Abstract
The bone marrow microenvironment in multiple myeloma is characterized by an increased microvessel density. The production of pro-angiogenic molecules is increased and the production of angiogenic inhibitors is suppressed, leading to an “angiogenic switch”. Here we present an overview of the role of angiogenesis in multiple myeloma, the pro-angiogenic factors produced by myeloma cells and the microenvironment, and the mechanisms involved in the myeloma-induced angiogenic switch. Current data suggest that the increased bone marrow angiogenesis in multiple myeloma is due to the aberrant expression of angiogenic factors by myeloma cells, the subsequent increase in pro-angiogenic activity of normal plasma cells as a result of myeloma cell angiogenic activity, and the increased number of plasma cells overall. Hypoxia also contributes to the angiogenic properties of the myeloma marrow microenvironment. The transcription factor hypoxia-inducible factor-1α is overexpressed by myeloma cells and affects their transcriptional and angiogenic profiles. In addition, potential roles of the tumor suppressor gene inhibitor of growth family member 4 and homeobox B7 have also been recently highlighted as repressors of angiogenesis and pro-angiogenic related genes, respectively. This complex pathogenetic model of myeloma-induced angiogenesis suggests that several pro-angiogenic molecules and related genes in myeloma cells and the microenvironment are potential therapeutic targets.
Keywords: Multiple myeloma, Angiogenesis, Angiogenic switch, Microenvironment, Hypoxia
Introduction
Angiogenesis is a multistep process characterized by the formation of new blood vessels from a pre-existing vasculature that occurs in physiologic conditions, such as normal growth and tissue healing, and pathologic states, such as cancer [1, 2]. The process begins with vasodilatation and increased permeability of existing vessels. The surrounding matrix degrades and allows activated and proliferating endothelial cells to migrate and form lumens. The endothelial cells subsequently differentiate and mature into a complex network of vessels supported by peri-endothelial cells and the matrix [1, 2]. Tumoral angiogenesis differs significantly from physiologic angiogenesis due to the chaotic and aberrant production of pro-angiogenic factors resulting in aberrant vascular structure, altered endothelial cell-pericyte interactions, abnormal blood flow, and increased permeability [4, 5]. Cancer cells induce an “angiogenic switch” by either a direct overproduction of pro-angiogenic molecules or by the induction of pro-angiogenic molecules in the microenvironment. Several years ago it was hypothesized that angiogenesis is involved in tumor growth and metastasis [3]. It is now well established that tumor cells stimulate vessel formation through the over-expression or induction of pro-angiogenic molecules in the tumor microenvironment, a process termed “angiogenic switch” [2–4]. Under normal conditions the angiogenic switch is “off,” and there is a balance between pro-angiogenic and anti-angiogenic molecules. When the switch is “on” the balance is tipped in favor of pro-angiogenic molecules leading to increased angiogenesis [2–4]. The angiogenic switch can occur at any stage of tumor progression, depending on the type of tumor and its microenvironment [2–4]. Many tumors initially have an avascular growth phase before reaching a steady state of proliferating cells. The angiogenic switch occurs at a later stage, resulting in perivascular detachment and vessel dilation followed by angiogenic sprouting, new vessel formation, and the recruitment of perivascular cells [4, 5], thus allowing for exponential tumor growth.
In the last years, increased angiogenesis has been demonstrated in the bone marrow (BM) microenvironment in hematologic malignancies, including multiple myeloma (MM), suggesting a potential pathophysiologic role for angiogenesis in MM. MM is a plasma cell malignancy characterized by a tight relationship between tumor cells and the BM microenvironment that supports myeloma cell growth and survival [6]. In MM, as in solid tumors, disease progression is characterized by a pre-angiogenic stage of slow tumor progression followed by an angiogenic switch and a subsequent angiogenic stage associated with progressive tumor growth [7]. Here we present an overview of the role of BM angiogenesis in MM, the production of pro-angiogenic factors by myeloma cells and the BM microenvironment, and the mechanisms involved in the MM-induced angiogenic switch.
Bone Marrow Angiogenesis in Multiple Myeloma Patients
Increased BM angiogenesis in patients MM was first demonstrated by Vacca et al. [8], who described increased in vitro pro-angiogenic activity of isolated plasma cells from patients with active MM as compared with inactive MM and monoclonal gammopathy of undetermined significance (MGUS). Thereafter, others confirmed this observation, showing that MM patients with active disease have increased BM angiogenesis compared to patients with smoldering MM or early stage MM [9, 10]. In a large cohort of patients with monoclonal gammopathies it has been shown that BM microvessel density (MVD) as assessed by immunohistochemical staining for CD 34 was significantly higher in patients with symptomatic MM as compared to MGUS and healthy controls. An increased incidence of high-grade angiogenesis was also demonstrated in patients with relapsed MM as compared to newly diagnosed MM [10]. In this study a relationship between BM angiogenesis and the number of BM plasma cells and plasma cell labeling index was demonstrated [10]. Others showed a correlation between MVD, the proliferation index Ki-67 and plasma cells burden [11]. Overall these results suggest that increased BM angiogenesis correlates with the progression of monoclonal gammopathy to overt MM and the extent of plasma cell infiltration.
Several groups have demonstrated a significant relationship between increased BM angiogenesis and prognosis in MM patients [10, 12–21]. In multivariate analysis of separate cohorts of MM patients, MVD emerged as an independent prognostic factor for overall survival together with beta2-microglobulin and C-reactive protein [10, 13, 14, 18, 20, 21]. A relationship between the increased BM microcirculation and the presence of deletion 13 [22], and the gain of 1q21 has also been demonstrated, but no relationship has been identified with the deletion of 17p13 [23]. The prognostic impact of angiogenesis in solitary bone plasmacytoma has been reported, showing that patients with high MVD were more likely to progress to MM with a shorter event-free survival [15]. Moreover, a significant relationship between increased angiogenesis, as assessed by immunohistochemistry, and a diffuse magnetic resonance imaging (MRI) pattern of infiltration was recently reported in newly diagnosed MM patients treated with novel agents and correlates with poor prognosis [17]. MVD has been also identified as a good predictor of complete response to therapy in MM.[13, 16]. Treatment related changes in BM angiogenesis have been reported in MM patients undergoing high dose therapy in relation to their response to therapy [24], although not all authors have show a significant reduction of MVD in MM patients responsive to treatment [25, 26]. Interestingly, recent data also shows a significant decrease of MVD in patients responding to treatment with thalidomide [27].
Vasculogenesis in MM
Together with angiogenesis, new blood vasculature in the BM of MM patients is due to a vasculogenic process [28, 29]. In contrast to angiogenesis, vasculogenesis is a de novo formation of blood vessels from endothelial progenitor cells (EPCs) recruited from the BM. EPCs are incorporated into nascent vessels at the angiogenic sites, and proliferate and differentiate into endothelial cells [30, 31]. The presence of EPCs has been demonstrated in the peripheral blood together with the circulating endothelial cells (CEC) in patients with malignancy. However, the real contribution of EPCs to the tumor vasculature and their role in the vasculogenic process in cancer is still unknown [30, 31]. Circulating EPCs and CEC have also been demonstrated in MM patients and their levels correlate with disease activity [32].
Peripheral mononuclear cells also contribute to vasculogenesis [29]. Both monocytes and macrophages may give rise to endothelial cells in presence of pro-angiogenic cytokines. Interestingly, it has been reported that the contribution of monocytes to the increased vasculature that occurs in the BM of MM patients is higher in MM patients with active disease as compared to those with MGUS or healthy subjects [33]. Recent data show that pleiothrophin production by myeloma cells induced transdifferantiation of monocytes into vascular endothelial cells in combination with macrophage colony stimulating factor (M-CSF), suggesting a critical role of this protein in vasculogenesis in MM [34].
Production of Pro-angiogenic Factors by Myeloma Cells and the Microenvironment
The increased BM angiogenesis in MM is sustained by an imbalance between the production of pro-angiogenic and anti-angiogenic factors by both myeloma cells and the microenvironment. Myeloma cells interact with several BM microenvironment cells including stromal cells, fibroblasts, osteoblasts, osteoclasts, T lymphocytes, monocytes/macrophages and mast cells that produce growth and survival factors that sustain myeloma cell survival and trigger endothelial cell proliferation and angiogenesis.
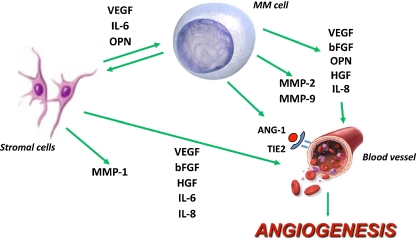
In the last years several factors produced by myeloma cells and the microenvironment have been identified as critical in MM-induced angiogenesis as summarized in Fig. 1.
Fig. 1.
