasing the HSC niche size (i.e. expansion of osteoblasts by parathyroid hormone [PTH] treatment) promoted skeletal localization of prostate cancer cells in the systemic circulation, while decreasing the niche size (using a conditional osteoblast-ablation mouse model) reduced tumor cell number localized in the bone marrow. In addition, an experimental treatment to mobilize HSCs (AMD3100, similarly to a clinical regimen used in autologous stem cell transplantation) could mobilize the cancer cells in the niche back into the circulation. Therefore, the HSC endosteal niche serves as a direct target for metastatic prostate cancer cells, and HSCs may function as competitors for metastatic cancer cells with strong bone tropism.
Contrary to the data demonstrating HSCs function as a competitor for niche occupancy, other data shows that HSCs may directly promote tumor growth and/or metastasis. Okamoto et al. demonstrated that HSCs regulate the angiogenic switch and promote tumor growth in the bone [41]. Furthermore, expansion of bone marrow cellularity by treatment with parathyroid hormone (PTH) resulted in significantly increased prostate cancer cell localization and subsequent growth in bone [42]. HSCs are pluripotent cells that can differentiate into any hematopoietic lineage cell types of tumorigenic potential. Accordingly, the direct roles of HSCs in tumor growth, particularly in the context of the bone microenvironment, need further investigation.
Megakaryocytes Attack Extravasating Tumor Cells in the Bone Marrow
As previously mentioned, megakaryocytes reside in parasinusoidal space with cytoplasmic invagination across the vascular barrier. As a result, platelets are released directly into the sinusoidal venous blood [43]. Because the sinusoidal endothelium is the main entry-exit point between the circulation and bone marrow tissue, bone metastatic cancer cells are thought to utilize the same route to extravasate. Therefore, megakaryocytes are potentially the first cells that tumor cells encounter upon arrival in the bone marrow microenvironment. Interestingly, Li et al. provided the first direct data that megakaryocytes suppress tumor cell proliferation and increase apoptosis in an experimental prostate cancer bone metastasis model [44]. In addition, expansion of the megakaryocyte population (by administering recombinant thrombopoietin) resulted in significantly reduced localization of tumor cells and subsequent growth in the bone in vivo. Direct contact between prostate cancer cells and megakaryocytic cells in vitro resulted in increased apoptosis as well as decreased proliferation of prostate cancer cells. These results demonstrated novel and specific inhibitory effects of megakaryocytes, a specialized hematopoietic lineage cell residing in the bone marrow, on metastatic cancer growth in the bone.
In parallel to tumor inhibitory effects based on direct cell-to-cell contact, secretory factors from megakaryocytes have been recently demonstrated to suppress osteoclast formation and activation [45–47]. In addition, megakaryocytes promote osteoblast synthesis of type I collagen, osteoprotegerin and receptor activator of nuclear factor kappa-B ligand (RANKL), all of which positively affect bone formation [48]. Reciprocally, osteoblasts directly influence hematopoiesis [49, 50] as well as megakaryopoiesis [51]. Consequently, production and activity of megakaryocytes are tightly coupled with bone remodeling, which in turn affects tumor growth in the bone. Given that the vicious cycle theory integrates activities of osteoblasts and osteoclasts as critical components [13], and that resorption is essential for tumor growth in bone [20, 52], megakaryocytes also alter the bone microenvironment (i.e. suppressing osteoclasts and activating osteoblasts) to affect metastatic tumor growth indirectly. Taken together, the current data suggest that megakaryocytes negatively regulate tumor cells in the bone marrow directly by suppressing tumor cell proliferation and inducing apoptosis, and also indirectly by suppressing osteoclasts and osteoblasts. However, detailed molecular mechanisms and clinical data are required to further characterize the role of megakaryocytes in skeletal metastasis.
