Abstract
Myeloma bone disease (BD) not only impairs quality of life, but is also associated with impaired survival. Studies of the biology underlying BD support the notion that the increased osteoclastogenesis and suppressed osteoblastogenesis, is both a consequence and a necessity for tumour growth and clonal expansion. Survival and expansion of the myeloma clone is dependent on its interactions with bone elements, thus targeting these interactions should have antimyeloma activities. Indeed both experimental and clinical findings indicate that bone-targeted therapies not only improve BD, but also create an inhospitable environment for myeloma cell growth and survival, favouring improved clinical outcome. This review summarizes recent progress in our understandings of the biology of myeloma BD, highlighting the role of osteoclasts and osteoblasts in this process and how they can be targeted therapeutically. Unravelling the mechanisms underlying myeloma-bone interactions will facilitate the development of novel therapeutic agents to treat BD, which as a consequence are likely to improve the clinical outcome of myeloma patients.
Keywords: Multiple myeloma, Bone disease, Osteoclast, Osteoblast, Therapy
Introduction
Multiple myeloma (MM) is the result of a clonal expansion of malignant plasma cells primarily located within the bone marrow (BM). Despite considerable therapeutic advances, the disease remains largely incurable with median survivals of 4–5 years dependent upon biological subgroup. Myeloma is unique among haematological malignancies, being characterized by osteolytic bone lesions and the development of skeletal related events (SREs). At presentation 70% of patients have bone disease (BD) and 60% patients report a pathologic fracture over the course of their disease [1]. The presence of BD is a defining characteristic of myeloma requiring treatment; moreover the extent of BD and bone resorption activity has been shown to be an important risk factor for overall survival (OS) [2–4]. As BD is the major contributor to morbidity and mortality in MM, in addition to chemotherapy targeting the myeloma cells, bone-supportive treatment is also an essential component of the therapy, and accumulating evidence suggests that bone targeted therapies not only reduce skeletal complications but also improve survival.
In the normal BM microenvironment, bone is constantly undergoing remodelling with a delicate balance between bone resorption and bone formation. The mechanism underlying myeloma BD is an uncoupling of bone resorption from bone formation as a consequence of increased osteoclastic activity and inhibition of osteoblast function [5]. We are now beginning to understand that this dysregulation is not only responsible for the bone destruction, but also for the initiation, maintenance and expansion of the myeloma clone. This pro-survival effect is thought to be mediated via direct cell-cell contact between myeloma and bone cells, as well as via positive cytokine feedback loops set up during the bone resorption process, creating a vicious cycle of bone resorption and tumour growth.
The complex interactions present in the BM microenvironment bring with them the potential for their therapeutic targeting. In this respect, in addition to therapies targeting the myeloma cells directly, additional benefit could be achieved by combination with agents targeting myeloma cell interactions with the BM microenvironment. This approach may not only be beneficial during induction treatment, by increasing the sensitivity of the myeloma cells to cytotoxic agents, but may also be important during the maintenance phase by targeting the plasma cell niche in the BM, such that the biology of residual clonal cells is modified. The complex interactions in the BM can be targeted in different ways including targeting the cell-cell contact of the MM cells with stromal elements, targeting the growth factors produced by both the MM cells and the stromal elements, as well as targeting the factors released from bone modelling process. Such changes in the emphasis of the treatment reflect our increasing ability to induce complete responses and the consequent requirement to develop approaches able to maintain these responses long term.
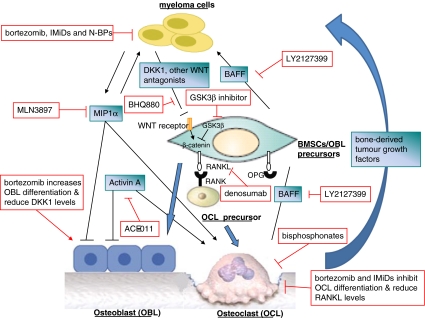
In this review, we discuss recent progress in the understanding of the myeloma-BM microenvironment interaction. We highlight the role of normal BM constituents, particularly osteoclasts, osteoblasts and their precursors, in myeloma pathogenesis and drug resistance. We also describe the antimyeloma activity of bone targeting therapies and how this could improve clinical outcome for MM patients (Fig. 1).
Fig. 1.
Bone targeted therapies. Recent progress in the pathogenesis of bone disease and myeloma biology have accelerated our understanding of the effects of current treatment on myeloma and associated bone disease, more importantly they have translated into the development of novel bone-targeted agents
Pathogenesis of Myeloma and its Interactions with the Bone Marrow Microenvironment
MM is currently viewed as a prototypical disease model for studying tumour–microenvironment interactions, based on the hypothesis that the biology of MM is highly dependent on the interactions with structural and soluble components within the microenvironment, which are also responsible for the generation of BD. Thus it should also be considered an excellent model in which we can understand whether targeting tumour-microenvironment interactions can have anticancer effects.
While the spectrum of genetic change in individual cases of myeloma is thought to determine the clinical behaviour of that case, this cannot be the whole story. The clonal plasma cells of all MM patients harbour genetic lesions, and these chromosome abnormalities are present in most cases of monoclonal gammopathy of undetermined significance (MGUS) [6], which is not characterized by lytic bone lesions. Thus the biology of MM cells is not determined exclusively by genetic abnormalities, but is also influenced by interactions with the local microenvironment, as well as by other epigenetic features in the myeloma cell. Thus considering the biology of the plasma cell and its interaction with the stromal microenvironment becomes important.
On binding to their stromal environment myeloma cells have been shown to induce changes in several cell types that are intimately involved in the induction of bone lesions, including BM stromal cells (BMSCs), BM endothelial cells, immune cells, osteoblasts and osteoclasts. The induced changes, in turn, offer the MM cells a supportive stromal environment, access to vascular networks, and locally produced growth factors and cytokines [7], favouring their growth and survival. Interestingly, although microenvironmental interactions are important, the genetic lesions associated with subgroups of myeloma also seem to modulate rates of BD possibly by modifying the interaction of the myeloma cells with the BM milieu. For example, MM cells harbouring the t(14;16) translocation overexpress the transcription factor c-maf, which activates cyclin D2 expression and increases MM cell proliferation. In addition, c-maf up-regulates b7-integrin expression and potentiates MM cell adhesion to BMSCs [8]. It has been shown that cases characterized by MAF deregulation have less BD as do cases with the t(4;14) [9, 10]. In contrast MM cells characterized by hyperdiploid karyotypes have more BD and seem to depend on the BM microenvironment for the induction of cyclin D1 expression [10, 11]. Furthermore, the hyperdiploid subgroup and cases with cyclin D1 overexpression are under-represented in plasma cell leukaemia [12], where microenvironmental interactions are clearly less important. These examples support the notion that the genetics of MM cells and their interactions with the BM microenvironment are not completely independent of each other, but rather functionally interact to influence the biology and clinical behaviour of the disease.
Osteoclasts are the principal resorptive cells of bone and play a key role in the regulation of bone mass. They are multinucleated cells formed by the fusion of the mononuclear progenitors of the monocyte/macrophage family [13]. Myeloma cells either directly produce or induce other cells to produce “osteoclast activating factors”, which drive the differentiation of haematopoietic stem/precursor cells to mature osteoclasts as well as increasing their bone resorbing activity. Osteoclast activation significantly increases the growth and survival of the myeloma clone, an effect that is mediated both by direct cell-cell contact and indirectly via the release of soluble factors. Soluble growth factors and cytokines in the MM microenvironment, such as interleukin 6 (IL-6), osteopontin, B cell activating factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL), have been implicated in osteoclast-induced myeloma cell survival [14].