Pro-angiogenic factors produced by myeloma cells and the microenvironment. Myeloma cells directly produce several pro-angiogenic molecules and induce their expression in bone marrow stromal cells. This in turn stimulates myeloma cell growth in a paracrine fashion. VEGF Vascular endothelial growth factor, bFGF basic fibroblast growth factor, HGF hepatocyte growth factor, OPN osteopontin, Ang-1 Angiopoietin-1, IL-6 interleukin-6, IL-8 interleukin-8. MMP-1,-2,-9 metalloproteinase-1,-2,-9
Vascular Endothelial Growth Factor (VEGF)
The pro-angiogenic molecule vascular endothelial growth factor (VEGF) is the primary growth and survival factor for endothelial cells and is essential for vascular development [1, 35]. Different isoforms of VEGF that share common receptors have been identified: VEGFR-1 or Flt-1, VEGFR-2 or KDR/Flk-1 and VEGFR-3 or Flt-4. VEGFR-1 and VEGFR-2 are expressed on endothelial cells and mediate the stimulatory effect of VEGF on endothelial cell proliferation, survival and migration [1, 35]. Placental growth factor (PlGF) also belongs to the VEGF family, binds VEGFR-1 and enhances the angiogenic effect of VEGF [1, 36]. In contrast, the soluble form of the VEGFR-1, (sVEGFR-1 or sFLT-1), generated by alternative splicing, acts as an inhibitor of VEGF.[1, 35].
The pro-angiogenic activity of myeloma cells is sustained by VEGF production. It has been demonstrated that myeloma cells directly produce VEGF [37–39]. Moreover, VEGF produced by myeloma cells stimulates interleukin-6 (IL-6) and VEGF secretion by BM stromal cells that in turn induce VEGF production by myeloma cells in a paracrine fashion [40, 41]. An increase in the angiogenic activity of mesenchymal stromal cells has also been demonstrated in MM with VEGF overexpression compared with healthy donors [42]. A VEGF autocrine loop has also been postulated based on the presence of VEGFR-1 on myeloma cells, by which VEGF stimulates myeloma cell proliferation through the MEK-1/ERK pathway [43]. MEK-1 specific inhibitors inhibit VEGF secretion by myeloma cells and by the BM microenvironment, suggesting that this pathway is involved in VEGF secretion by myeloma cells [44].
A potential correlation between VEGF secretion and CD45 expression by myeloma cells has been hypothesized on the basis of experimental data from the 5T2MM mouse model [7]. In this model the angiogenic switch is correlated with an increased number of CD45- myeloma cells, with a higher secretion of VEGF as compared to control [7]. Freshly purified CD138+ myeloma cells express VEGF mRNA in almost all MM patients (80–90%) and high VEGF levels are detected in BM samples of MM patients [37, 38, 45, 46]. However, only a few MM patients produce the VEGF soluble antagonist sVEGFR-1 and have a higher VEGF/sVEGFR-1 ratio as compared to healthy subjects [45]. This further supports the importance of VEGF in the increased BM angiogenesis in MM patients.
Basic-Fibroblast Growth Factor-2 (bFGF)
Basic fibroblast growth factor (bFGF) is another pro-angiogenic molecule that stimulates endothelial cell proliferation, survival, migration and mobilization in vascular development [1, 2, 4]. bFGF’s role in MM-induced angiogenesis has been demonstrated through the capacity of an anti-bFGF blocking antibody to inhibit the in vitro vessel formation induced by BM plasma cell samples [8]. Myeloma cells may produce bFGF, however not all MM patients’ plasma cells produce bFGF [41, 47, 48]. Bisping et al. [47] have reported that human myeloma cell lines and sorted CD138+ cells obtained from 12 of 15 MM patients produced bFGF, concluding that myeloma cells are the predominant source of bFGF. Others have shown that neither human myeloma cells nor EBV-positive B cell lines secret bFGF [41]. In our cohort of newly diagnosed MM patients we have demonstrated that 11 out of 35 patients expressed bFGF mRNA and a lower number of patients produced bFGF protein [48]. In contrast, it has been clearly shown that BM stromal cells express b-FGF that contributes to the pro-angiogenic activity of the microenvironment cells in MM patients [48].
Angiopoietins
The maturation and stabilization of the vascular wall is critical and is regulated by angiopoietin-1 (Ang-1), a factor that binds primarily to Tie2 receptor expressed on the endothelium [1, 2, 49]. Ang-1 does not induce endothelial cell proliferation directly [50], but acts as a survival factor for endothelial cells [51], induces vessel stabilization [52], tubule formation [53] and plays a key role in mediating interactions between endothelial and matrix cells [54]. Ang-1 is also produced by mast cells in the microenvironment that promotes plasma cell growth and stimulates angiogenesis together with tumor-derived VEGF [59].
Angiopoietin-2 (Ang-2) is the natural antagonist of Ang-1, and blocks Ang-1 mediated Tie2 activation on endothelial cells and induces vessel destabilization [1, 2, 49, 55]. This process may lead either to vessel regression or promote angiogenesis [55]. Because Ang-1 has a critical role in the angiogenic switch its potential role in myeloma-induced angiogenesis has been investigated [56]. First, it has been shown that myeloma cells express and secrete Ang-1 but not its antagonist Ang-2 [56]. Ang-1 is expressed in about 47% of patients of newly diagnosed MM patients. However, Ang-2 is not present in any patients tested [56]. In addition, the potential role of Ang-1 in MM-induced angiogenesis has been confirmed in an experimental model of angiogenesis. In this system, the conditioned medium of myeloma cells increased vessel formation in comparison with either control or VEGF treatment [56]. The presence of an anti-Tie-2 blocking antibody completely blunted tubule formation induced by myeloma cells [56]. In line with this evidence, other authors demonstrated inhibition of tumoral angiogenesis using a Tie2 blocking soluble receptor in solid cancer murine models [57].
In addition to the direct production of Ang-1, myeloma cells alter the expression of angiopoietins and the Tie2 receptor in the BM microenvironment. In an in vitro co-culture system it has been demonstrated that several human myeloma cell lines up-regulated expression of Tie-2 by endothelial cells at both the mRNA and protein level [56]. This finding has been confirmed in vivo, with the demonstration that over-expression of Tie-2 in isolated BM endothelial cells of MM patients as compared to normal endothelial cells [58], supporting the hypothesis that the response of MM endothelial cells are activated by Ang-1 and are more sensitive to the effect of Ang-1 produced by myeloma cells themselves. Higher levels of Ang-2 have been detected in MM patients as compared to controls, an observation with a potentially prognostic impact [60]. However, the source of circulating Ang-2 is not clear. It has been reported that myeloma cells lack expression of Ang-2 mRNA and protein [56], and consistent with this observation, the ANGPT2 gene expression levels in a large dataset of MM patients were very low [61]. In contrast, other authors have reported Ang-2 expression by myeloma cells [60]. Since endothelial cells and EPCs producion of Ang-2 is well-established, [49, 62] these results suggest that Ang-2 serum levels may be primarily derived from endothelial vascular cells.
Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are a family of enzymes that proteolytically degrade components of the extracellular matrix (ECM) promoting tumor invasion, metastasis and angiogenesis [63]. MMPs enhance angiogenesis by detaching pericytes from vessels undergoing angiogenesis, releasing and activating ECM-bound angiogenic factors, exposing cryptic pro-angiogenic integrin binding sites in the ECM, and by cleaving endothelial cell-cell adhesions [64]. MMPs also negatively regulate angiogenesis through the generation of endogenous angiogenesis inhibitors by proteolytic cleavage [64].
Myeloma cells produce both MMP-2 and MMP-9. MMP-2 expression by plasma cells from MM patients with active disease in increased as compared to patients with MGUS [8]. However MMP-9 expression is similar between MM and MGUS patients [8]. MMP-9 secretion by myeloma cells is enhanced by their interaction with endothelial cells in the microenvironment [65, 66]. Myeloma cells also upregulate MMP-1 secretion by BM stromal cells [67] and MMP-7 secreted by MM cells induces activation of the pro-MMP-2 [68].
Osteopontin
Osteopontin (OPN) is a pro-angiogenic factor that promotes endothelial cell migration and survival [60–70], adhesion of both endothelial and smooth muscle cells [71], and has been associated with tumoral angiogenesis in mouse models [72, 73]. OPN is produced directly by myeloma cells and is critical for the stimulation of endothelial cells. In an in vitro model of angiogenesis, OPN-immuno-depleted CM from myeloma cells failed to induce a pro-angiogenic effect and an anti-OPN Ab blocked myeloma-induced angiogenesis [74]. These observations support the potential role of OPN as a direct pro-angiogenic factor in MM. However, it is possible that OPN contributes to the increased angiogenesis in MM indirectly through the modulation of matrix metalloproteinase-2 (MMP-2). OPN increases MMP-2 expression and activates pro-MMP-2 in tumor cells [75]. OPN is secreted by osteoclasts in the myeloma microenvironment [76], suggesting that these cells may contribute to the angiogenic activity of this factor in MM. Interestingly, recent data suggest that osteoclasts act as angiogenic cells and regulate angiogenesis in the BM [77].
