Abstract
A variety of tumor cells preferentially home to the bone. The homing of cancer cells to the bone represents a multi-step process that involves malignant progression of the tumor, invasion of the tumor through the extracellular matrix and the blood vessels and settling of the tumor cells in the bone. Gaining a greater understanding as to the mechanisms used by cancer cells in these processes will facilitate the design of drugs which could specifically target the homing process. In this review we will discuss the properties of tumor cells and the bone microenvironment which promote homing of a cancer cell to the bone. We will highlight the different steps and the molecular pathways involved when a cancer cell metastasize to the bone. Since bone is the major home for hematopoietic stem cells (HSCs), we will also highlight the similarities between the homing of cancer and HSC to the bone. Finally we will conclude with therapeutic and early detection strategies which can prevent homing of a cancer cell to the bone.
Keywords: Homing, Skeletal metastasis, Chemotaxis, Matrix degradation, Cytokines, Tumor bone cross-talk, Bone microenvironment, HSC niche
Introduction
Cancer is a metastatic disease. Theoretically cancer cells can metastasize towards many organs of the body. Clinically however it has been demonstrated that cancer cells prefer to migrate to certain distant organs of the body such as bone, lung, liver, brain or the adrenal gland. Some cancers such as breast, prostate, lung and thyroid carcinomas have higher tendency to migrate to the bone. The frequency of bone metastasis is about 70% in breast, melanoma, lung and prostate cancer while it is 15–30% in carcinoma of the colon, stomach, bladder, uterus, rectum thyroid or kidney [1]. More than 350,000 people die each year in the United States with bone metastases [2]. The number is probably 2–3 times higher if European Union and Japan are also included. In advanced breast and prostate cancer bone metastases accounts for considerable morbidity. Early detection and treatment of breast and prostate cancer has increased the 5 year survival rate to 98% and 100% respectively [3]. However after metastases the survival rate of breast cancer drops to 26% while that of prostate cancer drops to 33%.
Bone metastases are frequently associated with severe bone pain. The reason for bone pain is still poorly understood [4] but is thought to be a side effect of the osteolytic process. Patients with overt bone metastases present with severe symptoms, including leukoerythroblastic anemia, bone deformity, nerve-compression syndromes such as spinal cord-nerve compression, hypercalcemia and pathological fractures, which considerably reduce the quality of life [5].
In many patients several years after the resection of the primary tumor, patients develop bone metastases. Tumor progression in these patients has been attributed to the presence of disseminated tumor cells (DTCs) which home to the bone marrow and initially enter a dormant phase to evade apoptosis induced by factors in a foreign microenvironment [6]. These dormant DTCs have been observed clinically to be resistant to chemotherapy, a phenomenon known as minimal residual disease [7]. At some point some dormant DTCs switch to a proliferative phenotype which is highly aggressive in nature. Approximately 70% of 569 men undergoing radical prostatectomy had DTCs detected in their bone marrow. Persistence of DTCs in these patients was an independent predictor of recurrence [7]. Analysis of 4,703 women with primary breast cancer revealed that approximately 30% of the women harbored DTCs in their bone marrow at primary diagnosis in the absence of any signs of overt bone metastasis. An extended 10 year follow up of these women revealed a poorer prognosis as compared to those without DTCs [8]. These observations suggest that homing of DTCs to the marrow is an early event and detection of DTCs is a predictor for unfavorable prognosis. Since blood is a common transport system for tumor cells to travel to distant sites such as bone, detection of circulating tumor cells (CTCs) which are present in the peripheral blood can also be a predictor for bone metastases among tumors which normally home to the bone. CTCs are likely to have a shorter half-life compared with DTCs and therefore provide only a snap-shot of tumor cell dissemination but have been used successfully in breast cancer to predict tumor relapse [9, 10].
Knowledge about the mechanisms by which a cancer cell migrates towards bone is of great significance as it will facilitate in the design of drugs which could specifically target the homing process. Preventing cancer cells metastases to the bone could really increase the quality of life of cancer patients and decrease cancer related morbidity. In this review we will discuss the properties of tumor cells and the bone microenvironment which promote homing of a cancer cell to the bone. We will highlight the different steps and the molecular pathways involved when a cancer cell metastasize to the bone. Since bone is the major home for hematopoietic stem cells (HSCs), we will also highlight the similarities between the homing of cancer and HSC to the bone. Finally we will conclude with therapeutic and early detection strategies which can prevent homing of a cancer cell to the bone.