Cell adhesion mediated drug resistance (CAMDR) is a feature of the myeloma cell interaction with osteoclasts in the BM. In this context it has been observed that co-culturing osteoclasts with myeloma cells is able to protect myeloma cells from drug-induced apoptosis [15]. Of interest in this respect is that after in vitro interaction with osteoclasts or stromal cells, myeloma cells acquire a more immature phenotype [16, 17]. Such observations are clinically relevant as high resolution imaging approaches have shown that plasma cells can persist in focal lesions in the BM of patients who have otherwise achieved a complete remission [18, 19]. Such a process could also be responsible for maintaining myeloma stem cells within a stromal cell niche in the BM, mediating chemo-resistance and subsequent disease relapse [20]. The effect of BMSCs to support proliferation, survival and drug resistance of MM should be viewed as pathological recapitulation of their natural role in supporting haematopoiesis.
Stromal cells are a heterogeneous assembly of mesenchymal stem cells (MSCs) that can differentiate into a variety of cell types including osteoblasts. MM cell adhesion to BMSCs (via adhesion molecules such as VLA-4 and VCAM-1) inhibits their differentiation into osteoblasts, at the same time, enhancing MM cell proliferation and survival through the direct activation of intracellular signal transduction pathways [7]. In addition, the increased production of pro-survival factors by stromal cells or myeloma cells, as consequence of their interaction, promotes myeloma cell growth in a paracrine or autocrine fashion. Important factors produced as a consequence of plasma cell-stromal cell interactions include IL-6, insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), tumour necrosis factor- α (TNF-α), stromal cell-derived factor 1α (SDF-1a), fibroblast growth factor (FGF), interleukin-1 (IL-1) and macrophage inflammatory protein 1 alpha (MIP-1α) [7]. In the myeloma cell, the cytokine stimulation and/or adhesion process activates diverse interdependent signalling cascades, including the Ras/Raf/MEK/MAPK pathway, the PI3K/Akt pathway, the JAK/Stat3 pathway, the nuclear factor-kappa B (NF-κB) pathway and the Wnt signalling pathway [21]. Taken together, these findings emphasize the role of protection provided by the BM microenvironment for myeloma cells, as well as providing a hint that modifying these interactions may improve the outcome of treatment.
A full understanding of the role of osteoblasts in mediating myeloma cell growth has been difficult to define, partially due to the use of ill-defined populations of osteoblasts [14]. Mature osteoblasts differ from BMSCs and immature osteoblasts by the expression of a different pattern of cytokines and “osteoclast activating factors”. Terminally differentiated osteoblasts produce high levels of OPG and reduced levels of RANKL, consequently reducing osteoclastogenesis and, therefore, bone resorption [22–24]. The production of pro-survival growth factors, such as IL-6 and IGF1, are reduced as BMSCs differentiate into osteoblast [25], also favouring an anti-myeloma effect. This effect may also be enhanced by the fact that mature osteoblasts can produce factors directly inhibiting the survival of myeloma cells as well as interfering with the pro-survival impact of osteoclasts [26]. One of the molecular mechanisms underlying such effects is mediated via decorin, a small leucine-rich proteoglycan. Decorin seem to directly induce MM cell apoptosis by activating caspase 3 and upregulating p21 [27]. In addition it indirectly inhibit tumour growth by degrading critical cell-surface growth-factor receptors such as MET and epidermal growth factor receptor (EGFR) [28] and by suppressing myeloma-induced osteoclastogenesis and angiogenesis [27]. These observations suggest that the inhibition of osteoblast differentiation in MM not only contributes to the induction of osteolytic lesions, but also creates favourable conditions for myeloma cell survival and proliferation. Thus therapeutic approaches to stimulate osteoblast differentiation may be able to reduce the levels of myeloma growth factors and osteoclastogenic factors in BM microenvironment, therefore, restraining myeloma clone growth and consequently improving clinical outcome.
Pathogenesis of Bone Disease in Myeloma
BD in myeloma is characterized by purely osteolytic lesions with no new bone formation, an effect that is mediated via increased osteoclastic activity and inhibition of osteoblast function. Interestingly in respect of the pattern of BD, myeloma cells lie in close proximity to the sites of active bone resorption and seem to play a key role in altering the balance of bone resorption and bone formation [29]. It is not surprising, therefore, that directly targeting the interactions of these local cellular components may have an impact on the growth of the myeloma clone with the potential for improving patients’ survival.
Osteoclast Activating Factors
Several osteoclast activating factors have been found to be important in regulating bone resorption. The most significant system, in this respect, consists of the receptor activator of NF-κB (RANK), its ligand RANKL and a soluble decoy receptor, osteoprotegerin (OPG) [29] (Fig. 1). RANK is a transmembrane receptor of the TNF superfamily which is expressed on the surface of osteoclastic cells and their precursors, whereas its ligand, RANKL, is expressed by BMSCs, osteoblasts and T-lymphocytes. RANK-RANKL signalling leads to the activation of NF-κB and Jun N-terminal kinase (JNK) pathways, resulting in the differentiation of osteoclast from their precursor cells [30]. Moreover, RANKL is also involved in enhanced osteoclast survival through inhibition of Fas-mediated apoptosis [31]. OPG, the soluble decoy receptor for RANKL, is produced by osteoblasts as well as by other cell types within the BM and balances the interaction between RANKL and RANK, therefore, limiting its role in osteoclast activation.
In the myeloma BM microenvironment, the interaction between BMSCs and MM cells results in increased RANKL expression and decreased OPG production, favouring bone resorption [32, 33]. Circulating levels of OPG and RANKL have been shown to correlate with the clinical activity of MM, the severity of BD and poor prognosis [34, 35]. The critical role of RANKL-OPG axis in MM-induced BD has been further demonstrated in several mouse models, showing that recombinant OPG or a RANKL inhibitor (RANK-Fc) both prevented bone destruction and reduced tumour burden [33, 36]. The importance of this system would suggest that if a strategy targeting BD as a therapy for myeloma is to be effective, an effect should be seen if this system is targeted. The availability of denosumab, an antibody against RANKL, has given us the ability to address this question and is discussed below.
MIP1α, largely produced by myeloma cells and osteoclasts, belongs to the C-C chemokine subfamily. It binds to G-protein-coupled receptors, CCR1 and CCR5, to activate ERK and AKT signalling pathways. It is a potent inducer of osteoclast formation independent of RANKL by promoting osteoclast precursor cell migration and fusion [37] (Fig. 1). It also has multiple roles in myeloma cell, including promoting myeloma cell growth, survival and migration [38]. High serum levels of MIP1α correlate with osteolytic lesions and survival in MM patients [39], and targeting it directly could have important effects on improving patients’ outcome. A clinical grade small molecule CCR1 antagonist, MLN3897, inhibits MIP1α-induced osteoclastogenesis and myeloma cell proliferation in vitro [40]. Recent report shows that MIP1α also inhibits osteoblast function (Fig. 1) [41], an effect mediated via downregulation of the osteogenic transcription factor osterix and downstream ERK signalling. MLN3897 blocks ERK phosphorylation and restores, at least partially, osteocalcin expression in vitro and in vivo. Evidence suggesting that targeting this molecule can improve clinical outcome comes from in vivo experiments showing that both antisense oligonucleotide and neutralizing antibody against MIP1α inhibit tumour growth and restore bone remodelling in a mouse model of MM BD [42, 43]. These observations highlight the important pathogenic role of MIP1α in MM and associated BD and warrant the need to assess the targeted inhibitors in upcoming clinical trials.
