Abstract
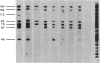
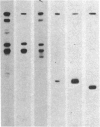
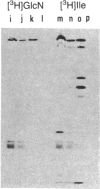
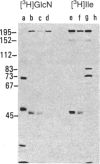
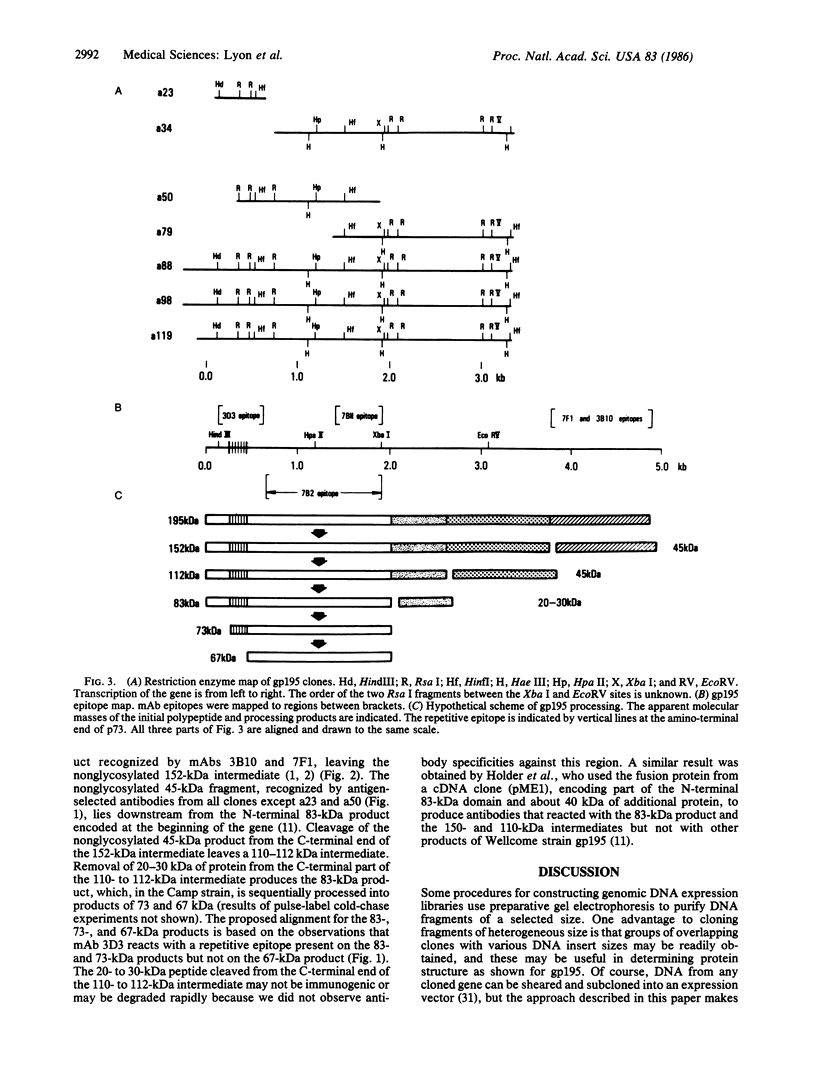
DNA fragments from human malaria parasites were cloned into lambda gt11 to produce a genomic DNA expression library. A pool of monoclonal antibodies (mAbs) recognizing three domains of the 195-kDa major merozoite surface glycoprotein (gp195) reacted with seven clones expressing malaria antigens. mAbs recognizing the 83-kDa product of gp195 reacted with the clones, but mAbs recognizing a glycosylated 45-kDa and a nonglycosylated 45-kDa domain did not. Restriction enzyme mapping revealed that the clones contained overlapping segments encoding about 70% of the gene beginning at the 5' end and ending at an EcoRI restriction enzyme site 3.3 kilobase pairs downstream. The mAbs recognizing the 83-kDa domain reacted differently with the clones, allowing the mapping of three epitopes, one of which was repetitive. Affinity-purified antibodies were selected from immune monkey serum with recombinant expression proteins adsorbed to nitrocellulose filters. When used to probe electrophoretic immunoblots of parasite extracts, these antigen-selected antibodies reacted with specific sets of processed products of gp195, including those associated with the 83- and the nonglycosylated 45-kDa domains. This information, combined with the mAb epitope map, allowed a tentative scheme for processing gp195 from the Camp strain to be proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aley S. B., Sherwood J. A., Howard R. J. Knob-positive and knob-negative Plasmodium falciparum differ in expression of a strain-specific malarial antigen on the surface of infected erythrocytes. J Exp Med. 1984 Nov 1;160(5):1585–1590. doi: 10.1084/jem.160.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A., Shaw A. R., Leban J., Perrin L. H. Cloning and expression in Escherichia coli of a surface antigen of Plasmodium falciparum merozoites. EMBO J. 1985 Apr;4(4):1007–1011. doi: 10.1002/j.1460-2075.1985.tb03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulay J. D., Aikawa M., Diggs C., Haynes J. D. Inhibitory effects of immune monkey serum on synchronized Plasmodium falciparum cultures. Am J Trop Med Hyg. 1981 Jan;30(1):12–19. doi: 10.4269/ajtmh.1981.30.12. [DOI] [PubMed] [Google Scholar]
- Chulay J. D., Haynes J. D., Diggs C. L. Inhibition of in vitro growth of Plasmodium falciparum by immune serum from monkeys. J Infect Dis. 1981 Sep;144(3):270–278. doi: 10.1093/infdis/144.3.270. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- David P. H., Hadley T. J., Aikawa M., Miller L. H. Processing of a major parasite surface glycoprotein during the ultimate stages of differentiation in Plasmodium knowlesi. Mol Biochem Parasitol. 1984 Apr;11:267–282. doi: 10.1016/0166-6851(84)90071-9. [DOI] [PubMed] [Google Scholar]
- Epstein N., Miller L. H., Kaushel D. C., Udeinya I. J., Rener J., Howard R. J., Asofsky R., Aikawa M., Hess R. L. Monoclonal antibodies against a specific surface determinant on malarial (Plasmodium knowlesi) merozoites block erythrocyte invasion. J Immunol. 1981 Jul;127(1):212–217. [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Hall R., McBride J., Morgan G., Tait A., Zolg J. W., Walliker D., Scaife J. Antigens of the erythrocytes stages of the human malaria parasite Plasmodium falciparum detected by monoclonal antibodies. Mol Biochem Parasitol. 