Types of Skeletal Metastasis
When a tumor cell grows in the bone it can either cause excess bone formation mediated by osteoblasts or excess bone resorption mediated by osteoclasts. Skeletal metastases are therefore classified as osteoblastic or osteolytic. Osteoblastic lesions are characteristic of prostate cancer while osteolytic lesions are characteristic of breast cancer. In spite of these classifications it is necessary to point out that bone formation and bone resorption events occur during both osteoblastic and osteolytic metastases. During autopsy bone metastasis are often heterogeneous within and between lesions [11] but copy number analysis indicate that they arise from a single precursor cancer cell [12].
Osteolytic bone metastasis is caused by factors secreted by the tumor which increase the activity of osteoclasts which are bone resorbing cells. Radiographically, osteolytic lesions appear as radiolucent areas, typically located in the skull or in the proximal end of the long bones. Histologically, one can observe tumor cells residing in the bone marrow and surrounded by osteoclasts [13, 14]. Osteolytic lesions may completely destroy the bone cortex allowing the tumor cells to infiltrate and move into the surrounding tissues. Osteoblastic lesions on the other hand are thought to result directly or indirectly from tumor derived factors that increase the activity of the bone forming cells, the osteoblasts. Radiographically, osteoblastic lesions appear as dense areas often located to the axial skeleton, particularly in vertebral bodies and the pelvis. Histologically, tumor cells residing in the bone marrow are surrounded by a high number of osteoblasts that form wide trabeculae of woven bone similar to that observed during primary ossifications [13, 14].
Homing, a Multistep Process
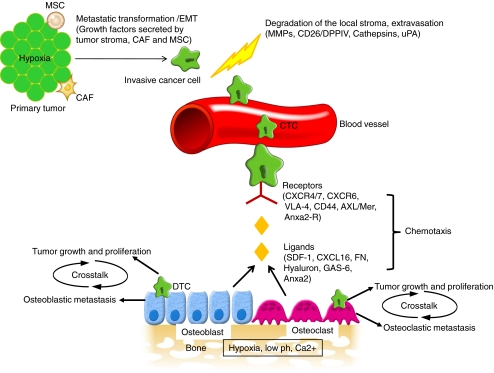
Homing of the cancer cell to the bone is a multi-step process. As shown in Fig. 1, cancer cells must at first detach from the primary tumor, invade the surrounding extracellular matrix and cross the endothelium to enter the blood stream. The cancer cells must move across the endothelium, invade more extracellular matrix before migrating and taking up residence in distant organs such as bone. The key events which lead to homing can be categorized as below.
Fig. 1.
Homing of the cancer cell to the bone is a multi-step process. Cancer cells from the primary tumor must first undergo metastatic transformation and EMT to acquire an invasive phenotype. Cells in the tumor stroma such as MSCs and CAFs, the hypoxic tumor micro-environment and the tumor itself promotes the release of growth factors which enables this transformation process. Several proteolytic enzymes such as MMPs, cathepsins, CD26/DPPIV and uPA help in the degradation process which allows the invasive cancer cells to detach from the basement membrane, invade through the extracellular matrix and extravasate across the endothelium to enter the blood stream. Cancer cells can survive in the blood stream as CTCs for a short period of time. This is followed by chemotaxis towards the bone. Certain receptors are over-expressed on the cancer cells such as CXCR4/CXCR7, CXCR6, CD44, VLA-4, Axl/Mer and Anxa2-R which during chemotaxis respond to ligands secreted or expressed by the bone marrow cells such as SDF-1, CXCL16, hyaluron, fibronection (FN), GAS-6, and Anxa2. After homing to the bone, cancer cells initially enter a dormant phase as DTCs to evade apoptosis in a foreign microenvironment. Years later these dormant DTCs can switch to a proliferative phenotype. Subsequently, crosstalk between the cancer cells and the bone microenvironment can lead to a vicious cycle of tumor proliferation and bone destruction
Metastatic Progression of the Primary Tumor
Primary tumors of epithelial origin often have interactions with the basement membrane that are very strong. In order to detach from the basement membrane the tumor cells undergo a process known as epithelial to mesenchymal transition (EMT). The process of EMT allows cancer cells to undergo biochemical changes to assume a mesenchymal phenotype [15]. This results in cancer cells which have enhanced migratory capacity, are highly invasive, have increased resistance to apoptosis and have an ability to degrade the extra cellular matrix (ECM) components. EMT is also an important phenomenon during embryogenesis and tissue regeneration [16–18]. Studies have shown that cancer cells undergoing EMT loose epithelial markers such as E-cadherin, N-cadherin, membrane bound β-catenin and gain mesenchymal markers such as α-smooth muscle actin (α-SMA) fibroblast-specific protein-1 (FSP1), vimentin and desmin [19]. A number of factors coming from the tumor stroma such as transforming growth factor-β (TGF-β), platelet derived growth factor (PDGF), epidermal growth factor (EGF) and hepatocyte growth factor (HGF) are thought to be important inducers of EMT. The process of EMT is not specific for bone metastasis but is an important event for the development of metastatic tumor cell lines. Very recently studies using immortalized human mammary epithelial cells have shown that the process of EMT increases the percent of cancer stem cells (CSC) within a tumor [20, 21]. CSCs are a rare population of tumor cells which possess self-renewal capacity that allows them to develop into any cells in the tumor. CSCs also have proliferative and invasive capacity which allows them to expand the tumor population and metastasize [22–25].