Contrary to anti-metastatic functions of megakaryocytes, the end-products, platelets, have been shown to have opposite roles. Firstly, platelet-derived growth factor (PDGF) is one of the first angiogenic factors discovered, and critical to vessel maturation [53]. In addition, aggregation of platelets surrounding tumor cells had been shown to protect tumor cell lysis by natural killer cells [54]. Most notably, Boucharaba et al. provided pivotal evidence supporting the role of platelets in breast cancer skeletal metastasis [55, 56]. Activation of platelets by tumor cells result in production of lysophosphatidic acid (LPA), which in turn promotes breast cancer growth and skeletal metastasis in mice [55]. However, there is currently no clear evidence supporting clinical benefits of anti-platelet agents (such as aspirin and heparin) in cancer patients [56].
Macrophages Promote Tumor Growth and Metastasis in Bone: More than a Scavenger
Increasing evidence now clearly supports that tumor-associated macrophages (TAMs) are important regulators of tumor progression in multiple types of cancers [57–61]. Clinical studies reveal that the density of TAMs in tumor tissue significantly correlates with poor prognosis in prostate, breast, ovarian and cervical cancers, and with controversial outcomes in stomach and lung cancers [62]. In comparison with classically activated macrophages (M1 macrophages) associated with inflammatory phagocytosis, TAMs are an alternatively activated and polarized population of macrophages (M2 macrophages) with tumorigenic potential [63]. The role of immune cells, particularly macrophages, in tumor progression is not a new idea. The first suggestion of their involvement dates back to 1863 [64]. Recent studies now provide the clinical correlations as well as potential molecular mechanisms of recruitment, activation and function of TAMs (M2 macrophages). In particular, most prominent molecules produced by tumors to affect TAMs include C-C chemokine ligand 2 (CCL2, also known as monocyte chemoattractant protein-1 [MCP-1]), macrophage colony stimulating factor (M-CSF, also known as CSF-1) and VEGF. For example, bone metastatic prostate cancer cells express CCL2 to recruit monocytes to tumor sites, which then differentiate into TAMs (M2 macrophages) and osteoclasts [58, 65–67]. In addition, CCL2 has been seen to increase prostate cancer growth and bone metastasis in an experimental metastasis model, which was accompanied by the recruitment of macrophages and osteoclasts [57, 68]. Lin et al. also demonstrated that macrophages switch on tumor angiogenesis, using the polyoma middle-T antigen mouse mammary tumor (PyMT) spontaneous breast cancer model [69]. Macrophages are highly specialized phagocytic cells, derived from monocytes. In tumor tissue, a wide variety of factors are secreted by tumor cells, including those that function as recruiting factors for monocyte-macrophages. The most prominent and widely investigated functions of TAMs (M2 macrophages) in tumor tissue are increased angiogenesis and tumor growth caused by growth factors and proteinases. Data of Harris et al. showed by immunohistochemical quantification that TAMs cluster in areas of increased angiogenesis in human breast cancer samples [70]. In addition, TAMs (M2 macrophages) produce many pro-angiogenic cytokines such as urokinase-type plasminogen activator (uPA)[71], tumor necrosis factor-alpha (TNF-α)[72], IL-1, VEGF [73] and nitric oxide (NO)[74]. Moreover, TAMs express a wide variety of growth factors and proteinases such as MMP-7 and 9; fibroblast growth factor (FGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF) and platelet-derived growth factor (PDGF), all of which have independent pro-tumorigenic functions [75–77].
Recently, interesting data from Pettit and colleagues demonstrated that a discrete population of macrophages, osteal tissue macrophages (termed ‘OsteoMacs’). Later, the authors also showed that OsteoMacs are required for physiological bone remodeling as well as intramembranous bone healing, suggesting that osteal macrophages are critical components in bone physiology [78, 79]. However, there is currently no definitive data showing the tumorigenic function of resident macrophages in tumor growth and/or metastasis in bone. To sum up, clinical and experimental data supports the tumorigenic roles of macrophages in primary tumor tissue, but further investigation is required for the potential roles in the bone microenvironment.