Hepatocyte Growth Factor (HGF), Syndecan-1 and Heparanase
Hepatocyte growth factor (HGF), a heparin-binding cytokine, is primarily expressed by mesenchymal cells and has pleiotropic effects mediated via its binding to the proto-oncogenic c-met receptor [78]. HGF has been shown to stimulate endothelial cell proliferation and survival, and acts as a pro-angiogenic molecule [79]. HGF is involved in tumoral angiogenesis and metastasis [80, 81]. In addition, HGF is synthesized by myeloma cells [82] and enhances MMP-9 secretion by myeloma cells [83]. HGF activity and signaling in myeloma cells is enhanced by syndecan-1, a cell surface heparan sulfate-bearing proteoglycan highly expressed by myeloma cells [84, 85]. Syndecan-1 can be cleaved and shed into the microenvironment and plays a role in myeloma cell growth, survival and angiogenesis [86, 87]. A relationship between HGF and syndecan-1 levels has also been observed with BM angiogenesis [12]. Heparanase is involved in syndecan-1 shedding through the upregulation of MMP-9 and in HGF expression and activity [88, 89], enhancing the pro-angiogenic properties of myeloma cells. Moreover, recent data suggest that heparanase expressed by myeloma cells upregulates VEGF production. Together these factors result in shedding of syndecan-1, which promotes MM-induced angiogenesis [90, 91].
Interleukin-6 and Interleukin-8
IL-6 is the major growth and survival factor for myeloma cells and is over-produced in a paracrine fashion by BM stromal cells in contact with myeloma cells [6]. IL-6 stimulates VEGF and OPN production by myeloma cells [6, 40, 54] and may exert a direct, pro-angiogenic effect [92], suggesting that this cytokine is involved both directly and indirectly in MM-induced angiogenesis.
IL-8, also called CXCL8, is a chemokine that exerts potent angiogenic activity through binding to the CXCR1 and CXCR2 receptors present on endothelial cells [93]. Studies indicate that myeloma cells and BM stromal cells [94, 95] directly produce IL-8, and elevated BM levels of IL-8 have been demonstrated in MM patients [95]. Tumor cell expression of IL-8 has been linked to the metastatic potential of many solid tumors [96, 97]. In myeloma cells, IL-8 expression has also been correlated with aberrant CD28 expression and consequently with MM progression and extra-medullary localization[98].
Relationship Between Angiogenic Factors and BM Angiogenesis in Multiple Myeloma
The over-production of pro-angiogenic factors by myeloma cells and the microenvironment is reflected by the overall increase in their levels in the BM plasma (i.e. VEGF, bFGF, OPN and HGF) and peripheral serum of MM patients [9, 12, 46, 99, 100]. Cytokine levels are generally higher in the BM. than in the peripheral blood [46]. However, there is not a strict relationship between BM angiogenesis and the levels of pro-angiogenic factors, suggesting that elevated cytokine levels may reflect myeloma cell burden rather than BM angiogenesis.
Different studies have investigated the potential relationship between the production of pro-angiogenic factors by myeloma cells and the increased BM angiogenesis observed in MM patients. Although VEGF and bFGF are considered the primary angiogenic growth factors produced by myeloma cells, recent evidence indicates that there is not a significant difference in the plasma cell expression levels of bFGF and VEGF and their receptor amongst MGUS, smoldering MM and active MM patients [101]. This suggests that the increased BM angiogenesis occurring in MM patients as compared to MGUS could be due to the higher numbers of plasma cells rather than the overexpression of pro-angiogenic molecules by myeloma cells.
Other authors have evaluated the expression of pro-angiogenic genes in normal BM plasma cells and in malignant myeloma cells by microarray analysis [102]. Expression of other angiogenic genes such as HGF, ANG, TGFA, was higher in malignant myeloma cells as compared to normal plasma cells [102]. Overall these data suggest that the increased BM angiogenesis in MM patients as compared to healthy subjects is due either to the increased number of BM plasma cells in MM patients or to the aberrant expression of angiogenic genes by myeloma cells that further increase the pro-angiogenic activity of normal plasma cells. In line with this hypothesis, a relationship between the expression of pro-angiogenic factors other than bFGF and VEGF and BM angiogenesis in MM patients has been reported. A significantly higher MVD and number of microvessels per field has been demonstrated in MM patients positive for Ang-1 expression as compared to those negative for Ang-1 [56]. Similar results have been obtained in comparisons of Ang-1 expression in myeloma cells versus healthy twin plasma cells. A 5.8 fold increase of Ang-1 transcripts was detected in myeloma cells, supporting an association with increased angiogenesis [103]. OPN expression by myeloma cells was also correlated with increased BM angiogenesis in MM patients. In particular, it has been demonstrated that OPN positive MM patients have a significantly higher MVD and number of microvessels per field as compared to OPN negative patients [74].
Finally, other investigators have noted that BM samples obtained from MGUS subjects have a decreased capacity to inhibit vessel formation in an in vitro model of angiogenesis as compared to BM samples from MM patients, and postulated that the increased angiogenesis that occurs in MM patients is a result of decreased production of normal angiogenesis inhibitors by myeloma cells, tilting the balance between pro-angiogenic and anti-angiogenic factors.[101].
Hypoxia and Hypoxia-Inducible Factor-1 in Myeloma-Induced Angiogenesis
Hypoxia is a common feature of solid tumors that is associated with angiogenesis and the malignant phenotype and is critical in the regulation of the angiogenic switch [104, 105]. Tumor adaptation to hypoxia is mainly due to the hypoxia-inducible factor (HIF)-1, a key transcription factor that regulates angiogenesis and tumor progression in solid cancers [106–111]. HIF-1 is a heterodimeric DNA binding complex composed of two basic helix-loop-helix proteins, including the constitutively expressed HIF-1β and the hypoxia-inducible α-subunit HIF-1α [106–109]. HIF-1α is over-expressed in many tumors [110, 111]. Under normoxic conditions, HIF-1α has a very short life and undergoes proteosomal degradation by oxygen-dependent hydroxylation.In contrast under hypoxic conditions, hydroxylation is suppressed and HIF-1α protein escapes proteasomal destruction and accumulates and translocates to the nucleus [106–109].
The role of hypoxia and HIF-1α has also been investigated in MM [112]. Myeloma cells grow in a hypoxic microenvironment. Myeloma BM has relatively low pO2 and sO2 as compared with healthy subjects [113]. It has also been reported that there is no significant difference in the reduced BM pO2 and sO2 in MM patients as compared with MGUS patients [112]. However, in a MM mouse model it has been reported that both normal and MM-infiltrated BM are hypoxic, although the level of oxygen was lower in MM BM [104]. This discrepancy could be due either to the different method used in the detection of hypoxia or to the lower physiological oxygen tension reported in mice as compared to human [112–114].
It is assumed that myeloma cells in the BM microenvironment are chronically exposed to low oxygen levels. This is supported by the finding that HIF-1α protein is highly expressed in myeloma cells [112]. Interestingly, the presence of HIF-1α protein was also observed in CD138+ purified cells of about 28% of MM patients analyzed under normoxic conditions [112], suggesting that hypoxia-independent stabilization of HIF-1α may occur in myeloma cells together with HIF-1α over-expression induced by the hypoxic microenvironment. Others also reported that myeloma cells express HIF-1α in normoxia, showing that constitutive expression of HIF-1α by myeloma cells is associated with oncogenic c-Myc, delineating a common signaling pathway in myeloma cells [115].
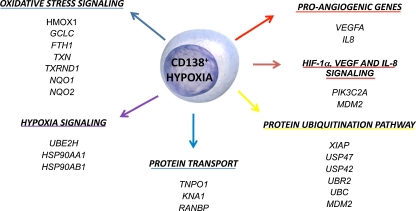
The role of hypoxia and HIF-1α in the production of pro-angiogenic molecules by myeloma cells has also been highlighted. By microarray analysis it has been reported that hypoxia affects both the transcriptional and angiogenic profiles of myeloma cells, as summarized in Fig. 2. Among the pro-angiogenic genes, VEGFA and IL8 were found to be significantly induced in CD138+ MM cases under hypoxic conditions [112]. Finally, it was reported that VEGF, IL-8, OPN and PGF are regulated by HIF-1α at both the mRNA and protein levels in myeloma cells [112], and that HIF-1α silencing consistently and significantly suppresses the pro-angiogenic properties of myeloma cells in vitro [112]. Overall these observations support the critical role of hypoxia and its transcription factor HIF-1α in the angiogenic switch induced by myeloma cells.
Fig. 2.
Effect of hypoxia on the transcriptional and pro-angiogenic profile of myeloma cells. Important gene and related pathways significantly up-regulated by hypoxia treatment in CD138+ purified cells. Among the pro-angiogenic genes VEGFA and IL8 were induced by hypoxia (Ref. 102)
Genes Involved in the Regulation of the Pro-angiogenic Profile of Myeloma Cells
Although HIF-1α seems to be a major regulator of the pro-angiogenic profile of myeloma cells and MM-induced angiogenesis, more recently the potential involvement of other genes in the production of pro-angiogenic factors by myeloma cells has been elucidated [95, 116].