The tumor stroma comprising of basement membrane, immune cells, capillaries and fibroblasts also plays an important role in progression of tumor metastasis [26, 27]. Over expression of TGFβ and/or HGF in mouse fibroblasts induces the initiation of breast cancer within the normal human epithelium [28]. Cancer associated fibroblasts (CAFs) derived stromal cell-derived factor-1 (SDF-1) promote angiogenesis by recruiting bone-marrow-derived endothelial cells into breast canrcinoma cells. CAF secreted SDF-1 also increases the proliferation of MCF-Ras breast cancer cells [29]. In addition to secreting growth factors that directly affect cell motility, activated fibroblasts are a source of extra-cellular matrix (ECM) degrading proteases such as the matrix metalloproteinases (MMPs). MMPs allow cancer cells to cross tissue boundaries and escape the primary tumor site [30, 31]. Recent work has shown that exogenously administered mesenchymal-stem cells (MSCs) can enter the tumor stroma and promote metastasis in human breast carcinoma cells [32]. In another more recent study tissue engineered bone was used to demonstrate that endogenous bone marrow stem cells can migrate to a primary breast tumor and affect its growth and frequency of metastasis [33].
Apart from the different types of cells surrounding a primary tumor, the tumor microenvironment of solid tumors also contains regions of poor oxygenation and high acidity. Hypoxia stabilizes the hypoxia-inducible factor 1-α (HIF1-α) protein which in turn regulates a number of genes involved in metastatic progression such as vascular endothelial growth factor (VEGF), PDGF, TGF-β, interleukin-8 (IL-8), MMP9 and urokinase-type plasminogen activator receptor (uPAR) [34].
Degradation of the Local Stroma in Preparation for Metastasis to Bone
In order to leave or extravasate from the primary site of origin and to enter into the bone, cancer cells need to degrade the surrounding ECM. To achieve this, cancer cells secrete a wide variety of matrix degrading enzymes, some of which will be highlighted here.
Matrix metalloproteinases or MMPs are a large family of membrane-bound and secreted zinc dependent proteinases which help degrade the ECM and the hard, mineralized matrix of the bone [35]. They are produced not only by the cancer cells but also by the surrounding fibroblasts. MMPs have been shown to contribute to angiogenesis, invasion, migration, and final colonization of a metastatic site [36]. High levels of MMPs are produced by breast cancer cells and are shown to be associated with poor prognosis of the breast cancer [37, 38]. Studies have shown that MDA-MB-231 breast cancer cell line produces abundant amount of MMP-1 which is necessary for initiation of osteoclastic bone resorption [39]. MMP-2 and MMP-13 were recently identified as two genes highly expressed in bone metastases compared to brain metastases from breast cancer [40]. MMP-9 was shown to be significantly higher in cancerous prostate tissue as compared to normal prostate. PC3 cells expressing MMP-9 can induce pre-osteoclast motility in vitro. Motility is diminished when MMP-9 is silenced by siRNA, thus corroborating a role for MMP-9 in osteoclast recruitment [41]. Bone marrow stromal cells stimulate the invasive capacity of PC3 prostate cancer cells by inducing the expression of several MMPs at the RNA level and MMP-12 at the protein level. MMP-12 increases the invasive capacity of PC3 cells by degradation of type 1 collagen [42]. Interestingly SDF-1 mediated secretion of MMP-2 and MMP-9 by CD34+ progenitor stem cells facilitates in vitro migration of these cells towards SDF-1[43].
Cathepsins are a group of cysteine proteinases that are capable of degrading several ECM components including collagen IV, fibronectin and laminin. Cathepsins are mostly lysosomal, although there are secreted and membrane-associated forms. Cathepsins B and L have been implicated in cancer metastasis [44]. Cathepsin B activity is elevated in prostate cancer tissue samples compared to benign hyperplastic prostate tissue (BPH) and normal tissue samples [45] and it has been reported that cathepsin K is expressed in prostate cancer tissue and not in normal prostate [46].