Myeloid-Derived Suppressor Cells and Monocytes: An Elusive Population with Confronting Functions
As frequently portrayed as ‘wounds that never heal’, cancer is comprised of multiple types of immune/inflammatory cells [80]. Clinical data have now accumulated indicating that human tumor samples positively correlate with infiltration of bone marrow-derived immune cells (BMDCs) such as macrophages and neutrophils. In particular, recent evidence collectively shows that bone marrow-derived macrophages and monocytes (collectively termed ‘myeloid lineage cells’) play crucial roles in tumor angiogenesis [76, 81, 82]. However, those pro-angiogenic myeloid cells are yet poorly defined, and show overlapping phenotypes [83]. The most widely accepted population of pro-tumorigenic BMDCs are myeloid-derived suppressor cells (MDSCs), expressing both CD11b (a myeloid cell marker) and Gr1 (a granulocyte marker). MDSCs were originally investigated for their roles in suppressing CD8+ T cell immunity, contributing to tumor escape from the host immune-surveillance [84–86]. Yang et al. demonstrated that CD11b+Gr1+ MDSCs promote vascular density and vascular maturation while decreasing necrosis [87, 88]. In addition, the authors showed that MDSCs express high levels of matrix metalloproteinase (MMP)-9, and also MDSCs acquire endothelial properties to incorporate into endothelium. Similarly, Kim et al. demonstrated that circulating monocytes in tumor-bearing hosts express an endothelial cell marker (CD31) and directly contribute to tumor angiogenesis [89]. However, the idea that MDSCs can differentiate into endothelial cells remains controversial and to be further investigated [90]. Interestingly, while tumors cannot grow in MMP-9 knockout mice, wild-type bone marrow transplantation can restore tumor growth in the same host, suggesting that BMDCs are the primary source of MMP-9 in tumor angiogenesis. CD11b+ myeloid cells, but not endothelial progenitor cells, are the main source of MMP-9 in the tumor tissue [91], which can increase the bioavailability of VEGF and other endothelial growth factors. In addition, neutrophils have been shown to secrete VEGF [92]. Recent data from Yang et al. suggest that MDSCs enhance tumor cell invasion and contribute to TGF-β-mediated breast cancer metastasis [93]. Furthermore, recruitment of CD11b+Gr1+ cells is mediated by the two chemokine axes, SDF-1/CXCR4 and CXCL5/CXCR2 [93].
Given the roles of MDSCs in tumor angiogenesis and invasion, it is likely that MDSCs promote tumor growth and/or metastasis in any organ site including bone. In addition, the surface markers of MDSCs overlap with those of osteoclast lineage cells, suggesting that MDSCs have potential to differentiate into osteoclasts. However, the role of MDSCs specifically in bone metastasis is not yet clearly understood. Some supporting evidence came from the work of Mundy and colleagues who discovered that MDSCs were increased in the bone marrow and spleen in a syngeneic myeloma mouse model, and the MDSCs from the myeloma-bearing mice had a greater capacity to form osteoclasts, compared to the MDSCs from control mice [88, 94]. Furthermore, these authors presented their preliminary data that MDSCs can be precursors of osteoclasts in myeloma bone lesions [95], and also that an osteoclast inhibitor, zoledronic acid, suppressed the differentiation of MDSCs into osteoclasts [96].
Discussion and Conclusions
Bone marrow is comprised of diverse populations of hematopoietic lineage cells as well as stromal cells. Increasing lines of evidence support pro-tumorigenic roles of individual bone marrow-derived cell populations in such processes as angiogenesis, tumor cell apoptosis, escape from immune-surveillance, etc. However, each cell population mediates distinct and sometimes contradictory (pro- or anti-tumorigenic) roles, and continued research endeavors are required to delineate the complexity. This review article summarized key evidence describing the differential roles of hematopoietic lineage cells, including HSCs, megakaryocytes, macrophages and MDSCs in bone metastasis (see Fig. 1 for a schematic summary of data). Briefly, expansion of bone marrow cellularity has been found to promote prostate cancer skeletal metastasis, suggesting in general that cells in the bone marrow have tumorigenic functions [42]. Indeed, HSCs switch on angiogenesis, promoting tumor growth and potentially metastasis [41]. However, recent data demonstrated that tumor cells compete with HSCs for niche occupancy, thus the presence of HSCs can negatively regulate tumor metastasis to bone [40]. More interestingly, HSCs have been shown to increase bone morphogenetic proteins (BMP)-2 and 6 in response to erythropoietin stimuli, potentially contributing to augmented osteoblastogenesis [97]. These data collectively support that even a single cell population entity (i.e. HSCs) can have a dual function in the context of tumor metastasis to bone. For example, data demonstrate that mesenchymal stem cells (MSCs), which give rise to multiple types of stromal cells including adipocytes, muscles, fibroblasts, chondrocytes, etc., contribute to the creation of a favorable tumor microenvironment in general as well as in bone [23]. Contrarily, Naveiras et al. demonstrated that bone marrow adipocytes, which frequently infiltrate red marrow spaces after chemotherapy or radiation, negatively regulate HSCs [98].