The new candidate tumor suppressor gene inhibitor of growth family member 4 (ING4) has been recently implicated in tumors as a repressor of tumor growth and angiogenesis through its association with NF-kB [117, 118]. Interestingly, it has been shown that tumors lacking ING4 showed increased vascularization compared with ING4 expressing tumors [118]. Moreover, ING4 down-regulated angiogenesis related molecules including IL-8 and HIF-1α [117, 119]. In line with these observations it has been shown that ING4 nuclear level is reduced in myeloma cells as compared to normal plasma cells, and that ING4 regulates IL-8 and OPN production by myeloma cells both in normoxia and hypoxia [95]. In addition, ING4 has a suppressive effect on HIF-1α activity in myeloma cells because blocking ING4 in myeloma cells increases both HIF-1α activity and the expression of its target gene NIP-3 [95]. The involvement of ING4 as an angiogenic repressor in MM was further confirmed in an established in vitro angiogenesis model showing that the supernatant of myeloma cells with suppressed ING4 levels significantly stimulates vessel formation in normoxic and hypoxic conditions [95]. Finally, these results were expanded in vivo by the finding in MM patients that ING4 expression levels are negatively correlated with the expression of pro-angiogenic molecules and the extenet of BM angiogenesis [95]. These observations support the hypothesis that ING4 may act as angiogenic repressor in MM, in a manner similar to that previously demonstrated in glioblastoma [117].
The deregulation of the homeobox (HOX) B genes has been previously correlated to the angiogenic process as well as to tumoral-induced neoangiogenesis and tumor progression in solid cancer [120–123]. Particularly, the overexpression of HOXB7 has been associated with the tumor-related angiogenic switch, cell proliferation, and the production of bFGF by breast cancer and melanoma cells [122, 123]. In addition, HOXB7 overexpression may also regulate VEGF, IL-8 and Ang-2 by tumor cells [121]. In MM it has recently been shown that HOXB7 overexpression in myeloma cells regulated VEGFA, FGF2, MMP2 and PDGFA gene expression and the corresponding proteins VEGF, bFGF, MMP-2 and PDGF-AA. The capacity of HOXB7 to regulate bFGF and VEGF was confirmed by silencing HOXB7 in myeloma cells that constitutively overexpressed HOXB7. The role of HOXB7 as a regulator of pro-agiogenic factors in myeloma cells was confirmed in a large independent dataset of primary MM tumors showing a significant relationship between HOXB7 expression and other angiogenic factors including bFGF and VEGF. These data suggest that HOXB7 could be an important gene in the regulation of the angiogenic profile of myeloma cells.
Anti Angiogenic Effect of the Novel Anti-MM Agents and Future Perspectives
New agents showing a significant anti-MM clinical activity such as thalidomide, its derivatives, the immunomodulatory drugs (IMiDs), and the proteasome inhibitor Bortezomib, have anti-angiogenic activity both in vitro and in vivo. Firstly it was demonstrated that thalidomide inhibits bFGF by a rabbit corneal assay [124]. Others showed that thalidomide inhibits VEGF, HGF, bFGF, IGF-1 and Ang-2 expression by BM endothelial cells isolated from MM patients [125, 126]. All IMiDs, including lenalidomide and pomalidomide, have anti-angiogenic activity independent of their immunomodulatory effects [125, 127, 128], although thalidomide is probably a more prominent inhibitor of angiogenesis than its derivatives. IMiDs inhibit VEGF and bFGF secretion from both myeloma and BM stromal cells and block endothelial cell migration rather than endothelial cell proliferation through the interference of PIk3/AKT signaling [128, 129]. Finally, in pre-clinical in vivo mouse MM models both thalidomide and lenalidomide induced a significant reduction of tumor-associated MVD [130, 131]. Although this evidence suggests that thalidomide and IMiDs have an anti-angiogenic activity, a reduction of MVD as well as VEGF levels is not always seen with thalidomide response in MM patients [100, 125, 127, 132], suggesting that both the in vivo anti angiogenic effect of these drugs and the contribution of their anti-angiogenic activity to the overall anti-tumor effect is uncertain.
In addition to a direct anti-MM effect, proteasome inhibitors have been shown to have an anti-angiogenic effect as well [125]. These drugs inhibit vascular formation on matrigel and induce apoptosis of endothelial cells in a dose-dependent manner [133]. Similarly, it has been shown that Bortezomib at clinically achievable concentrations inhibits endothelial cell proliferation and targets VEGF-induced caveoln-1 phosphorylation in endothelial cells [134]. Bortezomib also blocks MM interaction with BM stromal cells and indirectly blocks the production of pro-angiogenic factors [125]. Pre-clinical in vivo studies confirm the potential anti-angiogenic effect of the proteasome inhibitors [135] and it has been consistently reported that MM patients treated with Bortezomib show a significant reduction in MVD although serum VEGF are not consistently reduced [136]. However, a significant reduction in Ang-2 levels has been reported in MM patients treated with Bortezomib. Further studies are necessary to clarify whether the anti-angiogenic effect of Bortezomib is clinical relevant in vivo in MM patients [137].
Other new drugs with demonstrated anti-angiogenic activity in ongoing clinical phase I/II trials in myeloma include PI3K/AKT/mTOR inhibitors (i.e. Perifosine, Rapamycin), MAPK signal pathway inhibitors, and heat shock protein 90 (HSP90) inhibitors, either as single agents or in combination with bortezomib or IMiDs [138].
VEGF is a well known target of anti-angiogenic therapy in solid tumors. Drugs targeting VEGF and its receptor have been developed and include the anti-VEGF monoclonal antibody (Bevacizumab), a soluble fusion protein of the extracellular domain of VEGFR-1 and VEGFR-2 (Aflibercept), and several receptor tyrosine kinase (RTK) inhibitors targeting VEGFR [138]. Some of these have shown anti-MM activity in preclinical models (Sunitinib, Sorafenib, Semaxinib, Vandetanib, and the pan-VEGFR inhibitor Pazopanib) [138–140], however preliminary data as single agents has failed to show a relevant clinical effect despite their inhibitory effect on VEGF [141–143]. The lack of effect of VEGF inhibitors could be due either to the capacity of myeloma cells to produce other pro-angiogenic factors, allowing an escape from the block of VEGF and its signaling, or independence from the angiogenic switch of myeloma cells in end-stage disease. Future clinical studies should be done to explore the potential role of anti-VEGF drugs in combination with Bortezomib and IMiDs to improve their cytotoxic effects or in the early stage of disease.
Conclusions
MM is characterized by increased angiogenesis in the BM microenvironment that is related to myeloma cell infiltration and may support myeloma cell growth and survival. BM angiogenesis correlates with disease progression and prognosis in MM patients. The presence of an imbalance in the production of pro- and anti-angiogenic molecules in favor of pro-angiogenic factors leads to the angiogenic switch in MM. Myeloma cells directly produce or induce several pro-angiogenic molecules in the microenvironment, including VEGF, bFGF, Ang-1, OPN, HGF and IL-8. Current data suggests that the increased BM angiogenesis in MM patients is due either to the increased number of BM plasma cells or aberrant expression of angiogenic factors by myeloma cells that may further increase the pro-angiogenic activity of normal plasma cells. The lack of production by of angiogenic inhibitors by myeloma cells may contribute to the high grade of BM angiogenesis in MM patients. Molecular mechanisms underlying the angiogenic switch in MM have recently been identified. The BM microenvironment is hypoxic and the hypoxia induced transcription factor HIF-1α is critically involved in the production of angiogenic factors by myeloma cells. HIF-1α is overexpressed by myeloma cells and affects their transcriptional and angiogenic profiles. In addition, a potential role of ING4 as a repressor of angiogenesis and of HIF-1α activity, as well as the role of the pro-angiogenic gene HOXB7 has also been recently highlighted.