Urokinase type plaminogen activator or uPA is a serine proteinase that catalyses the conversion of inactive plasminogen to plasmin, a very potent, broad-spectrum protease which can degrade many of the ECM components and activate several latent proteases (such as pro-collagenases) [47]. Overexpression of uPA by rat prostate cancer cells has been shown to induce bone metastases in vivo [48], and an amino-terminal fragment of uPA has been shown to have mitogenic activity for osteoblasts [49].
CD26/dipeptidylpeptidase IV (DPPIV) is a membrane-bound extracellular peptidase that cleaves dipeptides from the N-terminus of polypeptide chains. CD26/DPPIV can cleave the chemokine SDF-1alpha at its position two proline. Interestingly CD26/DPPIV is expressed by both prostate cancer cells and by CD34+ stem progenitor cells. In prostate cancer it triggers metastasis to the bone marrow while in stem cells it plays a role in homing and mobilization [50–52].
Another proteolytic mechanism that may be important for the metastasis of prostate cancer cells involves prostate-specific antigen (PSA), a serine protease that is overproduced by prostate cancer cells and is used as a marker of tumor burden. PSA can cleave parathyroid hormone related peptide (PTHrP) at the amino terminus and could also potentially activate other growth factors such as TGF-β that are produced by prostate carcinomas [53–56]. Interestingly, over expression of PSA in osteosarcoma cell line Saos-2 results in induction of receptor activator of nuclear factor κ-B ligand (RANKL), runt-related transcription factor 2 (RUNX2) and wingless type (WNT) signaling all of which are important in bone remodeling, thus indirectly suggesting a role of PSA in prostate cancer metastasis [57].
Chemotaxis towards Bone
There have been a number of studies which demonstrate that chemokines and their receptors play a pivotal role in chemotaxis of cancer cells towards organs such as the bone and the lymph nodes. Chemokines are a group of cytokines which bind to G-protein coupled receptors. Chemokines have been known to be involved in homing of various subsets of hematopoietic cells to the bone but several studies have shown their role in organ specific metastasis. One of the most well studied chemokine is stromal cell-derived factor-1 or SDF-1 or CXCL12. SDF-1 is a homeostatic chemokine which is secreted by the bone marrow stromal cells and by epithelial cells in many organs [58], bone marrow SDF-1 is principally produced by the osteoblasts in the endosteum region of the bone marrow [59]. SDF-1 signals through CXCR4 which is a seven transmembrane, G protein coupled receptor. CXCR4 has been shown to be expressed by a variety of cell types including hematopoietic, endothelial, stromal and neuronal cells [60].
Early bone marrow transplantation studies demonstrated that SDF-1 and its receptor CXCR4 were critical for bone marrow engraftment in severe combined immune-deficient (SCID) mice. While treatment with antibodies to CXCR4 prevents engraftment, stem cell factor (SCF) and interleukin-6 (IL-6) increases CXCR4 expression and increases engraftment [61]. Early studies have also shown that CD34+CD38-/lowCXCR4+ stem progenitor cells are capable of engrafting into non-obese diabetes/SCID (NOD/SCID) mice while CD34-CD38-/lowLin- cells are not. Granulocyte-colony stimulating factor (G-CSF) potentiates the homing capabilities of CD34+CD38+ cells and homing of CD34+ cells is inhibited by pretreatment with antibodies for CXCR4, integrins such as very late activation antigens (VLA-4 and VLA-5) and lymphocyte function associated antigen-1 (LFA-1). Homing is also prevented by agents such as pertusis toxin (inhibitor of G proteins) and chelerythrine chloride (protein kinase C inhibitor). This indicates that primitive bone marrow cells migrate to the bone in an integrin mediated SDF-1/protein kinase C signaling dependent mechanism [62]. Engraftment studies on NOD/SCID also shows that SDF1/CXCR4 activates several integrins such as VLA-4, VLA-5; and to a lesser degree, LFA-1 on CD34+ progenitor cells which enhances the process of engraftment [63, 64]. Incorporation and co-localization of CXCR4 and Rac-1 into lipid rafts could enhance the chemotaxis of hematopoietic stem progenitor cells (HSPC) towards the bone [65].