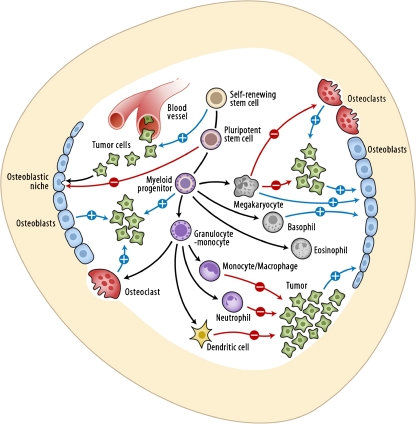
Fig. 1.
Interactions between metastatic tumor cells and bone marrow cells are illustrated. Hematopoietic stem cells increase tumor growth by promoting angiogenesis and osteoblastogenesis. Concurrently, hematopoietic stem cells compete with metastatic tumor cells for occupancy of the osteoblastic niche, resulting in negative regulation of skeletal metastasis. Osteoblasts and osteoclasts both contribute to the positive feedback leading to tumor growth in bone (a ‘vicious cycle’). Immune cells (phagocytic cells and dendritic cells) attack tumor cells. However, a subset of immune precursor cells (e.g. myeloid-derived suppressor cells) is implicated in promoting tumor growth and metastasis. Megakaryocytes induce apoptosis of extravasating tumor cells in bone, and also suppress tumor cell proliferation. (Legend: blue arrows with circled plus mark indicate positive regulation; red arrows with circled minus mark indicate negative regulation; and black arrows indicate differentiation. Cellular components of this cartoon are not proportionate to the actual size)
Other components of the bone marrow such as megakaryocytes and macrophages also have unique roles in tumor progression. Megakaryocytes are potentially the first cells that extravasating tumor cells encounter in the bone marrow, and megakaryocytes induce tumor cell apoptosis and decreased proliferation [44]. Despite the lack of definitive experimental results in bone metastasis, macrophages, particularly TAMs, are highly likely to play critical roles in tumor growth and angiogenesis in bone. Similarly, MDSCs are essential components for a favorable tumor microenvironment. Collectively, as the bone marrow is the primary supplying organ of macrophages, monocytes and other immune cells, precursors and the differentiated macrophages and MDSCs surely play essential roles in bone metastasis.