The complex pathogenetic model of myeloma-induced angiogenesis suggests that VEGF and other pro-angiogenic factors produced by myeloma cells and the microenvironment are potential targets for anti-angiogenic therapy in MM patients. Recent data suggests that the novel anti-MM agents such as thalidomide, IMiDs and Bortezomib have anti-angiogenic in vitro, either through the blockade of the production of pro-angiogenic factors or a direct effect on endothelial cells, although the clinical relevance of this effect as well as the role of anti-angiogenic therapy in MM patients are yet to be determined
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 4.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 5.Papetti M, Herman IM. Mechanism of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2001;282:947–970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;28(6):249–274. doi: 10.1146/annurev-pathol-011110-130249. [DOI] [PubMed] [Google Scholar]
- 7.Asosingh K, Raeve H, Menu E, Riet I, Marck E, Camp B, Vanderkerken K. Angiogenic switch during 5T2MM murine myeloma tumorigenesis: role of CD45 heterogeneity. Blood. 2004;103:3131–3137. doi: 10.1182/blood-2003-08-2946. [DOI] [PubMed] [Google Scholar]
- 8.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–3073. [PubMed] [Google Scholar]
- 9.Jakob C, Sterz J, Zavrski I, Heider U, Kleeberg L, Fleissner C, Kaiser M, Sezer O. Angiogenesis in multiple myeloma. Eur J Cancer. 2006;42:1581–1590. doi: 10.1016/j.ejca.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Mesa RA, Fonseca R, Schroeder G, Plevak MF, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Gertz MA, Kyle RA, Russell SJ, Greipp PR. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res. 2002;8:2210–2216. [PubMed] [Google Scholar]
- 11.Alexandrakis MG, Passam FH, Dambaki C, Pappa CA, Stathopoulos EN. The relation between bone marrow angiogenesis and the proliferation index Ki-67 in multiple myeloma. J Clin Pathol. 2004;57:856–860. doi: 10.1136/jcp.2003.013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen NF, Standal T, Nielsen JL, Heickendorff L, Borset M, Sørensen FB, Abildgaard N. Syndecan-1 and angiogenic cytokines in multiple myeloma: correlation with bone marrow angiogenesis and survival. Br J Haematol. 2005;128:210–217. doi: 10.1111/j.1365-2141.2004.05299.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhatti SS, Kumar L, Dinda AK, Dawar R. Prognostic value of bone marrow angiogenesis in multiple myeloma: use of light microscopy as well as computerized image analyzer in the assessment of microvessel density and total vascular area in multiple myeloma and its correlation with various clinical, histological, and laboratory parameters. Am J Hematol. 2006;81:649–656. doi: 10.1002/ajh.20639. [DOI] [PubMed] [Google Scholar]
- 14.Hillengass J, Wasser K, Delorme S, Kiessling F, Zechmann C, Benner A, Kauczor HU, Ho AD, Goldschmidt H, Moehler TM. Lumbar bone marrow microcirculation measurements from dynamic contrast-enhanced magnetic resonance imaging is a predictor of event-free survival in progressive multiple myeloma. Clin Cancer Res. 2007;13:475–481. doi: 10.1158/1078-0432.CCR-06-0061. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Wellik L, Witzig TE, Gertz MA, Kyle RA, Greipp PR, Rajkumar SV. Prognostic value of angiogenesis in solitary bone plasmacytoma. Blood. 2003;101:1715–1717. doi: 10.1182/blood-2002-08-2441. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Gertz MA, Dispenzieri A, Lacy MQ, Wellik LA, Fonseca R, Lust JA, Witzig TE, Kyle RA, Greipp PR, Rajkumar SV. Prognostic value of bone marrow angiogenesis in patients with multiple myeloma undergoing high-dose therapy. Bone Marrow Transplant. 2004;34:235–239. doi: 10.1038/sj.bmt.1704555. [DOI] [PubMed] [Google Scholar]
- 17.Moulopoulos LA, Dimopoulos MA, Christoulas D, Kastritis E, Anagnostou D, Koureas A, Roussou M, Gavriatopoulou M, Migkou M, Iakovaki M, Gkotzamanidou M, Tasidou A, Terpos E. Diffuse MRI marrow pattern correlates with increased angiogenesis, advanced disease features and poor prognosis in newly diagnosed myeloma treated with novel agents. Leukemia. 2010;24:1206–1212. doi: 10.1038/leu.2010.70. [DOI] [PubMed] [Google Scholar]
- 18.Munshi NC, Wilson C. Increased bone marrow microvessel density in newly diagnosed multiple myeloma carries a poor prognosis. Semin Oncol. 2001;28:565–569. doi: 10.1016/S0093-7754(01)90025-9. [DOI] [PubMed] [Google Scholar]
- 19.Pruneri G, Ponzoni M, Ferreri AJ, Decarli N, Tresoldi M, Raggi F, Baldessari C, Freschi M, Baldini L, Goldaniga M, Neri A, Carboni N, Bertolini F, Viale G. Microvessel density, a surrogate marker of angiogenesis, is significantly related to survival in multiple myeloma patients. Br J Haematol. 2002;118:817–820. doi: 10.1046/j.1365-2141.2002.03654.x. [DOI] [PubMed] [Google Scholar]
- 20.Sezer O, Niemöller K, Eucker J, Jakob C, Kaufmann O, Zavrski I, Dietel M, Possinger K. Bone marrow microvessel density is a prognostic factor for survival in patients with multiple myeloma. Ann Hematol. 2000;79:574–577. doi: 10.1007/s002770000236. [DOI] [PubMed] [Google Scholar]
- 21.Sezer O, Niemöller K, Jakob C, Zavrski I, Heider U, Eucker J, Kaufmann O, Possinger K. Relationship between bone marrow angiogenesis and plasma cell infiltration and serum beta2-microglobulin levels in patients with multiple myeloma. Ann Hematol. 2001;80:598–601. doi: 10.1007/s002770100361. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber S, Ackermann J, Obermair A, Kaufmann H, Urbauer E, Aletaha K, Gisslinger H, Chott A, Huber H, Drach J. Multiple myeloma with deletion of chromosome 13q is characterized by increased bone marrow neovascularization. Br J Haematol. 2000;110:605–609. doi: 10.1046/j.1365-2141.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- 23.Hillengass J, Zechmann CM, Nadler A, Hose D, Cremer FW, Jauch A, Heiss C, Benner A, Ho AD, Bartram CR, Kauczor HU, Delorme S, Goldschmidt H, Moehler TM. Gain of 1q21 and distinct adverse cytogenetic abnormalities correlate with increased microcirculation in multiple myeloma. Int J Cancer. 2008;122:2871–2875. doi: 10.1002/ijc.23455. [DOI] [PubMed] [Google Scholar]
- 24.Sezer O, Niemöller K, Kaufmann O, Eucker J, Jakob C, Zavrski I, Possinger K. Decrease of bone marrow angiogenesis in myeloma patients achieving a remission after chemotherapy. Eur J Haematol. 2001;66:238–244. doi: 10.1034/j.1600-0609.2001.066004238.x. [DOI] [PubMed] [Google Scholar]
- 25.Rajkumar SV, Fonseca R, Witzig TE, Gertz MA, Greipp PR. Bone marrow angiogenesis in patients achieving complete response after stem cell transplantation for multiple myeloma. Leukemia. 1999;13:469–472. doi: 10.1038/sj.leu.2401336. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Gertz MA, Kyle RA, Greipp PR, Rajkumar SV. Bone marrow angiogenesis in multiple myeloma: effect of therapy. Br J Haematol. 2002;119:665–671. doi: 10.1046/j.1365-2141.2002.03871.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Witzig TE, Dispenzieri A, Lacy MQ, Wellik LE, Fonseca R, Lust JA, Gertz MA, Kyle RA, Greipp PR, Rajkumar SV. Effect of thalidomide therapy on bone marrow angiogenesis in multiple myeloma. Leukemia. 2004;18:624–627. doi: 10.1038/sj.leu.2403285. [DOI] [PubMed] [Google Scholar]
- 28.Vacca A, Ribatti D. Bone marrow angiogenesis in multiple myeloma. Leukemia. 2006;20:193–199. doi: 10.1038/sj.leu.2404067. [DOI] [PubMed] [Google Scholar]
- 29.Ribatti D, Vacca A. The role of monocytes-macrophages in vasculogenesis in multiple myeloma. Leukemia. 2009;23:1535–1536. doi: 10.1038/leu.2009.55. [DOI] [PubMed] [Google Scholar]
- 30.Patenaude A, Parker J, Karsan A. Involvement of endothelial progenitor cells in tumor vascularization. Microvasc Res. 2010;79:217–223. doi: 10.1016/j.mvr.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Ahn GO, Brown JM. Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis. 2009;12:159–164. doi: 10.1007/s10456-009-9135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Vakil V, Braunstein M, Smith EL, Maroney J, Chen L, Dai K, Berenson JR, Hussain MM, Klueppelberg U, Norin AJ, Akman HO, Ozçelik T, Batuman OA. Circulating endothelial progenitor cells in multiple myeloma: implications and significance. Blood. 2005;105:3286–3294. doi: 10.1182/blood-2004-06-2101. [DOI] [PubMed] [Google Scholar]