The SDF-1/CXCR4 pathway has also been shown to play a significant role in metastases of prostate cancer to the bone. Several prostate cancer cells lines PC3, DU145, C42B and LNCAP express high levels of CXCR4 [66]. Prostate cancer cells were observed migrating across bone marrow endothelial cell monolayers in response to SDF-1. Pretreatment of the prostate cancer cells with SDF-1 significantly increases their adhesion to osteosarcomas and endothelial cell lines in a dose-dependent manner. Invasion of the prostate cancer cell lines through basement membranes is supported by SDF-1 and inhibited by antibody to CXCR4 [66]. To confirm the role of SDF-1/CXCR4 axis in skeletal metastasis SDF-1 levels have been established in various mouse tissues by ELISA, immunohistochemistry, and in situ hybridization. Tissues harboring metastatic prostate cancer lesions express higher levels of SDF-1. SDF-1 levels are highest in the pelvis, tibia, femur, liver, and adrenal/kidneys compared with the lungs, tongue, and eye. Antibody to CXCR4 significantly reduces skeletal metastases [67]. High-density tissue microarrays constructed from clinical samples obtained from a cohort of over 600 patients demonstrates that CXCR4 protein expression is significantly elevated in localized and metastatic cancers [68]. It has also been demonstrated that metastatic human prostate cell lines DU145, LNCAP and PC3 express functional CXCR4 receptor and that SDF-1 enhances their migratory capabilities [69]. Another receptor for SDF-1 is CXCR7. Studies have shown that CXCR7 level increases as tumor becomes more aggressive. Both in vitro and in vivo experiments have shown that CXCR7 plays a role adhesion and invasiveness in prostate cancer cells [70]. CXCR7 regulates expression of CD44 and cadherin-11 both thought to be involved in invasiveness and IL-8 and VEGF, involved in angiogenesis in prostate cancer cells [70].
Breast cancer cells, malignant breast tumors and metastases highly express the chemokine receptors CXCR4 and CXCR7. Signaling through these receptors mediate actin polymerization, pseudopodia formation and induce chemotactic and invasive responses. Neutralizing antibodies against CXCR4 and CXCR7 significantly impaires breast cancer metastasis in vivo [71]. SDF-1 is highly secreted by bone and other common sites of breast cancer metastasis [72]. CXCR4 is one of a set of five genes over expressed in highly metastatic breast cancer cells and over expression of CXCR4 in MDA-MB-231 breast cancer cells significantly increases bone metastasis in nude mice [73]. CXCR4 expression by breast cancer cells has been shown to be regulated by VEGF [74] and the hypoxia induced HIF1-α pathway [75]. Increased expression of CXCR4 has been shown to be associated with poor prognosis in breast cancer [76]. Studies in other solid tumors such as rhabdomysarcoma and esophageal cancer have also illustrates that the SDF-1/CXCR4 axis plays a major role in skeletal metastasis.
Apart from solid tumors the SDF-1/CXCR4 pathway has been shown to be crucial for chemotaxis in leukemic cells. In B-cell acute lymphoblastic leukemia (ALL), B-cell precursor cells line NALM-6 and REH and even primary ALL cells have been shown to express CXCR4 and VLA-4 integrin and respond to the SDF-1 gradient while migrating towards bone marrow fibroblasts [77]. The migratory response of ALL cells to SDF-1 is dependent on p38 mitogen activated protein signaling [78]. Using NOD/SCID mice it was shown that CXCR4 participates in the homing of leukemic cells to the marrow [79]. Acute myeloid leukemia (AML) cells express CXCR4 which induces chemotaxis and invasion to the bone marrow stromal cells [80–82]. CXCR4 may also play a role in homing in AML [83] however, this study in particular is contradicted by other reports [84]. In chronic lymphoblastic leukemia, co-culture with SDF-1 induces chemotaxis to stromal cells in vitro [85]. Small peptide CXCR4 antagonists effectively blocks SDF-1 induced migration [86].
CXCL16, yet another chemokine and its receptor CXCR6 also may play a role in metastasis of prostate cancer cells. Highly metastatic cancer cell lines PC3 and C4-2B express more CXCR6 and CXCL16 mRNA than less aggressive prostate cancer LNCaP cells, nonneoplastic PrEC and RWPE-1 cells, and benign prostate tissues. Immunohistochemical examination of CXCR6 expression shows strong epithelial staining that correlates with Gleason score. Interleukin-1β and tumor necrosis factor α (TNF-α) significantly induces CXCL16 production by prostate epithelial cells, thereby indicating that inflammatory cytokines may play a role in the CXCL16 induction. CXCL16 was found to promote prostate cancer cell migration and invasion in vitro. [87].