Even with the data described in this article, elucidating the roles of bone marrow cells in the metastatic bone microenvironment remain a rich area of research opportunity. For example, one emerging question is how solid tumors in a primary organ site or in circulation regulate bone marrow cells before the occurrence of bone metastasis. The tumor microenvironment is comprised of primary tumor cells mixed with multiple types of stromal cells, of which a significant fraction originates from the bone marrow. Increasing evidence supports the critical roles of those bone marrow-derived cells (BMDCs) in tumor progression. As BMDCs are such critical components, it is likely that primary tumors somehow communicate with the cells in the bone marrow to supply the indispensable components to enhance metastatic capacity. In addition, the data demonstrating that tumor cells prime the metastatic soil (termed ‘pre-metastatic niche’) before arrival of tumor cells in the metastatic recipient organ by VEGF-receptor 1-positive bone marrow cells [99, 100], suggest similar mechanisms may occur in the bone marrow before arrival of breast or prostate cancer cells in the bone marrow. Particularly, the unique bone-tropism of metastatic prostate or breast cancer cells may be due to breast or prostate tumor-derived factors modulating bone and bone marrow cells. One potential candidate molecule mediating crosstalk between tumor cells and bone marrow cells is parathyroid hormone-related peptide (PTHrP). PTHrP was first discovered as an etiologic factor for malignancy-induced hypercalcemia, and was later implicated in pro-tumorigenic roles such as cellular proliferation, angiogenesis as well as stimulating osteoblasts and osteoclasts. Similar to parathyroid hormone (PTH), a physiological counterpart, PTHrP promotes bone turnover and anabolic response, which can promote tumor growth in bone. In addition, PTHrP up-regulates cytokine expression from the bone marrow stromal cells (i.e. osteoblasts), including VEGF, IL-6 and C-C chemokine ligand (CCL)-2 (also known as monocyte chemoattractant protein [MCP]-1) all of which have the potential to promote bone marrow cells. Therefore, tumor cells in their primary organ site may secrete PTHrP to prime the cells in the bone marrow indirectly via up-regulating cytokines from osteoblasts, leading to expansion and/or potentiation of fractions of bone marrow cells (e.g. MDSCs). In turn, the primed bone marrow cells either cultivate the metastatic recipient site, and/or travel back to the primary tumor tissue to promote growth, invasion and angiogenesis. However, there is currently no data supporting this potential loop of crosstalk between tumor tissue and the bone marrow. Continued research in this field may yield potential mechanisms that could be targeted for the treatment and prevention of metastasis, thereby providing a means to increase length and quality of life for cancer patients.
Acknowledgements
This work was financially supported by the Department of Defense Prostate Cancer Research Program Grants W81XWH-10-1-0546 (Serk In Park) and W81XWH-08-1-0037 (Laurie K. McCauley); and the National Cancer Institute Program Project Grant P01CA093900 (Laurie K. McCauley). The authors thank Janice E. Berry, Amy J. Koh and Matthew Eber for their assistance with preparation of this manuscript.
Conflict of Interest The authors declare no financial conflict of interest.
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Canc. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Canc Res. 2010;70(14):5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidler IJ. Critical determinants of metastasis. Semin Canc Biol. 2002;12(2):89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- 4.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Canc Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 5.Ewing J (1928) Neoplastic diseases; a treatise on tumors. 3d ed rev. and enl., with 546 illustrations. edn. W.B. Saunders, Philadelphia, London
- 6.Geldof AA. Models for cancer skeletal metastasis: a reappraisal of Batson’s plexus. Anticancer Res. 1997;17(3A):1535–1539. [PubMed] [Google Scholar]
- 7.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 8.Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Canc Res. 1980;40(7):2281–2287. [PubMed] [Google Scholar]
- 9.Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Clinicopathological observations on metastasis in man studied in patients treated with peritoneovenous shunts. Br Med J (Clin Res Ed) 1984;288(6419):749–751. doi: 10.1136/bmj.288.6419.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Canc Res. 1984;44(8):3584–3592. [PubMed] [Google Scholar]
- 11.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 12.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 13.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Canc. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 14.Roodman GD. Mechanisms of bone metastasis. New Engl J Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 15.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Canc Res. 2000;6(3):1038–1045. [PubMed] [Google Scholar]