Osteoblast Inhibitors
Myeloma BD seems to be related not only to the increased activity of osteoclast but also to a lack of an appropriate compensatory osteoblastic response. Bone formation is inhibited in myeloma via two distinct mechanisms, the first being the functional inhibition of already existing osteoblasts [44–46] and the second via the impaired differentiation of MSCs into new mature osteoblasts [44, 47, 48]. In addition to reducing new bone formation, the excess of immature osteoblasts provides a rich source of the osteoclastogenic factor RANKL, favouring bone resorption and myeloma cell survival [49]. The differentiation to a mature osteoblast is inhibited by factors secreted by both myeloma cells (e.g. DKK1, sFRP2-3, sclerostin, IL-7 and HGF) and microenvironmental cells (e.g. IL-3, activin A), as well as by direct cell-cell contact between MM cells and osteoblast precursors. A full understanding of the mechanisms underlying this process should provide a rich source of therapeutic targets able to treat BD and also to improve the survival of myeloma patients.
A positive signal delivered by the Wnt signaling pathway is crucial in osteoblast differentiation [50] (Fig. 1). Classically, Wnt binds to the coreceptors LRP5 and 6, and the complex then binds to the frizzled receptor. Signal transduction from the frizzled receptor results in stabilization of beta-catenin, which translocates to the nucleus and stimulates osteoblast differentiation [51]. A variety of secreted molecules can inhibit Wnt proteins by direct binding and associated inactivation (e.g. sFRP) or by competitive antagonism on Wnt surface receptors (e.g. DKK-1 and sclerostin). These antagonists may serve as potential therapeutic targets to increase bone formation, and consequently restrain the growth of the myeloma clone.
DKK1 binds to LRP5/6, thereby inhibiting downstream Wnt signalling. A role for DKK1 in the inhibition of osteoblast activity in MM was first suggested based on gene expression profiling (GEP) of myeloma patients [52], an observation which has been confirmed by various other detection methods (ELISA, qRT-PCR, western blot and immunostaining) [53, 54]. In addition, DKK1 has also been shown to enhance osteoclast activity via an increase in RANKL/OPG ratio [23, 24]. The serum DKK-1 levels have been shown increased in MM patients and correlate with the extent of BD [55, 56], and BM plasma, from MM patients, can inhibit osteoblast differentiation in vitro, an effect that is neutralized by an anti-DKK-1 antibody [52, 55]. Importantly in terms of targeting BD as a potential anti-myeloma therapy, it has been shown that administration of an anti-DKK-1 antibody in a mouse model of myeloma inhibits bone destruction, increases bone formation and also inhibits tumour growth [57].
The sFRP family proteins inhibit Wnt signalling via binding to Wnt proteins, resulting in reduced osteoblast function. Same as DKK1, FRZB is upregulated in MM [52, 58, 59], and a low BD group is characterized by its downregulation [4]. sFRP-2 has also been reported to inhibit osteoblast differentiation in myeloma patients [60], but conflicting results have also been found [61].
Sclerostin has, more recently, been identified as another major Wnt signalling pathway inhibitor via a mechanism similar to DKK1 [62]. Mutations of SOST, the gene encoding sclerostin, are linked to high-bone mass disorders [49], whereas transgenic mice overexpressing SOST are osteopenic [63]. Sclerostin levels are increased in the serum of patients with MM, correlating with advanced ISS stage, increased bone resorption, reduced osteoblast function and poor survival [64]. While sclerostin was thought to be exclusively expressed in osteocytes; it has recently been shown to be expressed in myeloma cells. Sclerostin has been demonstrated to reduce bone formation marker in an in-vitro co-culture system of BMSCs and myeloma cells and a neutralizing sclerostin antibody has been shown to improve bone formation markers [65]. These results suggest that anti-sclerostin represents a promising therapy for the anabolic treatment and warrant the assessment of this approach in myeloma BD.
Although there is compelling evidence that targeting Wnt signaling prevents myeloma BD in experimental models and that this may translate into improved survival outcome, concern has been raised over the implications for tumour growth. Activation of the Wnt signaling pathway through β-catenin has been linked to tumourigenesis [66] and expression of β-catenin has been demonstrated in myeloma cells [67]. Currently, published data are conflicting as to the role of Wnt signaling in myeloma cells [23, 67, 68]. Importantly, in all studies in vivo, when the tumour cells were present within the BM microenvironment, activation of Wnt signaling resulted in a reduction in tumour burden and prevention of myeloma BD [23, 68]. These data highlight the importance of the local microenvironment and demonstrate that, despite the potential to increase tumour growth at extramedullary sites, increasing Wnt signaling in the BM microenvironment seems to be able to prevent the development of myeloma BD and reduce tumour burden. Overall Wnt signalling pathway is a promising potential target for treatment of myeloma BD with the aim of not only to increase bone mass but also to improve patients’ survival. However, further studies are necessary to clarify the role of Wnt signaling in myeloma growth, particularly at extramedullary sites.
Why Might There be a Survival Benefit for Targeting Bone
BD not only significantly impairs quality of life as consequence of skeletal complications such as bone pain and pathological fracture, but also has been linked to poor prognosis in myeloma patients. Bone resorption activity has been shown to be an independent risk factor for OS in MM patients [3]. Results from the MRC myeloma IX trial show that patients with presenting BD have a significantly shorter OS compared to those without BD, with a shorter survival from relapse being the main contributor to this effect (median 12.2 vs 23.4 months) [unpublished data]. In contrast, a low BD group has favourable survival [4]. These observations are not surprising as the dysregulated BM microenvironment components, which are responsible for BD in myeloma, have been demonstrated to contribute to disease progression and resistance to chemotherapy; although BD-associated poor quality of life may also contribute to the impaired outcome.
The important contributions of the BM microenvironment to disease progression can explain, to a certain extent, why the “novel drugs” that target the bone microenvironment as well as myeloma cells, have been more effective than conventional approaches. Apart from their direct anti-tumour activities, the immunomodulatory agents (IMiDs) thalidomide and lenalidomide and the proteasome inhibitor bortezomib, seem to affect osteoclast and osteoblast activity in myelomatous bones [69–72] (Fig. 1).
Bisphosphonates (BPs) are the current standard care for the prevention and treatment of malignant BD [73, 74], strong preclinical evidences from various models of MM suggest that nitrogen-containing BPs (N-BPs) such as zoledronic acid (ZOL) may have anticancer activity including inhibiting angiogenesis, enhancing antitumour immune responses, and directly or indirectly modulating the proliferation and survival of myeloma cells [74]. This has been confirmed by a number of clinical trials showing bisphosphonates improve both OS and PFS in myeloma patients [75–79]. These findings further support the notion that the interactions between myeloma cell and surrounding BM microenvironment constitutes an important factor that needs to be taken into account in the development of novel therapeutic strategies. Here we summarize the current therapies for myeloma BD and their possible anti-tumour activities.
Targeting Bone Resorption and the Osteoclast
Bisphosphonates (BPs)
BPs naturally bind to mineralized surfaces such as bone and inhibit osteoclast-mediated bone resorption (Fig. 1). The BPs pamidronate, ZOL, and clodronate (CLO; in the European Union but not in the United States) are approved for the treatment of patients with osteolytic lesions from MM for the prevention of skeletal-related events (SREs). The second generation N-BPs (e.g. ZOL, pamidronate) have been proven more effective at reducing SREs compared to the first generation BPs (e.g. CLO) [80]. Moreover strong preclinical evidence, from various models of MM, suggest that the N-BPs may have anticancer activity (Fig. 1) via the inhibition of angiogenesis, enhanced anti-tumour immune responses, inhibition of tumour cell migration and directly modulating the proliferation and survival of myeloma cells [81–86]. In vivo N-BPs may also affect MM progression by blocking the release of cytokines and growth factors from the bone matrix, thereby breaking the vicious cycle of bone destruction and cancer growth [74]. In addition, the anticancer effects of BPs have been demonstrated to have synergy with agents that are used in the treatment of myeloma, including dexamethasone, thalidomide, and bortezomib [84, 87, 88]. Preclinical mouse models of MM indicate that the anti-myeloma effect of N-BPs may be mediated via the inhibition of protein prenylation and consequent inhibition of the RAS-RAF-MAPK pathway [89], a mechanism of action not shared by non-N-BPs.