- 33.Scavelli C, Nico B, Cirulli T, Ria R, Pietro G, Mangieri D, Bacigalupo A, Mangialardi G, Coluccia AM, Caravita T, Molica S, Ribatti D, Dammacco F, Vacca A. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27:663–674. doi: 10.1038/sj.onc.1210691. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Campbell RA, Chang Y, Li M, Wang CS, Li J, Sanchez E, Share M, Steinberg J, Berenson A, Shalitin D, Zeng Z, Gui D, Perez-Pinera P, Berenson RJ, Said J, Bonavida B, Deuel TF, Berenson JR. Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: a novel mechanism of tumor-induced vasculogenesis. Blood. 2009;113:1992–2002. doi: 10.1182/blood-2008-02-133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 36.Tjwa M, Luttun A, Autiero M, Carmeliet P. VEGF and PlGF: two pleiotropic growth factors with distinct roles in development and homeostasis. Cell Tissue Res. 2003;314:5–14. doi: 10.1007/s00441-003-0776-3. [DOI] [PubMed] [Google Scholar]
- 37.Bellamy WT. Expression of vascular endothelial growth factor and its receptors in multiple myeloma and other hematopoietic malignancies. Semin Oncol. 2001;28:551–559. doi: 10.1016/S0093-7754(01)90023-5. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Witzig TE, Timm M, Haug J, Wellik L, Fonseca R, Greipp PR, Rajkumar SV. Expression of VEGF and its receptors by myeloma cells. Leukemia. 2003;17:2025–2031. doi: 10.1038/sj.leu.2403084. [DOI] [PubMed] [Google Scholar]
- 39.Ria R, Roccaro AM, Merchionne F, Vacca A, Dammacco F, Ribatti D. Vascular endothelial growth factor and its receptors in multiple myeloma. Leukemia. 2003;17:1961–1966. doi: 10.1038/sj.leu.2403076. [DOI] [PubMed] [Google Scholar]
- 40.Dankbar B, Padro T, Leo R, Feldmann B, Kropff M, Mesters RM, Serve H, Berdel WE, Kienast J. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–2636. [PubMed] [Google Scholar]
- 41.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, Schlossman RL, Richardson P, Ralph P, Wu L, Payvandi F, Muller G, Stirling DI, Anderson KC. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Zhang Z, Yao C. Angiogenic activity of mesenchymal stem cells in multiple myeloma. Cancer Invest. 2011;29:37–41. doi: 10.3109/07357907.2010.496758. [DOI] [PubMed] [Google Scholar]
- 43.Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, Lin BK, Gupta D, Shima Y, Chauhan D, Mitsiades C, Raje N, Richardson P, Anderson KC. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98:428–435. doi: 10.1182/blood.V98.2.428. [DOI] [PubMed] [Google Scholar]
- 44.Giuliani N, Lunghi P, Morandi F, Colla S, Bonomini S, Hojden M, Rizzoli V, Bonati A. Downmodulation of ERK protein kinase activity inhibits VEGF secretion by human myeloma cells and myeloma-induced angiogenesis. Leukemia. 2004;18:628–635. doi: 10.1038/sj.leu.2403269. [DOI] [PubMed] [Google Scholar]
- 45.Giuliani N, Colla S, Rizzoli V. Angiogenic switch in multiple myeloma. Hematology. 2004;9:377–381. doi: 10.1080/10245330400018524. [DOI] [PubMed] [Google Scholar]
- 46.Raimondo F, Azzaro MP, Palumbo G, Bagnato S, Giustolisi G, Floridia P, Sortino G, Giustolisi R. Angiogenic factors in multiple myeloma: higher levels in bone marrow than in peripheral blood. Haematologica. 2000;85:800–805. [PubMed] [Google Scholar]
- 47.Bisping G, Leo R, Wenning D, Dankbar B, Padro T, Kropff M, Scheffold C, Kroger M, Mesters RM, Berdel WE, Kienast J. Paracrine interactions of basic fibroblast growth factor and interleukin-6 in multiple myeloma. Blood. 2003;101:2775–2783. doi: 10.1182/blood-2002-09-2907. [DOI] [PubMed] [Google Scholar]
- 48.Colla S, Morandi F, Lazzaretti M, Polistena P, Svaldi M, Coser P, Bonomini S, Hojden M, Martella E, Chisesi T, Rizzoli V, Giuliani N. Do human myeloma cells directly produce basic FGF? Blood. 2003;102:3071–3072. doi: 10.1182/blood-2003-06-1883. [DOI] [PubMed] [Google Scholar]
- 49.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 50.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/S0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 51.Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Letter. 1999;448:249–253. doi: 10.1016/S0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- 52.Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- 53.Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res. 1999;58:224–237. doi: 10.1006/mvre.1999.2179. [DOI] [PubMed] [Google Scholar]
- 54.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–941. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 55.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 56.Giuliani N, Colla S, Lazzaretti M, Sala R, Roti G, Mancini C, Bonomini S, Lunghi P, Hojden M, Genestreti G, Svaldi M, Coser P, Fattori PP, Sammarelli G, Gazzola GC, Bataille R, Almici C, Caramatti C, Mangoni L, Rizzoli V. Proangiogenic properties of human myeloma cells: production of angiopoietin-1 and its potential relationship to myeloma-induced angiogenesis. Blood. 2003;102:638–645. doi: 10.1182/blood-2002-10-3257. [DOI] [PubMed] [Google Scholar]
- 57.Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest. 1997;100:2072–2078. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vacca A, Ria R, Semeraro F, Merchionne F, Coluccia M, Boccarelli A, Scavelli C, Nico B, Gernone A, Battelli F, Tabilio A, Guidolin D, Petrucci MT, Ribatti D, Dammacco F. Endothelial cells in the bone marrow of patients with multiple myeloma. Blood. 2003;102:3340–3348. doi: 10.1182/blood-2003-04-1338. [DOI] [PubMed] [Google Scholar]
- 59.Nakayama T, Yao L, Tosato G. Mast cell-derived angiopoietin-1 plays a critical role in the growth of plasma cell tumors. J Clin Invest. 2004;114:1317–1325. doi: 10.1172/JCI22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terpos E, Anargyrou K, Katodritou E, Kastritis E, Papatheodorou A, Christoulas D, Pouli A, Michalis E, Delimpasi S, Gkotzamanidou M, Nikitas N, Koumoustiotis V, Margaritis D, Tsionos K, Stefanoudaki E, Meletis J, Zervas K, Dimopoulos MA, Greek Myeloma Study Group, Greece (2011) Circulating angiopoietin-1 to angiopoietin-2 ratio is an independent prognostic factor for survival in newly diagnosed patients with multiple myeloma who received therapy with novel antimyeloma agents. Int J Cancer. doi:10.1002/ijc.26062 [DOI] [PubMed]
- 61.Agnelli L, Bicciato S, Mattioli M, Fabris S, Intini D, Verdelli D, Baldini L, Morabito F, Callea V, Lombardi L, Neri A. Molecular classification of multiple myeloma: a distinct transcriptional profile characterizes patients expressing CCND1 and negative for 14q32 translocations. J Clin Oncol. 2005;23:7296–7306. doi: 10.1200/JCO.2005.01.3870. [DOI] [PubMed] [Google Scholar]
- 62.Plank MJ, Sleeman BD, Jones PF. The role of the angiopoietins in tumour angiogenesis. Growth Factors. 2004;22:1–11. doi: 10.1080/08977190310001643218. [DOI] [PubMed] [Google Scholar]
- 63.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valckenborgh E, Bakkus M, Munaut C, Noël A, St Pierre Y, Asosingh K, Riet I, Camp B, Vanderkerken K. Upregulation of matrix metalloproteinase-9 in murine 5 T33 multiple myeloma cells by interaction with bone marrow endothelial cells. Int J Cancer. 2002;101:512–518. doi: 10.1002/ijc.10642. [DOI] [PubMed] [Google Scholar]
- 66.Valckenborgh E, Croucher PI, Raeve H, Carron C, Leenheer E, Blacher S, Devy L, Noël A, Bruyne E, Asosingh K, Riet I, Camp B, Vanderkerken K. Multifunctional role of matrix metalloproteinases in multiple myeloma: a study in the 5T2MM mouse model. Am J Pathol. 2004;165:869–878. doi: 10.1016/S0002-9440(10)63349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barillé S, Akhoundi C, Collette M, Mellerin MP, Rapp MJ, Harousseau JL, Bataille R, Amiot M. Metalloproteinases in multiple myeloma: production of matrix metalloproteinase-9 (MMP-9), activation of proMMP-2, and induction of MMP-1 by myeloma cells. Blood. 1997;90:1649–1655. [PubMed] [Google Scholar]
- 68.Barillé S, Bataille R, Rapp MJ, Harousseau JL, Amiot M. Production of metalloproteinase-7 (matrilysin) by human myeloma cells and its potential involvement in metalloproteinase-2 activation. J Immunol. 1999;163:5723–5728. [PubMed] [Google Scholar]
- 69.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 71.Liaw L, Almeida M, Hart CE, Schwartz SM, Giachelli CM. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994;74:214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi F, Akutagawa S, Fukumoto H, Tsukiyama S, Ohe Y, Takahashi K, Fukuchi Y, Saijo N, Nishio K. Osteopontin induces angiogenesis of murine neuroblastoma cells in mice. Int J Cancer. 2002;98:707–712. doi: 10.1002/ijc.10261. [DOI] [PubMed] [Google Scholar]