- 16.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Canc. 2011;11(6):411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol. 2009;310(1–2):71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Investig. 1996;98(7):1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Investig. 1999;103(2):197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Canc Res. 2009;69(4):1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao J, Li X, Koh AJ, Berry JE, Thudi N, Rosol TJ, Pienta KJ, McCauley LK. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Canc. 2008;123(10):2267–2278. doi: 10.1002/ijc.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao J, McCauley LK. Skeletal metastasis: established and emerging roles of parathyroid hormone related protein (PTHrP) Canc Metastasis Rev. 2006;25(4):559–571. doi: 10.1007/s10555-006-9033-z. [DOI] [PubMed] [Google Scholar]
- 23.Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Canc Metastasis Rev. 2010;29(2):249–261. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- 24.Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Canc. 2010;46(7):1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul WE. Fundamental immunology. 4. Philadelphia: Lippincott-Raven; 1999. [Google Scholar]
- 26.Schermer S. The blood morphology of laboratory animals. 3. Philadelphia: Davis; 1967. [Google Scholar]
- 27.Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicol Pathol. 2006;34(5):548–565. doi: 10.1080/01926230600939856. [DOI] [PubMed] [Google Scholar]
- 28.Shirota T, Tavassoli M. Cyclophosphamide-induced alterations of bone marrow endothelium: implications in homing of marrow cells after transplantation. Exp Hematol. 1991;19(5):369–373. [PubMed] [Google Scholar]
- 29.Shirota T, Tavassoli M. Alterations of bone marrow sinus endothelium induced by ionizing irradiation: implications in the homing of intravenously transplanted marrow cells. Blood Cell. 1992;18(2):197–214. [PubMed] [Google Scholar]
- 30.Weiss L, Geduldig U. Barrier cells: stromal regulation of hematopoiesis and blood cell release in normal and stressed murine bone marrow. Blood. 1991;78(4):975–990. [PubMed] [Google Scholar]
- 31.Tavassoli M, Hardy CL. Molecular basis of homing of intravenously transplanted stem cells to the marrow. Blood. 1990;76(6):1059–1070. [PubMed] [Google Scholar]
- 32.Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188(3):465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frenette PS, Subbarao S, Mazo IB, Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci U S A. 1998;95(24):14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 35.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 36.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Canc Res. 2002;62(6):1832–1837. [PubMed] [Google Scholar]
- 37.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Canc Metastasis Rev. 2010;29(4):709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung Y, Wang J, Song J, Shiozawa Y, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110(1):82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105(2):370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Investig. doi:10.1172/JCI43414 [DOI] [PMC free article] [PubMed]
- 41.Okamoto R, Ueno M, Yamada Y, Takahashi N, Sano H, Suda T, Takakura N. Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood. 2005;105(7):2757–2763. doi: 10.1182/blood-2004-08-3317. [DOI] [PubMed] [Google Scholar]
- 42.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146(4):1727–1736. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 43.Lichtman MA, Chamberlain JK, Simon W, Santillo PA. Parasinusoidal location of megakaryocytes in marrow: a determinant of platelet release. Am J Hematol. 1978;4(4):303–312. doi: 10.1002/ajh.2830040402. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Koh AJ, Wang Z, Soki FN, Park SI, Pienta KJ, McCauley LK. Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J Bone Miner Res. 2011;26(1):125–134. doi: 10.1002/jbmr.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kacena MA, Gundberg CM, Horowitz MC. A reciprocal regulatory interaction between megakaryocytes, bone cells, and hematopoietic stem cells. Bone. 2006;39(5):978–984. doi: 10.1016/j.bone.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Kacena MA, Nelson T, Clough ME, Lee SK, Lorenzo JA, Gundberg CM, Horowitz MC. Megakaryocyte-mediated inhibition of osteoclast development. Bone. 2006;39(5):991–999. doi: 10.1016/j.bone.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res. 2004;19(4):652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- 48.Bord S, Frith E, Ireland DC, Scott MA, Craig JI, Compston JE. Megakaryocytes modulate osteoblast synthesis of type-l collagen, osteoprotegerin, and RANKL. Bone. 2005;36(5):812–819. doi: 10.1016/j.bone.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 50.Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E, Higashio K, Gillespie MT, Martin TJ, Suda T. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone. 1999;25(5):517–523. doi: 10.1016/S8756-3282(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed N, Khokher MA, Hassan HT. Cytokine-induced expansion of human CD34+ stem/progenitor and CD34 + CD41+ early megakaryocytic marrow cells cultured on normal osteoblasts. Stem Cell. 1999;17(2):92–99. doi: 10.1002/stem.170092. [DOI] [PubMed] [Google Scholar]
- 52.Roato I, D'Amelio P, Gorassini E, Grimaldi A, Bonello L, Fiori C, Delsedime L, Tizzani A, Libero A, Isaia G, Ferracini R. Osteoclasts are active in bone forming metastases of prostate cancer patients. PLoS One. 2008;3(11):e3627. doi: 10.1371/journal.pone.0003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Canc Res. 1999;59(6):1295–1300. [PubMed] [Google Scholar]