Although no significant differences in survival with BPs were observed in earlier clinical trials in MM, BPs seemed to improve survival in high-risk subsets of patients [76, 77, 90]. For example, in a trial of patients with newly diagnosed or relapsed/refractory MM (N = 392), pamidronate significantly increased survival in the subset of MM patients receiving second-line antimyeloma therapy in comparison to placebo [77]. Based on the BP anticancer theory and promising early results, a large randomized trial (N = 1960) was conducted to evaluate the role of BPs in newly diagnosed MM patients receiving either intensive or non-intensive regimens [78]. Results show that ZOL significantly reduces skeletal morbidity and significantly improves both PFS and OS (HR 1.19, 95% CI 1.04–1.35) versus CLO. Notably, the survival benefit with ZOL remained significant after adjustment for SREs, consistent with clinically meaningful antimyeloma activity.
Denosumab
As described above, bone resorptive osteoclastic activity is magnified by RANK/RANKL signalling and is inhibited by OPG, a soluble decoy receptor for RANKL. In the myeloma BM microenvironment the interactions between MM cells and BMSCs result in increased RANKL expression and decreased OPG production, and consequently enhances osteoclast differentiation by triggering NF-κB/JNK signalling in osteoclast precursors. In preclinical studies, the critical role of RANKL in myeloma-induced BD was confirmed in mouse models of human MM bone using RANKL-specific inhibitors or OPG.
A human neutralizing antibody against RANKL, denosumab, which mimics the endogenous effect of OPG, has been tested in myeloma patients (Fig. 1). A single subcutaneous administration of denosumab induces a sustained inhibition of bone resorption markers lasting about 80 days versus 30 days of bisphosphonates [91]. Denosumab has been investigated in two phase II studies of myeloma patients previously treated with BPs, and both studies confirmed its efficacy in reducing SREs [92, 93]. In one of the trials using denosumab as a single agent in patients with plateau phase or progressive MM, although denosumab did not significantly decrease tumour burden, some patients with progressive disease experienced disease stabilization [93]. This observation raised the possibility that denosumab could influence MM growth through alteration of the microenvironment instead of via a direct cytotoxic effect. However, recently Henry et al. [94] reported the results of a phase III randomized trial that directly compared denosumab with ZOL on SRE development and survival in patients with myeloma. Consistent with the other studies denosumab was at least as effective as ZOL in reducing the time to first SREs; however, the ZOL treated group had a more favourable survival (HR 2.26; 95% CI 1.13 to 4.50). Therefore, current findings indicate that both BPs and denosumab can effectively reduce SREs, but ZOL was superior to denosumab in terms of survival benefit in MM patients. It is important, therefore, to be able to rationalize these differences given the importance of the RANK/RANKL system and the ability of denosumab to target it. The explanation for these differences may relate to the mode of action of these two agents. While they both target osteoclasts and consequently may indirectly restrain tumour growth, ZOL also has a range of both direct and indirect anti-myeloma activities, which seems to contribute to its survival benefit in MM patients over and above that which can be achieved by simply targeting one element of the known interactions. Alternatively as RANKL is also involved in dendritic cell maturation and the regulation of T cell-dependent immune response, the anti-RANKL strategy may have an effect on the immune system and a possible decreased T cell-mediated cytotoxic effect on myeloma cells.
Anti-BAFF-Neutralizing Antibody
Osteoclasts and myeloma cells interact by stimulating each others’ growth and survival, and a critical mediator in this interplay is a TNF family member BAFF (Fig. 1). BAFF is a MM growth factor derived from osteoclast and BMSC that mediates both myeloma cell survival and myeloma cell-BMSC adhesion [95, 96]. A neutralizing antibody against BAFF has been shown to significantly inhibit tumour burden in vivo and, importantly, reduce the number of lytic lesions and osteoclast differentiation [97]. On the basis of these results, a phase I study of this BAFF-neutralizing antibody (LY2127399) combined to bortezomib is currently ongoing in drug resistant MM patients (NCT00689507) [98].
Immunomodulatory Drugs
Immunomodulatory drugs (IMiDs), such as thalidomide, pomalidomide and lenalidomide, are effective agents for treating MM. Apart from their well-known anti-tumour activity, these drugs may directly interfere with osteoclast differentiation via the reduction of PU.1 expression, a critical transcription factor during osteoclast development [70, 72]. Moreover, the exposure of BMSCs derived from MM patients to lenalidomide decreases their secretion of RANKL, consequently impairs osteoclastogenesis and favours myeloma cell suppression [72] (Fig. 1). These data were related to the clinical findings that in the serum of MM patients treated with lenalidomide, RANK/RANKL levels were reduced, whereas OPG levels were increased. However both thalidomide and lenalidomide have been shown to induce DKK1 expression of myeloma cells after 48 h of treatment in a GEP study, which could potentially impair osteoblast function [99].
Targeting Bone Formation and the Osteoblast
The N-BPs ZOL and pamidronate are the current gold standard for the treatment of MM BD; however, not all patients respond to the treatment. Despite being on ZOL up to 30% newly diagnosed MM patients still develop SREs within 2 years [78]. This observation has led researchers to focus activity on agents able to promote anabolic bone activity as a way of treating BD. The biological pathways outlined above provide a framework against which to describe evaluation of such agents.
Bortezomib
Possible informative data on the role of stimulating bone formation as a therapeutic strategy has come indirectly from the use of the proteosome inhibitor Bortezomib which was incidentally shown to have anabolic bone activities. Bortezomib is a proteasome and NF-κB signalling inhibitor with potent anti-MM activity. Since RANKL enhances osteoclast differentiation by activating NF-κB pathway, it is not surprising that bortezomib, by reducing NF-κB activity, can impair osteoclast survival and differentiation [100, 101]. In addition to decreasing osteoclast activity in MM, bortezomib also has “anabolic” bone effects by inducing osteoblast differentiation [69, 102] (Fig. 1). Although the mechanism(s) by which proteasome inhibition leads to increased osteoblast activity has not been firmly established, previous work suggests that proteasome inhibition can upregulate Runx2 and osterix, two critical transcription factors in osteoblast differentiation [102–104]. Bortezomib also appears to decrease the serum levels of DKK-1 and RANKL in patients with MM [105] (Fig. 1). Taken together, these preclinical data provide an explanation for the increased bone formation markers and reduced bone resorption markers seen in MM patients treated with bortezomib [106–109]. In a study of mouse MM model the effect of bortezomib treatment was compared with that of melphalan, which has direct anti-myeloma effects but no effect on bone [110]. Only bortezomib prevented myeloma BD suggesting that the effects of bortezomib on BD were not a consequence of reduced tumour burden but a direct effect on bone cells. Although these findings suggest that bortezomib has the capacity to prevent myeloma BD, at present there is only one report showing the effectiveness of bortezomib on SREs in myeloma patients [111], and interestingly radiological evidence of bone healing was observed in some of the bortezomib-treated patients.