- 73.Hirama M, Takahashi F, Takahashi K, Akutagawa S, Shimizu K, Soma S, Shimanuki Y, Nishio K, Fukuchi Y. Osteopontin overproduced by tumor cells acts as a potent angiogenic factor contributing to tumor growth. Cancer Lett. 2003;198:107–117. doi: 10.1016/S0304-3835(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 74.Colla S, Morandi F, Lazzaretti M, Rizzato R, Lunghi P, Bonomini S, Mancini C, Pedrazzoni M, Crugnola M, Rizzoli V, Giuliani N. Human myeloma cells express the bone regulating gene Runx2/Cbfa1 and produce osteopontin that is involved in angiogenesis in multiple myeloma patients. Leukemia. 2005;19:2166–2176. doi: 10.1038/sj.leu.2403976. [DOI] [PubMed] [Google Scholar]
- 75.Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-kappa B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001;276:44926–44935. doi: 10.1074/jbc.M103334200. [DOI] [PubMed] [Google Scholar]
- 76.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 77.Cackowski FC, Anderson JL, Patrene KD, Choksi RJ, Shapiro SD, Windle JJ, Blair HC, Roodman GD. Osteoclasts are important for bone angiogenesis. Blood. 2010;115:140–149. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galimi F, Brizzi MF, Comoglio PM. The hepatocyte growth factor and its receptor. Stem Cells. 1993;11(Suppl 2):22–30. doi: 10.1002/stem.5530110805. [DOI] [PubMed] [Google Scholar]
- 79.Rosen EM, Lamszus K, Laterra J, Polverini PJ, Rubin JS, Goldberg ID. HGF/SF in angiogenesis. Ciba Found Symp. 1997;212:215–226. doi: 10.1002/9780470515457.ch14. [DOI] [PubMed] [Google Scholar]
- 80.Gao CF, Vande Woude GF. HGF/SF-Met signaling in tumor progression. Cell Res. 2005;15:49–51. doi: 10.1038/sj.cr.7290264. [DOI] [PubMed] [Google Scholar]
- 81.Lesko E, Majka M. The biological role of HGF-MET axis in tumor growth and development of metastasis. Front Biosci. 2008;13:1271–1278. doi: 10.2741/2760. [DOI] [PubMed] [Google Scholar]
- 82.Borset M, Hjorth-Hansen H, Seide C, Sundan A, Waage A. Hepatocyte growth factor and its receptor c-met in multiple myeloma. Blood. 1996;88:3998–4004. [PubMed] [Google Scholar]
- 83.Vande Broek I, Vanderkerken K, Camp B, Riet I. Extravasation and homing mechanisms in multiple myeloma. Clin Exp Metastasis. 2008;25:325–334. doi: 10.1007/s10585-007-9108-4. [DOI] [PubMed] [Google Scholar]
- 84.Derksen PW, Keehnen RM, Evers LM, Oers MH, Spaargaren M, Pals ST. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma. Blood. 2002;99:1405–1410. doi: 10.1182/blood.V99.4.1405. [DOI] [PubMed] [Google Scholar]
- 85.Seidel C, Børset M, Hjertner O, Cao D, Abildgaard N, Hjorth-Hansen H, Sanderson RD, Waage A, Sundan A. High levels of soluble syndecan-1 in myeloma-derived bone marrow: modulation of hepatocyte growth factor activity. Blood. 2000;96:3139–3146. [PubMed] [Google Scholar]
- 86.Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, Jourdan E, Pantesco V, Baudard M, Vos J, Larroque M, Moehler T, Rossi JF, Rème T, Goldschmidt H, Klein B. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109:4914–4923. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khotskaya YB, Dai Y, Ritchie JP, MacLeod V, Yang Y, Zinn K, Sanderson RD. Syndecan-1 is required for robust growth, vascularization, and metastasis of myeloma tumors in vivo. J Biol Chem. 2009;284:26085–26095. doi: 10.1074/jbc.M109.018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 89.Ramani VC, Yang Y, Ren Y, Nan L, Sanderson RD. Heparanase Plays a Dual Role in Driving Hepatocyte Growth Factor (HGF) Signaling by Enhancing HGF Expression and Activity. J Biol Chem. 2011;286:6490–6499. doi: 10.1074/jbc.M110.183277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Biol Chem. 2008;283:32628–32636. doi: 10.1074/jbc.M806266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115:2449–2457. doi: 10.1182/blood-2009-07-234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci USA. 1990;87:3092–3096. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 94.Kline M, Donovan K, Wellik L, Lust C, Jin W, Moon-Tasson L, Xiong Y, Witzig TE, Kumar S, Rajkumar SV, Lust JA. Cytokine and chemokine profiles in multiple myeloma; significance of stromal interaction and correlation of IL-8 production with disease progression. Leuk Res. 2007;31:591–598. doi: 10.1016/j.leukres.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 95.Colla S, Tagliaferri S, Morandi F, Lunghi P, Donofrio G, Martorana D, Mancini C, Lazzaretti M, Mazzera L, Ravanetti L, Bonomini S, Ferrari L, Miranda C, Ladetto M, Neri TM, Neri A, Greco A, Mangoni M, Bonati A, Rizzoli V, Giuliani N. The new tumor-suppressor gene inhibitor of growth family member 4 (ING4) regulates the production of proangiogenic molecules by myeloma cells and suppresses hypoxia-inducible factor-1 alpha (HIF-1alpha) activity: involvement in myeloma-induced angiogenesis. Blood. 2007;110:4464–4475. doi: 10.1182/blood-2007-02-074617. [DOI] [PubMed] [Google Scholar]
- 96.Brat DJ, Bellail AC, Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 98.Shapiro VS, Mollenauer MN, Weiss A. Endogenous CD28 expressed on myeloma cells up-regulates interleukin-8 production: implications for multiple myeloma progression. Blood. 2001;98:187–193. doi: 10.1182/blood.V98.1.187. [DOI] [PubMed] [Google Scholar]
- 99.Alexandrakis MG, Passam FJ, Ganotakis E, Dafnis E, Dambaki C, Konsolas J, Kyriakou DS, Stathopoulos E. Bone marrow microvascular density and angiogenic growth factors in multiple myeloma. Clin Chem Lab Med. 2004;42:1122–1126. doi: 10.1515/CCLM.2004.230. [DOI] [PubMed] [Google Scholar]
- 100.Cibeira MT, Rozman M, Segarra M, Lozano E, Rosiñol L, Cid MC, Filella X, Bladé J. Bone marrow angiogenesis and angiogenic factors in multiple myeloma treated with novel agents. Cytokine. 2008;41:244–253. doi: 10.1016/j.cyto.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 101.Kumar S, Witzig TE, Timm M, Haug J, Wellik L, Kimlinger TK, Greipp PR, Rajkumar SV. Bone marrow angiogenic ability and expression of angiogenic cytokines in myeloma: evidence favoring loss of marrow angiogenesis inhibitory activity with disease progression. Blood. 2004;104:1159–1165. doi: 10.1182/blood-2003-11-3811. [DOI] [PubMed] [Google Scholar]
- 102.Hose D, Moreaux J, Meissner T, Seckinger A, Goldschmidt H, Benner A, Mahtouk K, Hillengass J, Rème T, Vos J, Hundemer M, Condomines M, Bertsch U, Rossi JF, Jauch A, Klein B, Möhler T. Induction of angiogenesis by normal and malignant plasma cells. Blood. 2009;114:128–143. doi: 10.1182/blood-2008-10-184226. [DOI] [PubMed] [Google Scholar]
- 103.Munshi NC, Hideshima T, Carrasco D, Shammas M, Auclair D, Davies F, Mitsiades N, Mitsiades C, Kim RS, Li C, Rajkumar SV, Fonseca R, Bergsagel L, Chauhan D, Anderson KC. Identification of genes modulated in multiple myeloma using genetically identical twin samples. Blood. 2004;103:1799–1806. doi: 10.1182/blood-2003-02-0402. [DOI] [PubMed] [Google Scholar]
- 104.Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia and cancer. J Mol Med. 2007;85:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- 105.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 106.Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217–257. doi: 10.1016/S0070-2153(06)76007-0. [DOI] [PubMed] [Google Scholar]
- 107.Hirota K, Semenza GL. ReguIation of angiogenesis by hypoxia-inducibIe factor 1. Crit Rev OncoI Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Lisy K, Peet DJ. Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 2008;15:642–649. doi: 10.1038/sj.cdd.4402315. [DOI] [PubMed] [Google Scholar]
- 109.Weidemann A, Johnson RS. Biology of HIF-1α. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 110.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhong H, Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 112.Colla S, Storti P, Donofrio G, Todoerti K, Bolzoni M, Lazzaretti M, Abeltino M, Ippolito L, Neri A, Ribatti D, Rizzoli V, Martella E, Giuliani N. Low bone marrow oxygen tension and hypoxia-inducible factor-1α overexpression characterize patients with multiple myeloma: role on the transcriptional and proangiogenic profiles of CD138(+) cells. Leukemia. 2010;24:1967–1970. doi: 10.1038/leu.2010.193. [DOI] [PubMed] [Google Scholar]