Several cell to cell adhesion molecules also play a key role in chemotaxis of cancer cells to the bone. One such molecule is annexin2 (Anxa2). Anxa2 is a 36 kd peripheral membrane protein expressed by endothelial cells, early myeloid cells and osteoblasts [88]. Anxa2 expressed by osteoblasts and marrow endothelial cells, facilitate adhesion and homing of HSCs to the bone marrow niche and also regulates engraftment of HSCs following transplantation. Fewer HSCs are found in the marrow of Anxa2 deficient mice thus indicating that Anxa2 acts as an adhesion ligand for HSC homing [89]. Anxa2 is also associated with proliferative and invasive cancers including lung, pancreatic, brain, colon and gastric carcinomas, and is associated with poor prognosis [90–93]. It was shown that Anxa2 expressed by the osteoblasts serves as an adhesion molecule for prostate cancer cells. Prostate cancer cells express Anxa2 receptor (Anxa2-R) and blocking Anxa2 or Anxa2-R limits prostate cancer metastasis to the bone in animal models. Anxa2 also regulates prostate cancer proliferation and survival via the mitogen-activated protein kinase (MAPK) signaling pathway. [94].
Osteoblasts in the bone marrow secrete a ligand called growth arrest-specific 6 (GAS-6). An E2A/PBX1-positive ALL cell line RCH-ACV over expresses its tyrosine kinase receptor Mer. RCV-ACH cells stimulates GAS-6 production by osteoblasts and the GAS-6/Mer axis is important for the homing and survival of ALL cells [95]. Interestingly, gene expression analysis in prostate cancer cells treated with Anxa2 shows increase in expression of Axl which is also a tyrosine kinase receptor for GAS-6. Axl expression is correlated with metastasis and poor prognosis in a wide range of cancers such as breast cancer, prostate cancer, pancreatic cancer, ovarian cancer, renal cell carcinoma and esophageal adenocarcinoma [96–100]. Signaling through GAS-6 and its receptor Axl promotes invasion of prostate cancer cells towards the bone and regulates prostate cancer proliferation and survival [101].
CD44 and its ligand hyaluron may also play key role in metastasis of hematological malignancies and solid tumors to the bone marrow [102] and in homing and engraftment in CD34+ stem and progenitor cells [103]. Osteopontin (OPN) is produced mainly by the osteoblasts is a multi-domain phosphorylated glycoprotein responsible for cell to extracelluar matrix adhesion. OPN is highly expressed in trabecular bone along the endosteum [104]. Elevated OPN levels are observed in cancer of the breast, colon prostate and lung [105]. High OPN levels are associated with poorer outcomes in patients with breast cancer metastasis [106] and have been shown to promote metastasis in prostate [107] and mammary carcinoma [108, 109]. OPN regulates homing and invasion of tumor cells by binding with integrin and CD44 receptors [110]. Interestingly OPN has been implicated as an important regulator of migration of HSCs through the marrow and functionality of the HSC niche. In OPN knockout mice, HSCs are unable to engraft in the endosteal region, revealing that OPN is critical for HSC homing and retention [104].
Integrins are cell-surface proteins that faciliate cellular adhesion, and are comprised of non-covalently associated with alpha and beta subunits. Integrins bind to cell and mediate signals that regulate the activities of cytoplasmic kinases, growth factor receptors, and ion channels and control the organization of the intracellular actin cytoskeleton. [111]. Expression of alpha-v-beta-3(α5β3) integrins correlates with highly aggressive and metastatic breast and prostate cancer [112, 113]. VLA-4 integrin deregulation is consistently found in solid tumors and in AML and has shown to be responsible for tumor drug resistance [114–116][117]. VLA-4 and its ligand fibronectin are also important for HSC localization and retention to osteoblasts and the endosteal niche [118, 119].
In addition to integrins, cadherins are calcium-dependent binding proteins that facilitate cell-cell adhesion, and exhibit hemophilic binding (i.e. they serve as both a ligand and receptor). Knockdown of cadherin-11(also known as osteoblast cadherin) in bone greatly reduces metastasis of PC3 cells. On comparing human prostate cancer specimens it is observed that cadherin-11 is not expressed by normal prostate epithelial cells but is detected in prostate cancer [120]. Cadherin-11 expression increases from primary to metastatic prostate lesion found in bone and the lymph nodes. Exogenous expression of cadherin-11 in cadherin-11-negative C4-2B4 prostate cancer cells increases their spreading and intercalation into an osteoblast layer and also stimulates migration and invasiveness [120, 121]. In breast cancer cells cadherin-11 promotes homing and ostepclastogenesis. Cadherin-11 over expression in MDA-MB-231 breast cancer cells increases bone metastases and promoted bone resorption. Co-culture of MDA-MB-231 cells with MC3T3-E1 osteoblastic cells that constitutively express cadherin-11 results in an up-regulation of PTH-rP production in MDA-MB-231 cells. Co-culture also promotes osteoclastogeneis which is blocked by a PTHrP neutralizing peptide [122]. HSCs have been shown to express N-cadherin and cadherin-11 and therefore may maintain interactions with the mesenchymal stromal cells [123]. However their role in HSC homing remains controversial [124].