Anti-DKK1 and Other Agents Targeting WNT Pathway
Canonical Wnt signalling has been identified as an important pathway in normal osteoblast differentiation and is tightly regulated by a combination of positive induction through the binding of the Wnt ligand and negative regulation through secreted Wnt inhibitors. The two basic therapeutic strategies for enhancing bone regeneration through the Wnt signalling pathways are adding agonists or blocking naturally occurring antagonists. The delivery of a canonical Wnt ligand, Wnt3a, to a SCID mouse model of human intramedullary MM can inhibit bone destruction and tumour growth, but has no effect when myeloma cells were grown subcutaneously [23]. However, Wnt ligands are glycoproteins that are difficult and expensive to produce and, therefore, the alternative strategy of blocking the effect of natural antagonists is a more feasible approach that is currently being explored.
The inhibition of GSK3β is necessary for effective canonical Wnt signalling and subsequent osteogenic differentiation, GSK3β inhibitors are, therefore, good candidates for bone anabolic therapy in MM (Fig. 1). Similar to the effect of Wnt ligand delivery, reduced bone destruction and tumour growth have been achieved in the 5TGM mouse model of MM treated with lithium chloride, a suppressor of GSK3β and activator of Wnt signalling [68]. An in-vitro study in myeloma cell lines has shown that GSK3β inhibition sensitizes myeloma cells to a histone deacetylase inhibitor [112]. GSK3β inhibition has also been shown to reduce myeloma–induced BD as well as inducing tumour cell death in a murine plasmacytoma model [113]. An orally active, small molecule GSK3β inhibitor, 603281-31-8, has been reported to increase bone mass and bone formation markers, lower adiposity and reduce fracture risk in ovariectimized mice [114, 115]. These promising results from preclinical studies warrant the testing of GSK3β inhibitors in clinical settings.
DKK1 is a soluble inhibitor of the Wnt pathway produced by MM cells. Recently, a DKK1 neutralizing antibody (BHQ880) has been tested in myeloma in the context of the bone microenvironment [116] (Fig. 1). This antibody was able to enhance osteoblast differentiation, inhibit osteoclast differentiation, as well as reduce IL-6 levels in a co-culturing system, which are potentially therapeutically relevant. While the antibody did not demonstrate direct cytotoxic effects on MM cells, it did inhibit MM cell growth when the MM cells were cocultured with BMSCs and this was associated with reduced IL-6 secretion by BMSCs, suggesting that it may have anti-myeloma effects in vivo. Indeed a few studies using murine MM models show that DKK1-neutralizing antibody increases osteoblast numbers and bone formation, as well as inhibits MM cell growth [57, 116]. BHQ880 is being tested in an ongoing phase I/II clinical trial for patients with relapsed and refractory MM who are receiving ZOL and anti-MM therapy (NCT00741377) [98].
Activin A Inhibitor
Activin A is a member of the transforming growth factor-β (TGF-β) superfamily and is released from BMSCs and osteoclasts. It signals through the activin type 2A receptor and has dual effects of stimulating osteoclast activity and inhibiting osteoblast differentiation (Fig. 1). Activin A levels have been demonstrated to be elevated in the BM of MM patients and correlate with the extent of osteolytic lesions [117]. Effects of Activin A inhibition in MM were investigated using a soluble receptor RAP-011. RAP-011 treatment leads to increased OB differentiation and inhibits OC development in vitro. It has also been shown to increase bone volume and decrease MM tumour burden in a number of murine models of MM [117, 118]. Furthermore, RAP-011 also increased bone formation in macaques, demonstrating the capacity of this agent to enhance bone formation in vivo [119]. As a result of these studies, a phase II trial of the humanized counterpart of RAP-011, ACE-011, in bisphosphonate naive MM patients with osteolytic lesions has been carried out, the results show that the bone formation markers are increased while the bone resorption markers are decreased in patients treated with the antagonist [120].
Conclusions
As we have increasingly recognized that tumour burden and BD are inextricably linked in MM, understanding the biology of MM BD and its roles in tumour growth and drug resistance is crucial for the development of novel anti-myeloma strategies. Some of the current therapies, such as N-BPs, IMiDs and bortezomib, have been shown being able to target both the tumour and bone cells (e.g. osteoblast and osteoclast), and consequently reduce the tumour burden and BD. More novel bone-targeted agents, such as BHQ880 (anti-DKK1), denosumab (anti-RANKL), ACE-011 (anti-activin A) and LY2127399 (anti-BAFF) are under development, and will significantly improve the care of MM patients in the future.
Acknowledgements
Thanks to these organizations who have provided funding support to this article: Myeloma UK, Cancer Research UK, the Bud Flanagan Leukaemia Fund and the Biological Research Centre of the National Institute for Health Research at the Royal Marsden Hospital
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Melton LJ, 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005;20(3):487–493. doi: 10.1359/JBMR.041131. [DOI] [PubMed] [Google Scholar]
- 2.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Jakob C, Sterz J, Liebisch P, Mieth M, Rademacher J, Goerke A, Heider U, Fleissner C, Kaiser M, Metzler I, Muller C, Sezer O. Incorporation of the bone marker carboxy-terminal telopeptide of type-1 collagen improves prognostic information of the International Staging System in newly diagnosed symptomatic multiple myeloma. Leukemia. 2008;22(9):1767–1772. doi: 10.1038/leu.2008.159. [DOI] [PubMed] [Google Scholar]
- 4.Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B, Anaissie E, Hollmig K, Pineda-Roman M, Tricot G, Rhee F, Walker R, Zangari M, Crowley J, Barlogie B, Shaughnessy JD., Jr The molecular classification of multiple myeloma. Blood. 2006;108(6):2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bataille R, Chappard D, Marcelli C, Dessauw P, Sany J, Baldet P, Alexandre C. Mechanisms of bone destruction in multiple myeloma: the importance of an unbalanced process in determining the severity of lytic bone disease. J Clin Oncol. 1989;7(12):1909–1914. doi: 10.1200/JCO.1989.7.12.1909. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca R, Bailey RJ, Ahmann GJ, Rajkumar SV, Hoyer JD, Lust JA, Kyle RA, Gertz MA, Greipp PR, Dewald GW. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100(4):1417–1424. [PubMed] [Google Scholar]
- 7.Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG, Munshi NC, Anderson KC. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am. 2007;21(6):1007–1034. doi: 10.1016/j.hoc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Hurt EM, Wiestner A, Rosenwald A, Shaffer AL, Campo E, Grogan T, Bergsagel PL, Kuehl WM, Staudt LM. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5(2):191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 9.Robbiani DF, Chesi M, Bergsagel PL. Bone lesions in molecular subtypes of multiple myeloma. N Engl J Med. 2004;351(2):197–198. doi: 10.1056/NEJM200407083510223. [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Walker BA, Boyd KD, Wardell CP, Johnson DC, Gregory WM, Davies FE, Brewer D, Morgan GJ (2010) Defining myeloma patients at high risk of developing bone disease while on bisphosphonate treatment blood (ASH Meeting Abstracts) 116:1
- 11.Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J., Jr Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106(1):296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergsagel PL, Kuehl WM. Critical roles for immunoglobulin translocations and cyclin D dysregulation in multiple myeloma. Immunol Rev. 2003;194:96–104. doi: 10.1034/j.1600-065x.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 13.Hodge JM, Kirkland MA, Aitken CJ, Waugh CM, Myers DE, Lopez CM, Adams BE, Nicholson GC. Osteoclastic potential of human CFU-GM: biphasic effect of GM-CSF. J Bone Miner Res. 2004;19(2):190–199. doi: 10.1359/JBMR.0301232. [DOI] [PubMed] [Google Scholar]