- 113.Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.V99.1.394. [DOI] [PubMed] [Google Scholar]
- 114.Asosingh K, Raeve H, Ridder M, Storme GA, Willems A, Riet I, Camp B, Vanderkerken K. Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematologica. 2005;90:810–817. [PubMed] [Google Scholar]
- 115.Zhang J, Sattler M, Tonon G, Grabher C, Lababidi S, Zimmerhackl A, Raab MS, Vallet S, Zhou Y, Cartron MA, Hideshima T, Tai YT, Chauhan D, Anderson KC, Podar K. Targeting angiogenesis via a c-Myc/hypoxia-inducible factor-1alpha-dependent pathway in multiple myeloma. Cancer Res. 2009;69(12):5082–5090. doi: 10.1158/0008-5472.CAN-08-4603. [DOI] [PubMed] [Google Scholar]
- 116.Storti P, Donofrio G, Colla S, Airoldi I, Bolzoni M, Agnelli L, Abeltino M, Todoerti K, Lazzaretti M, Mancini C, Ribatti D, Bonomini S, Franceschi V, Pistoia V, Lisignoli G, Pedrazzini A, Cavicchi O, Neri A, Rizzoli V, Giuliani N. HOXB7 expression by myeloma cells regulates their pro-angiogenic properties in multiple myeloma patients. Leukemia. 2011;25:527–537. doi: 10.1038/leu.2010.270. [DOI] [PubMed] [Google Scholar]
- 117.Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004;428:328–332. doi: 10.1038/nature02329. [DOI] [PubMed] [Google Scholar]
- 118.Gunduz M, Nagatsuka H, Demircan K, Gunduz E, Cengiz B, Ouchida M, Tsujigiwa H, Yamachika E, Fukushima K, Beder L, Hirohata S, Ninomiya Y, Nishizaki K, Shimizu K, Nagai N. Frequent deletion and down-regulation of ING4, a candidate tumor suppressor gene at 12p13, in head and neck squamous cell carcinomas. Gene. 2005;356:109–117. doi: 10.1016/j.gene.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 119.Ozer A, Wu LC, Bruick RK. The candidate tumor suppressor ING4 represses activation of the hypoxia inducible factor (HIF) Proc Natl Acad Sci USA. 2005;102:7481–7486. doi: 10.1073/pnas.0502716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gorski DH, Walsh K. The role of homeobox genes in vascular remodeling and angiogenesis. Circ Res. 2000;87:865–872. doi: 10.1161/01.res.87.10.865. [DOI] [PubMed] [Google Scholar]
- 121.Carè A, Felicetti F, Meccia E, Bottero L, Parenza M, Stoppacciaro A, Peschle C, Colombo MP. HOXB7: a key factor for tumor-associated angiogenic switch. Cancer Res. 2001;61:6532–6539. [PubMed] [Google Scholar]
- 122.Caré A, Silvani A, Meccia E, Mattia G, Peschle C, Colombo MP. Transduction of the SkBr3 breast carcinoma cell line with the HOXB7 gene induces bFGF expression, increases cell proliferation and reduces growth factor dependence. Oncogene. 1998;16:3285–3289. doi: 10.1038/sj.onc.1201875. [DOI] [PubMed] [Google Scholar]
- 123.Caré A, Silvani A, Meccia E, Mattia G, Stoppacciaro A, Parmiani G, Peschle C, Colombo MP. HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol Cell Biol. 1996;16:4842–4851. doi: 10.1128/mcb.16.9.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anargyrou K, Dimopoulos MA, Sezer O, Terpos E. Novel anti-myeloma agents and angiogenesis. Leuk Lymphoma. 2008;49:677–689. doi: 10.1080/10428190701861686. [DOI] [PubMed] [Google Scholar]
- 126.Vacca A, Scavelli C, Montefusco V, Pietro G, Neri A, Mattioli M, Bicciato S, Nico B, Ribatti D, Dammacco F, Corradini P. Thalidomide downregulates angiogenic genes in bone marrow endothelial cells of patients with active multiple myeloma. J Clin Oncol. 2005;23:5334–5346. doi: 10.1200/JCO.2005.03.723. [DOI] [PubMed] [Google Scholar]
- 127.Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dredge K, Marriott JB, Macdonald CD, Man HW, Chen R, Muller GW, Stirling D, Dalgleish AG. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87:1166–1172. doi: 10.1038/sj.bjc.6600607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, Dalgleish AG, Bartlett JB. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1–2):56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 130.Yaccoby S, Johnson CL, Mahaffey SC, Wezeman MJ, Barlogie B, Epstein J. Antimyeloma efficacy of thalidomide in the SCID-hu model. Blood. 2002;100:4162–4168. doi: 10.1182/blood-2002-03-0939. [DOI] [PubMed] [Google Scholar]
- 131.Lentzsch S, LeBlanc R, Podar K, Davies F, Lin B, Hideshima T, Catley L, Stirling DI, Anderson KC. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia. 2003;17:41–44. doi: 10.1038/sj.leu.2402745. [DOI] [PubMed] [Google Scholar]
- 132.Mileshkin L, Honemann D, Gambell P, Trivett M, Hayakawa Y, Smyth M, Beshay V, Ritchie D, Simmons P, Milner AD, Zeldis JB, Prince HM. Patients with multiple myeloma treated with thalidomide: evaluation of clinical parameters, cytokines, angiogenic markers, mast cells and marrow CD57+ cytotoxic T cells as predictors of outcome. Haematologica. 2007;92:1075–1082. doi: 10.3324/haematol.11208. [DOI] [PubMed] [Google Scholar]
- 133.Drexler HC, Risau W, Konerding MA. Inhibition of proteasome function induces programmed cell death in proliferating endothelial cells. FASEB J. 2000;14:65–77. doi: 10.1096/fasebj.14.1.65. [DOI] [PubMed] [Google Scholar]
- 134.Podar K, Shringarpure R, Tai YT, Simoncini M, Sattler M, Ishitsuka K, Richardson PG, Hideshima T, Chauhan D, Anderson KC. Caveolin-1 is required for vascular endothelial growth factor-triggered multiple myeloma cell migration and is targeted by bortezomib. Cancer Res. 2004;64:7500–7506. doi: 10.1158/0008-5472.CAN-04-0124. [DOI] [PubMed] [Google Scholar]
- 135.LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien CS, Adams J, Gupta D, Richardson PG, Munshi NC, Anderson KC. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 136.Politou M, Naresh K, Terpos E, Crawley D, Lampert I, Apperley JF, Rahemtulla A. Anti-angiogenic effect of bortezomib in patients with multiple myeloma. Acta Haematol. 2005;114:170–173. doi: 10.1159/000087894. [DOI] [PubMed] [Google Scholar]
- 137.Anargyrou K, Terpos E, Vassilakopoulos TP, Pouli A, Sachanas S, Tzenou T, Masouridis S, Christoulas D, Angelopoulou MK, Dimitriadou EM, Kalpadakis C, Tsionos K, Panayiotidis P, Dimopoulos MA, Pangalis GA, Kyrtsonis MC, Greek Myeloma Study Group Normalization of the serum angiopoietin-1 to angiopoietin-2 ratio reflects response in refractory/resistant multiple myeloma patients treated with bortezomib. Haematologica. 2008;93:451–454. doi: 10.3324/haematol.11852. [DOI] [PubMed] [Google Scholar]
- 138.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ramakrishnan V, Timm M, Haug JL, Kimlinger TK, Wellik LE, Witzig TE, Rajkumar SV, Adjei AA, Kumar S. Sorafenib, a dual Raf kinase/vascular endothelial growth factor receptor inhibitor has significant anti-myeloma activity and synergizes with common anti-myeloma drugs. Oncogene. 2010;29:1190–1202. doi: 10.1038/onc.2009.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Podar K, Catley LP, Tai YT, Shringarpure R, Carvalho P, Hayashi T, Burger R, Schlossman RL, Richardson PG, Pandite LN, Kumar R, Hideshima T, Chauhan D, Anderson KC. GW654652, the pan-inhibitor of VEGF receptors, blocks the growth and migration of multiple myeloma cells in the bone marrow microenvironment. Blood. 2004;103:3474–3479. doi: 10.1182/blood-2003-10-3527. [DOI] [PubMed] [Google Scholar]
- 141.Zangari M, Anaissie E, Stopeck A, Morimoto A, Tan N, Lancet J, Cooper M, Hannah A, Garcia-Manero G, Faderl S, Kantarjian H, Cherrington J, Albitar M, Giles FJ. Phase II study of SU5416, a small molecule vascular endothelial growth factor tyrosine kinase receptor inhibitor, in patients with refractory multiple myeloma. Clin Cancer Res. 2004;10(1 Pt 1):88–95. doi: 10.1158/1078-0432.CCR-0221-3. [DOI] [PubMed] [Google Scholar]
- 142.Kovacs MJ, Reece DE, Marcellus D, Meyer RM, Mathews S, Dong RP, Eisenhauer E. A phase II study of ZD6474 (Zactima, a selective inhibitor of VEGFR and EGFR tyrosine kinase in patients with relapsed multiple myeloma–NCIC CTG IND.145. Invest New Drugs. 2006;24:529–535. doi: 10.1007/s10637-006-9022-7. [DOI] [PubMed] [Google Scholar]
- 143.Prince HM, Hönemann D, Spencer A, Rizzieri DA, Stadtmauer EA, Roberts AW, Bahlis N, Tricot G, Bell B, Demarini DJ, Benjamin Suttle A, Baker KL, Pandite LN. Vascular endothelial growth factor inhibition is not an effective therapeutic strategy for relapsed or refractory multiple myeloma: a phase 2 study of pazopanib (GW786034) Blood. 2009;113:4819–4820. doi: 10.1182/blood-2009-02-207209. [DOI] [PubMed] [Google Scholar]