Cross Talk between the Cancer Cell and the Bone Microenvironment
Cancer has an inherent ability to metastasize to different organs of the body, yet certain cancer preferentially metastasizes to distant sites such as the bone. This phenomenon was first observed by Steven Paget who studied autopsy samples from 735 women who had died of breast cancer and found that a high percentage of them had skeletal metastasis. This led him to develop a “seed and soil hypothesis” which basically meant that tumor cells are seeds which will grow in favorable micro-environment provided by the bone which serves as the soil [reviewed in 125].
Bone is a highly enriched organ comprising of osteoblasts, osteoclasts, stromal cells, stems cells and mineralized bone matrix all surrounding a rich vascular bed. This makes bone marrow an ideal home for tumor cells. Crosstalk between the tumor cells and the bone microenvironment promotes several signaling pathways which trigger tumor growth and bone destruction. While, factors secreted by the tumor can stimulate osteoblasts or osteotoclasts in the bone, the bone in response secretes growth factors which drive tumor proliferation. This autocrine loop can overtime create a very aggressive form of tumor. Several growth factors and molecules may play a role in this cross-talk (e.g. TGF-β, insulin-such as growth factors 1 and 2 (IGF-1,2), fibroblast growth factors (FGF), PDGF, bone morphogenetic proteins (BMP), RUNX2, RANKL/Osteoprotegerin (RANKL/OPG),VEGF, IL-6, endothelins (ET) and WNT signaling [126]). A few of the critical players are reviewed as follows.
RANKL is a transmembrane signaling receptor of the TNF receptor superfamily that is expressed on the surface of osteoclast precursors [127]. RANKL binds with RANK and mediates osteoclast induced bone resorption which is important for bone remodeling [128]. Osteoprotogerin or OPG is a soluble member of the TNF receptor superfamily that is secreted by the osteoblasts. OPG acts as a decoy receptor for RANKL, preventing its interaction with RANK and thus inhibiting osteoclastogenesis. The ratio of RANKL to OPG determines the degree of osteoclastogenesis [129]. RANKL has been identified as a potential mediator of cancer-induced bone destruction in humans [130]. RANKL and OPG are increased in prostate cancer bone metastases versus those in primary tumors and soft-tissue metastases [131]. Soluble RANKL released from prostate cancer cells by MMP-7 is also thought to have a role in establishment of prostate cancer bone metastases and osteolysis associated with prostate cancer bone lesions [132]. In a SCID mouse model of multiple myeloma tumor RANKL inhibition with recombinant RANK-Fc protein not only reduces multiple myeloma induced osteolysis, but also causes a marked decline in tumor burden, as measured by serum protein levels and histology [133]. RANKL triggers migration of human epithelial cancer cells and melanoma cells that express the receptor RANK. Neutralization of RANKL by OPG in a mouse model of melanoma resulted in a marked reduction of tumor burden in bones but not in other organs. This further supports the role if RANKL in bone specific metastasis [134].
RUNX2 is a transcription factor that belongs to the runt domain gene family and functions by binding to the DNA as a heterodimer with Cbfb (core binding factor). RUNX2 is an essential factor for osteoblast differentiation [135, 136]. Metastatic breast cancer cells express bone sialoprotein, an important regulator of osteoblast differentiation under the control of RUNX2 and msh homeobox-2 (Msx2) transcription factors [137]. In normal bone marrow cells RUNX2 has been shown to promote osteoclast differentiation by inducing RANKL expression and suppressing OPG expression [138]. In MCF-7 and MDA-MB-231 breast cancer cell lines and prostate cancer cell lines RUNX2 transactivates expression of MMP-9, MMP-13 and VEGF indicating that RUNX2 contributes to the metastatic property of cancer cells [139]. RUNX2 mediates the responses of cells to signaling pathways hyperactive in tumors, including BMP/TGF-β and other growth factor signals. Inhibition of RUNX2 in MDA-MB-231 cells transplanted to bone decreases tumorigenesis and prevents osteolysis [140]. In the intra-tibial metastasis model, high RUNX2 levels is associated with development of large tumors and with increased expression of metastasis-related genes (MMP9, MMP13, VEGF, OPN) and secreted bone-resorbing factors (PTHrP, IL-8) [141]. RUNX2 siRNA treatment of PC3 cells decreases cell migration and invasion through matrigel in vitro. In vivo RUNX2 knockdown in PC3 cells blocks their ability to survive in the bone microenvironment. In co-cultures RUNX2 promotes osteoclastogenesis in PC3 cells and inhibits osteoblast activity. [141]. Induction of RUNX2 in C4-2B cells enhances their invasiveness and promotes cellular quiescence by blocking the G1/S phase transition during cell cycle progression [142]. All these results indicate that RUNX2 promotes new bone formation, invasion and homing of cancer cells to the bone.