- 14.Yaccoby S. Advances in the understanding of myeloma bone disease and tumour growth. Br J Haematol. 2010;149(3):311–321. doi: 10.1111/j.1365-2141.2010.08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, Epstein J. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 2004;64(6):2016–2023. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 16.Yaccoby S. The phenotypic plasticity of myeloma plasma cells as expressed by dedifferentiation into an immature, resilient, and apoptosis-resistant phenotype. Clin Cancer Res. 2005;11(21):7599–7606. doi: 10.1158/1078-0432.CCR-05-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dezorella N, Pevsner-Fischer M, Deutsch V, Kay S, Baron S, Stern R, Tavor S, Nagler A, Naparstek E, Zipori D, Katz BZ. Mesenchymal stromal cells revert multiple myeloma cells to less differentiated phenotype by the combined activities of adhesive interactions and interleukin-6. Exp Cell Res. 2009;315(11):1904–1913. doi: 10.1016/j.yexcr.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD, Jr, Epstein J, Hemert R, Erdem E, Hoering A, Crowley J, Ferris E, Hollmig K, Rhee F, Zangari M, Pineda-Roman M, Mohiuddin A, Yaccoby S, Sawyer J, Angtuaco EJ. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25(9):1121–1128. doi: 10.1200/JCO.2006.08.5803. [DOI] [PubMed] [Google Scholar]
- 19.Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr, Rhee F, Anaissie E, Alpe T, Angtuaco E, Walker R, Epstein J, Crowley J, Barlogie B. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114(10):2068–2076. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zipori D. The hemopoietic stem cell niche versus the microenvironment of the multiple myeloma-tumor initiating cell. Cancer Microenviron. 2010;3(1):15–28. doi: 10.1007/s12307-009-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23(1):10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, Barlogie B, Shaughnessy JD., Jr Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112(1):196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119(Pt 7):1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 25.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24(4):986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 26.Yaccoby S, Wezeman MJ, Zangari M, Walker R, Cottler-Fox M, Gaddy D, Ling W, Saha R, Barlogie B, Tricot G, Epstein J. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91(2):192–199. [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112(1):159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldoni S, Iozzo RV. Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123(11):2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 29.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 30.Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev. 2005;208:252–266. doi: 10.1111/j.0105-2896.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Pan G, McKenna MA, Zayzafoon M, Xiong WC, McDonald JM. RANKL regulates Fas expression and Fas-mediated apoptosis in osteoclasts. J Bone Miner Res. 2005;20(1):107–116. doi: 10.1359/JBMR.041022. [DOI] [PubMed] [Google Scholar]
- 32.Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98(13):3527–3533. doi: 10.1182/blood.v98.13.3527. [DOI] [PubMed] [Google Scholar]
- 33.Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci U S A. 2001;98(20):11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, Viniou N, Yataganas X, Goldman JM, Rahemtulla A. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102(3):1064–1069. doi: 10.1182/blood-2003-02-0380. [DOI] [PubMed] [Google Scholar]
- 35.Seidel C, Hjertner O, Abildgaard N, Heickendorff L, Hjorth M, Westin J, Nielsen JL, Hjorth-Hansen H, Waage A, Sundan A, Borset M. Serum osteoprotegerin levels are reduced in patients with multiple myeloma with lytic bone disease. Blood. 2001;98(7):2269–2271. doi: 10.1182/blood.v98.7.2269. [DOI] [PubMed] [Google Scholar]
- 36.Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, Brabbs AC, Beek EJ, Holen I, Skerry TM, Dunstan CR, Russell GR, Camp B, Vanderkerken K. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98(13):3534–3540. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- 37.Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97(11):3349–3353. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- 38.Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101(9):3568–3573. doi: 10.1182/blood-2002-08-2383. [DOI] [PubMed] [Google Scholar]
- 39.Terpos E, Politou M, Szydlo R, Goldman JM, Apperley JF, Rahemtulla A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br J Haematol. 2003;123(1):106–109. doi: 10.1046/j.1365-2141.2003.04561.x. [DOI] [PubMed] [Google Scholar]
- 40.Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, Pozzi S, Breitkreutz I, Kiziltepe T, Yasui H, Ocio EM, Shiraishi N, Jin J, Okawa Y, Ikeda H, Mukherjee S, Vaghela N, Cirstea D, Ladetto M, Boccadoro M, Anderson KC. MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood. 2007;110(10):3744–3752. doi: 10.1182/blood-2007-05-093294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden DT, Anderson KC, Raje N (2011) A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. doi:10.1038/leu.2011.43 [DOI] [PMC free article] [PubMed]
- 42.Choi SJ, Oba Y, Gazitt Y, Alsina M, Cruz J, Anderson J, Roodman GD. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108(12):1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, Grubbs B, Zhao M, Chen D, Sherry B, Mundy GR. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 2003;102(1):311–319. doi: 10.1182/blood-2002-12-3905. [DOI] [PubMed] [Google Scholar]
- 44.Bataille R, Chappard D, Alexandre C, Dessauw P, Sany J. Importance of quantitative histology of bone changes in monoclonal gammopathy. Br J Cancer. 1986;53(6):805–810. doi: 10.1038/bjc.1986.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bataille R, Delmas PD, Chappard D, Sany J. Abnormal serum bone Gla protein levels in multiple myeloma. Crucial role of bone formation and prognostic implications. Cancer. 1990;66(1):167–172. doi: 10.1002/1097-0142(19900701)66:1<167::aid-cncr2820660130>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Evans CE, Galasko CS, Ward C. Does myeloma secrete an osteoblast inhibiting factor? J Bone Joint Surg Br. 1989;71(2):288–290. doi: 10.1302/0301-620X.71B2.2925748. [DOI] [PubMed] [Google Scholar]
- 47.Bataille R, Chappard D, Marcelli C, Dessauw P, Baldet P, Sany J, Alexandre C. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest. 1991;88(1):62–66. doi: 10.1172/JCI115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bataille R, Chappard D, Marcelli C, Rossi JF, Dessauw P, Baldet P, Sany J, Alexandre C. Osteoblast stimulation in multiple myeloma lacking lytic bone lesions. Br J Haematol. 1990;76(4):484–487. doi: 10.1111/j.1365-2141.1990.tb07904.x. [DOI] [PubMed] [Google Scholar]
- 49.Atkins GJ, Kostakis P, Pan B, Farrugia A, Gronthos S, Evdokiou A, Harrison K, Findlay DM, Zannettino AC. RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res. 2003;18(6):1088–1098. doi: 10.1359/jbmr.2003.18.6.1088. [DOI] [PubMed] [Google Scholar]
- 50.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 51.Bain G, Muller T, Wang X, Papkoff J. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003;301(1):84–91. doi: 10.1016/s0006-291x(02)02951-0. [DOI] [PubMed] [Google Scholar]
- 52.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 53.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, Bonomini S, Sala R, Mangoni M, Rizzoli V. Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res. 2007;67(16):7665–7674. doi: 10.1158/0008-5472.CAN-06-4666. [DOI] [PubMed] [Google Scholar]
- 54.Haaber J, Abildgaard N, Knudsen LM, Dahl IM, Lodahl M, Thomassen M, Kerndrup GB, Rasmussen T. Myeloma cell expression of 10 candidate genes for osteolytic bone disease. Only overexpression of DKK1 correlates with clinical bone involvement at diagnosis. Br J Haematol. 2008;140(1):25–35. doi: 10.1111/j.1365-2141.2007.06871.x. [DOI] [PubMed] [Google Scholar]
- 55.Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119(7):1728–1731. doi: 10.1002/ijc.22033. [DOI] [PubMed] [Google Scholar]
- 56.Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, Stover D, Sezer O, Heider U. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol. 2008;80(6):490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 57.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109(5):2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davies FE, Dring AM, Li C, Rawstron AC, Shammas MA, O’Connor SM, Fenton JA, Hideshima T, Chauhan D, Tai IT, Robinson E, Auclair D, Rees K, Gonzalez D, Ashcroft AJ, Dasgupta R, Mitsiades C, Mitsiades N, Chen LB, Wong WH, Munshi NC, Morgan GJ, Anderson KC. Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. Blood. 2003;102(13):4504–4511. doi: 10.1182/blood-2003-01-0016. [DOI] [PubMed] [Google Scholar]