1983 Mar;7(3):247–265. doi: 10.1016/0166-6851(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Harn D. A., Mitsuyama M., David J. R. Schistosoma mansoni. Anti-egg monoclonal antibodies protect against cercarial challenge in vivo. J Exp Med. 1984 May 1;159(5):1371–1387. doi: 10.1084/jem.159.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R., Newbold C. I. Serological cross-reaction between high molecular weight proteins synthesized in blood schizonts of Plasmodium yoelii, Plasmodium chabaudi and Plasmodium falciparum. Mol Biochem Parasitol. 1983 Nov;9(3):191–196. doi: 10.1016/0166-6851(83)90096-8. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984 Aug 1;160(2):624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Cowman A. F., Saint R. B., Brown G. V., Anders R. F. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: detection with antibodies from immune humans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3787–3791. doi: 10.1073/pnas.80.12.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lanar D. E., Pearce E. J., Sher A. Expression in Escherichia coli of two Schistosoma mansoni genes that encode major antigens recognized by immune mice. Mol Biochem Parasitol. 1985 Oct;17(1):45–60. doi: 10.1016/0166-6851(85)90127-6. [DOI] [PubMed] [Google Scholar]
- Lyon J. A., Haynes J. D., Diggs C. L., Chulay J. D., Pratt-Rossiter J. M. Plasmodium falciparum antigens synthesized by schizonts and stabilized at the merozoite surface by antibodies when schizonts mature in the presence of growth inhibitory immune serum. J Immunol. 1986 Mar 15;136(6):2252–2258. [PubMed] [Google Scholar]
- Lyon J. A., Haynes J. D. Plasmodium falciparum antigens synthesized by schizonts and stabilized at the merozoite surface when schizonts mature in the presence of protease inhibitors. J Immunol. 1986 Mar 15;136(6):2245–2251. [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- McBride J. S., Newbold C. I., Anand R. Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J Exp Med. 1985 Jan 1;161(1):160–180. doi: 10.1084/jem.161.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Hansen J. L., Dame J. B., Mullins J. A. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. doi: 10.1126/science.6330899. [DOI] [PubMed] [Google Scholar]
- McElroy J. F., Miller J. M., Meyer J. S. Fenfluramine, p-chloroamphetamine and p-fluoroamphetamine stimulation of pituitary-adrenocortical activity in rat: evidence for differences in site and mechanism of action. J Pharmacol Exp Ther. 1984 Mar;228(3):593–599. [PubMed] [Google Scholar]
- Nunberg J. H., Rodgers G., Gilbert J. H., Snead R. M. Method to map antigenic determinants recognized by monoclonal antibodies: localization of a determinant of virus neutralization on the feline leukemia virus envelope protein gp70. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3675–3679. doi: 10.1073/pnas.81.12.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirson P. J., Perkins M. E. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J Immunol. 1985 Mar;134(3):1946–1951. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vernes A., Haynes J. D., Tapchaisri P., Williams J. L., Dutoit E., Diggs C. L. Plasmodium falciparum strain-specific human antibody inhibits merozoite invasion of erythrocytes. Am J Trop Med Hyg. 1984 Mar;33(2):197–203. doi: 10.4269/ajtmh.1984.33.197. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Hockmeyer W. T. Structure of the circumsporozoite protein gene in 18 strains of Plasmodium falciparum. Mol Biochem Parasitol. 1985 Jun;15(3):305–316. doi: 10.1016/0166-6851(85)90092-1. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]