- 55.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Investig. 2004;114(12):1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta GP, Massague J. Platelets and metastasis revisited: a novel fatty link. J Clin Investig. 2004;114(12):1691–1693. doi: 10.1172/JCI23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11(11):1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9(7):556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halin S, Rudolfsson SH, Rooijen N, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11(2):177–186. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 61.Hiraoka K, Zenmyo M, Watari K, Iguchi H, Fotovati A, Kimura YN, Hosoi F, Shoda T, Nagata K, Osada H, Ono M, Kuwano M. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Canc Sci. 2008;99(8):1595–1602. doi: 10.1111/j.1349-7006.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 63.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Canc Biol. 2008;18(5):349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 65.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Canc Res. 2007;67(19):9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 66.Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008;283(36):25057–25073. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b + peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284(49):34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Canc Inst. 2010;102(8):522–528. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Canc Res. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 70.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Canc Res. 1996;56(20):4625–4629. [PubMed] [Google Scholar]
- 71.Hildenbrand R, Dilger I, Horlin A, Stutte HJ. Urokinase and macrophages in tumour angiogenesis. Br J Canc. 1995;72(4):818–823. doi: 10.1038/bjc.1995.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR. Expression of tumour necrosis factor (TNF alpha) and its receptors in benign and malignant breast tissue. Int J Canc. 1994;56(6):777–782. doi: 10.1002/ijc.2910560603. [DOI] [PubMed] [Google Scholar]
- 73.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17(14):2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 74.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 75.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Canc. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 76.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Canc Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 78.Alexander K, Chang M, Maylin E, Kohler T, Muller R, Wu A, Rooijen N, Sweet M, Hume D, Raggatt L, Pettit A. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26(7):1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 79.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181(2):1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 80.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. New Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 81.Zumsteg A, Christofori G. Corrupt policemen: inflammatory cells promote tumor angiogenesis. Curr Opin Oncol. 2009;21(1):60–70. doi: 10.1097/CCO.0b013e32831bed7e. [DOI] [PubMed] [Google Scholar]
- 82.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Canc. 2008;8(8):618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 83.Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, Palma M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010;176(4):1564–1576. doi: 10.2353/ajpath.2010.090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13(7):828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Canc Res. 2008;68(8):2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 87.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr + CD11b + cells in tumor-bearing host directly promotes tumor angiogenesis. Canc Cell. 2004;6(4):409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 88.Yang L, Edwards CM, Mundy GR. Gr-1 + CD11b + myeloid-derived suppressor cells (MDSCs): formidable partners in tumor metastasis. J Bone Miner Res. 2010;25(8):1701–1706. doi: 10.1002/jbmr.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim SJ, Kim JS, Papadopoulos J, Wook Kim S, Maya M, Zhang F, He J, Fan D, Langley R, Fidler IJ. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol. 2009;174(5):1972–1980. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18(8):372–378. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 91.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Canc Cell. 2008;13(3):193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taichman NS, Young S, Cruchley AT, Taylor P, Paleolog E. Human neutrophils secrete vascular endothelial growth factor. J Leukoc Biol. 1997;62(3):397–400. doi: 10.1002/jlb.62.3.397. [DOI] [PubMed] [Google Scholar]
- 93.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1 + CD11b + myeloid cells that promote metastasis. Canc Cell. 2008;13(1):23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.ASBMR 29th Annual Meeting J Bone Miner Res 200722S1s2–s51.18050512 [Google Scholar]
- 95.Zhuang J, Yang L, Lwin ST, Edwards CM, Edwards JR, Mundy GR. Osteoclasts in myeloma are derived from Gr-1 + CD11b + myeloid immune suppressor cells of the bone marrow niche in vivo. ASH Annu Meet Abstr. 2008;112(11):736. [Google Scholar]
- 96.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Canc Res. 2007;67(23):11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 2010;5(5):e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Canc Metastasis Rev. 2006;25(4):521–529. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 100.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]