- 59.Vos J, Couderc G, Tarte K, Jourdan M, Requirand G, Delteil MC, Rossi JF, Mechti N, Klein B. Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood. 2001;98(3):771–780. doi: 10.1182/blood.v98.3.771. [DOI] [PubMed] [Google Scholar]
- 60.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106(9):3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 61.Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106(7):2472–2483. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24(4):578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 63.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Lindner B, Harmelin S, Hawa G, Boutsikas G, Migkou M, Gavriatopoulou M, Michalis E, Pouli A, Kastritis E, Zervas K, Dimopoulos MA.High serum sclerostin correlates with advanced stage, increased bone resporption, reduced osteoblast function, and poor survival in newly-diagnosed patients with multiple myeloma Blood 2009114119574477 [Google Scholar]
- 65.Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Liso V, Curci P, Miccolis RM, Rinaldi E, Specchia G, Passeri G, Zallone A, Rizzi R, Grano M (2010) Myeloma cells induce osteoblast suppression through sclerostin secretion blood (ASH Meeting Abstracts) 116:1 [DOI] [PMC free article] [PubMed]
- 66.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116(5):1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, Neut R, Spaargaren M, Pals ST. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004;101(16):6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111(5):2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zangari M, Esseltine D, Lee CK, Barlogie B, Elice F, Burns MJ, Kang SH, Yaccoby S, Najarian K, Richardson P, Sonneveld P, Tricot G. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131(1):71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 70.Anderson G, Gries M, Kurihara N, Honjo T, Anderson J, Donnenberg V, Donnenberg A, Ghobrial I, Mapara MY, Stirling D, Roodman D, Lentzsch S. Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood. 2006;107(8):3098–3105. doi: 10.1182/blood-2005-08-3450. [DOI] [PubMed] [Google Scholar]
- 71.Terpos E, Dimopoulos MA, Sezer O. The effect of novel anti-myeloma agents on bone metabolism of patients with multiple myeloma. Leukemia. 2007;21(9):1875–1884. doi: 10.1038/sj.leu.2404843. [DOI] [PubMed] [Google Scholar]
- 72.Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, Chauhan D, Okawa Y, Munshi NC, Richardson PG, Anderson KC. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22(10):1925–1932. doi: 10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- 73.Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, Orlowski RZ, Roodman DG, Twilde P, Anderson K. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25(17):2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 74.Morgan GJ. Can bisphosphonates improve outcomes in patients with newly diagnosed multiple myeloma? Crit Rev Oncol Hematol. 2011;77(Suppl 1):S24–S30. doi: 10.1016/S1040-8428(11)70005-1. [DOI] [PubMed] [Google Scholar]
- 75.Aviles A, Nambo MJ, Neri N, Castaneda C, Cleto S, Huerta-Guzman J. Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol. 2007;24(2):227–230. doi: 10.1007/BF02698044. [DOI] [PubMed] [Google Scholar]
- 76.McCloskey EV, Dunn JA, Kanis JA, MacLennan IC, Drayson MT. Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. Br J Haematol. 2001;113(4):1035–1043. doi: 10.1046/j.1365-2141.2001.02851.x. [DOI] [PubMed] [Google Scholar]
- 77.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs M, Blacklock H, Bell R, Simeone JF, Reitsma DJ, Heffernan M, Seaman J, Knight RD. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol. 1998;16(2):593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 78.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376(9757):1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berenson J, Dimopoulos M, Chen YM (2006) Improved survival in patients with multiple myeloma and high BALP levels treated with zoledronic acid compared with pamidronate: univariate and multivariate models of hazard ratios. Blood (ASH Meeting Abstracts) 1
- 80.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ, Seaman JJ, Chen BL, Ambros Y. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7(5):377–387. [PubMed] [Google Scholar]
- 81.Baulch-Brown C, Molloy TJ, Yeh SL, Ma D, Spencer A. Inhibitors of the mevalonate pathway as potential therapeutic agents in multiple myeloma. Leuk Res. 2007;31(3):341–352. doi: 10.1016/j.leukres.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 82.Shipman CM, Rogers MJ, Apperley JF, Russell RG, Croucher PI. Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol. 1997;98(3):665–672. doi: 10.1046/j.1365-2141.1997.2713086.x. [DOI] [PubMed] [Google Scholar]
- 83.Tassone P, Forciniti S, Galea E, Morrone G, Turco MC, Martinelli V, Tagliaferri P, Venuta S. Growth inhibition and synergistic induction of apoptosis by zoledronate and dexamethasone in human myeloma cell lines. Leukemia. 2000;14(5):841–844. doi: 10.1038/sj.leu.2401770. [DOI] [PubMed] [Google Scholar]
- 84.Ural AU, Yilmaz MI, Avcu F, Pekel A, Zerman M, Nevruz O, Sengul A, Yalcin A. The bisphosphonate zoledronic acid induces cytotoxicity in human myeloma cell lines with enhancing effects of dexamethasone and thalidomide. Int J Hematol. 2003;78(5):443–449. doi: 10.1007/BF02983818. [DOI] [PubMed] [Google Scholar]
- 85.Corso A, Ferretti E, Lunghi M, Zappasodi P, Mangiacavalli S, Amici M, Rusconi C, Varettoni M, Lazzarino M. Zoledronic acid down-regulates adhesion molecules of bone marrow stromal cells in multiple myeloma: a possible mechanism for its antitumor effect. Cancer. 2005;104(1):118–125. doi: 10.1002/cncr.21104. [DOI] [PubMed] [Google Scholar]
- 86.Zwolak P, Manivel JC, Jasinski P, Kirstein MN, Dudek AZ, Fisher J, Cheng EY. Cytotoxic effect of zoledronic acid-loaded bone cement on giant cell tumor, multiple myeloma, and renal cell carcinoma cell lines. J Bone Joint Surg Am. 2010;92(1):162–168. doi: 10.2106/JBJS.H.01679. [DOI] [PubMed] [Google Scholar]
- 87.Schmidmaier R, Simsek M, Baumann P, Emmerich B, Meinhardt G. Synergistic antimyeloma effects of zoledronate and simvastatin. Anticancer Drugs. 2006;17(6):621–629. doi: 10.1097/01.cad.0000215058.85813.02. [DOI] [PubMed] [Google Scholar]
- 88.Moschetta M, Pietro G, Ria R, Gnoni A, Mangialardi G, Guarini A, Ditonno P, Musto P, D’Auria F, Ricciardi MR, Dammacco F, Ribatti D, Vacca A. Bortezomib and zoledronic acid on angiogenic and vasculogenic activities of bone marrow macrophages in patients with multiple myeloma. Eur J Cancer. 2010;46(2):420–429. doi: 10.1016/j.ejca.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 89.Guenther A, Gordon S, Tiemann M, Burger R, Bakker F, Green JR, Baum W, Roelofs AJ, Rogers MJ, Gramatzki M. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int J Cancer. 2010;126(1):239–246. doi: 10.1002/ijc.24758. [DOI] [PubMed] [Google Scholar]
- 90.Lipton A, Cook RJ, Coleman RE, Smith MR, Major P, Terpos E, Berenson JR. Clinical utility of biochemical markers of bone metabolism for improving the management of patients with advanced multiple myeloma. Clin Lymphoma Myeloma. 2007;7(5):346–353. doi: 10.3816/CLM.2007.n.011. [DOI] [PubMed] [Google Scholar]
- 91.Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011;17(6):1278–1286. doi: 10.1158/1078-0432.CCR-10-1804. [DOI] [PubMed] [Google Scholar]
- 92.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 93.Vij R, Horvath N, Spencer A, Taylor K, Vadhan-Raj S, Vescio R, Smith J, Qian Y, Yeh H, Jun S. An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. Am J Hematol. 2009;84(10):650–656. doi: 10.1002/ajh.21509. [DOI] [PubMed] [Google Scholar]
- 94.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, Moos R, Willenbacher W, Woll PJ, Wang J, Jiang Q, Jun S, Dansey R, Yeh H. Randomized, double-blind study of denosumab versus zoledronic Acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 95.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104(8):2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 96.Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, Podar K, Hideshima T, Chauhan D, Raje N, Schlossman R, Richardson P, Munshi NC, Anderson KC. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66(13):6675–6682. doi: 10.1158/0008-5472.CAN-06-0190. [DOI] [PubMed] [Google Scholar]
- 97.Neri P, Kumar S, Fulciniti MT, Vallet S, Chhetri S, Mukherjee S, Tai Y, Chauhan D, Tassone P, Venuta S, Munshi NC, Hideshima T, Anderson KC, Raje N. Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res. 2007;13(19):5903–5909. doi: 10.1158/1078-0432.CCR-07-0753. [DOI] [PubMed] [Google Scholar]
- 98.www.clinicaltrials.gov.
- 99.Shaugnessy J, Zhan F, Kordsmeier B, Randolph C, McCastlain K, Barlogie B. Gene expression profiling (GEP) after short term in-vivo treatment identifies potential mechanisms of action of current drugs used to treat multiple myeloma. Blood. 2002;100:1. [Google Scholar]
- 100.Zavrski I, Krebbel H, Wildemann B, Heider U, Kaiser M, Possinger K, Sezer O. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem Biophys Res Commun. 2005;333(1):200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 101.Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, Kaiser M, Jakob C, Sterz J, Kleeberg L, Heider U, Sezer O. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21(9):2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 102.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118(2):491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, Mancini C, Martella E, Ferrari L, Tabilio A, Rizzoli V. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110(1):334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 104.Matteo M, Brunetti AE, Maiorano E, Cafforio P, Dammacco F, Silvestris F. Constitutive down-regulation of Osterix in osteoblasts from myeloma patients: in vitro effect of Bortezomib and Lenalidomide. Leuk Res. 2010;34(2):243–249. doi: 10.1016/j.leukres.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 105.Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A, Pouli A, Katodritou E, Verrou E, Vervessou EC, Dimopoulos MA, Croucher PI. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135(5):688–692. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 106.Shimazaki C, Uchida R, Nakano S, Namura K, Fuchida SI, Okano A, Okamoto M, Inaba T. High serum bone-specific alkaline phosphatase level after bortezomib-combined therapy in refractory multiple myeloma: possible role of bortezomib on osteoblast differentiation. Leukemia. 2005;19(6):1102–1103. doi: 10.1038/sj.leu.2403758. [DOI] [PubMed] [Google Scholar]
- 107.Heider U, Kaiser M, Muller C, Jakob C, Zavrski I, Schulz CO, Fleissner C, Hecht M, Sezer O. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77(3):233–238. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 108.Boissy P, Andersen TL, Lund T, Kupisiewicz K, Plesner T, Delaisse JM. Pulse treatment with the proteasome inhibitor bortezomib inhibits osteoclast resorptive activity in clinically relevant conditions. Leuk Res. 2008;32(11):1661–1668. doi: 10.1016/j.leukres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 109.Uy GL, Trivedi R, Peles S, Fisher NM, Zhang QJ, Tomasson MH, DiPersio JF, Vij R. Bortezomib inhibits osteoclast activity in patients with multiple myeloma. Clin Lymphoma Myeloma. 2007;7(9):587–589. doi: 10.3816/clm.2007.n.045. [DOI] [PubMed] [Google Scholar]
- 110.Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol. 2009;84(1):6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delforge M, Terpos E, Richardson PG, Shpilberg O, Khuageva NK, Schlag R, Dimopoulos MA, Kropff M, Spicka I, Petrucci MT, Samoilova OS, Mateos MV, Magen-Nativ H, Goldschmidt H, Esseltine DL, Ricci DS, Liu K, Deraedt W, Cakana A, van de Velde H, San Miguel JF (2011) Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol. doi:10.1111/j.1600-0609.2011.01599.x [DOI] [PubMed]
- 112.Kuhns MA, Kalaycio M, Reu FJ, Maciejewski JP, Cheriyath V.GSK-3β inhibitors in overcoming chemoresistance in multiple myeloma Blood 2010116120616222 [Google Scholar]
- 113.Gunn WG, Krause U, Lee N, Gregory CA. Pharmaceutical inhibition of glycogen synthetase kinase-3beta reduces multiple myeloma-induced bone disease in a novel murine plasmacytoma xenograft model. Blood. 2011;117(5):1641–1651. doi: 10.1182/blood-2010-09-308171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kulkarni NH, Onyia JE, Zeng Q, Tian X, Liu M, Halladay DL, Frolik CA, Engler T, Wei T, Kriauciunas A, Martin TJ, Sato M, Bryant HU, Ma YL. Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res. 2006;21(6):910–920. doi: 10.1359/jbmr.060316. [DOI] [PubMed] [Google Scholar]
- 115.Kulkarni NH, Wei T, Kumar A, Dow ER, Stewart TR, Shou J, N’Cho M, Sterchi DL, Gitter BD, Higgs RE, Halladay DL, Engler TA, Martin TJ, Bryant HU, Ma YL, Onyia JE. Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of PTH and small molecule GSK-3 inhibitor. J Cell Biochem. 2007;102(6):1504–1518. doi: 10.1002/jcb.21374. [DOI] [PubMed] [Google Scholar]
- 116.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S, Santo L, Cirstea D, Patel K, Sohani AR, Guimaraes A, Xie W, Chauhan D, Schoonmaker JA, Attar E, Churchill M, Weller E, Munshi N, Seehra JS, Weissleder R, Anderson KC, Scadden DT, Raje N. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci U S A. 2010;107(11):5124–5129. doi: 10.1073/pnas.0911929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chantry A, Heath D, Coulton L, Gallagher O, Evans H, Seehra J, Vanderkerken KICP. A soluble activin type II receptor prevents myeloma bone disease. Haematologica. 2007;92(suppl2):1. [Google Scholar]
- 119.Lotinun S, Pearsall RS, Davies MV, Marvell TH, Monnell TE, Ucran J, Fajardo RJ, Kumar R, Underwood KW, Seehra J, Bouxsein ML, Baron R. A soluble activin receptor Type IIA fusion protein (ACE-011) increases bone mass via a dual anabolic-antiresorptive effect in Cynomolgus monkeys. Bone. 2010;46(4):1082–1088. doi: 10.1016/j.bone.2010.01.370. [DOI] [PubMed] [Google Scholar]
- 120.Abdulkadyrov KM, Salogub GN, Khuazheva NK, Woolf R, Haltom E, Borgstein NG, Knight R, Renshaw G, Yang Y, Sherman ML.ACE-011, a soluble activin receptor type Iia IgG-Fc fusion protein, increases hemoglobin (Hb) and improves bone lesions in multiple myeloma patients receiving myelosuppressive chemotherapy: preliminary analysis Blood 2009114119574477 [Google Scholar]