Transforming growth factor-beta or TGF-β is known to promote several pathways which drives tumor genesis including EMT, bone remodeling, angiogenesis and suppression of the immune surveillance [143, 144]. There are 3 isoforms of TGF-β in humans, TGF-β1, TGF-β2 and TGF-β3. Active TGF-β isoforms bind to their transmembrane serine/threonine kinase receptor, namely type I and type II TGF-β receptors (TβRI and TβRII), that phosphorylates and activates the TGF-β-specific intracellular signaling mediators Smad2 and Smad3. Phosphorylated Smad2/3 then interacts with the Smad4, translocates to the nucleus, binds to specific DNA sequences and recruits co-activators or co-repressors to regulate the transcription of TGF-β target genes [145, 146].TGF-β is released by the bone during osteoclastic bone resorption [147]. Some of the known regulatory networks include TGF-β which increases PTHrP secretion from MDA-MB-231 breast cancer cells and thus promotes osteolytic metastasis in these cells [148]. PTHrP is known to induce osteoclastic bone resorption through the trans-activation of the RANKL gene [149, 150]. Neutralizing antibodies against PTHrP or inhibitors of its gene transcription decreases osteolytic metastases and tumor burden in cancer models [151]. TGF-β promotes osteolytic bone metastasis in 1205lu melanoma cells and inhibition of TGF-β signaling in these cells prevents osteolytic bone metastases and reduces expression of osteolytic factors, PTHrP and IL-11, chemotactic receptor CXCR4, and OPN [152]. Recently, glioblastoma associated oncogene family zinc finger 2 (Gli2) a mediator of the sonic hedgehog pathway was identified as a transcriptional target for TGF-β in melanoma. Gli2 expression in melanoma cell lines associates with loss of E-cadherin expression, increased matrigel invasion and higher bone metastases [153]. BMPs are a family of growth factors that belong to the TGF-β superfamily. They promote the differentiation of mesenchymal stem cells towards an osteoblastic lineage and are required for skeletal development and maintenance of adult bone homeostasis. BMPs signal through the Smad and MAPK pathways [154]. Mouse xenograft models of MDA-231-D, a highly metastatic human breast cancer cell shows higher phospho-Smad2 and phospho-Smad1/5/8 staining in the nuclei of primary tumor and in bone metastatic lesions indicative of both BMP and TGF-β signaling [155]. Functional in vivo bioluminescence imaging system shows that TGF-β and BMP-induced transcriptional pathways are active in these bone metastatic lesions. Furthermore, expression of dominant-negative receptors for TGF-β and BMPs in the MDA-231-D cells inhibits invasiveness in vitro and bone metastasis in vivo [155]. Prostate cancer cells co-cultured with osteoblast conditioned media has increased migration, higher cell surface expression of β1 or β3 integrin and increased MAPK and nuclear factor kappa-B (NF-κB) activation. However this is not observed when conditioned media from osteoblasts treated with BMP-2 siRNA are used [156]. Use of the BMP antagonist noggin and a RANKL antagonist effectively delays the development of osteolytic lesions, reducing bone loss and tumor burden in a mouse model of metastatic prostate cancer [157].
IGF-1 and IGF-2 are among the most abundant non-structural proteins present in the bone matrix [158]. KM1468, a novel antibody directed against human IGF-1 and IGF-2 given intraperitoneally to mice after inoculation of MDA PCa 2b prostate cancer markedly and dose-dependently suppresses the development of new bone tumors and the progression of established tumor foci and it also decreases serum PSA levels, compared with the control [159]. In neuroblastoma cells high IGF-I receptor expression resulted in increase in migration towards bone, adhesion with the bone marrow stromal cells and formation of osteolytic lesions [160].
Apart from the above mentioned growth factors physical factors of the bone such as hypoxia, acidic pH and calcium can also affect homing of the cancer cells to the bone. Bone is a hypoxic environment. Hypoxia in the bone normally functions in promoting hematopoiesis, maintaining stem cells in a pluripotent stage and also chondrocyte differentiation. Hypoxia is also known to promote tumorigenesis. At the primary tumor stage hypoxia induces EMT while in the bone hypoxia could increase transcription of factors which can further the vicious cycle of skeletal metastasis [161]. For example HIF1-α over expression was detected in lymph node and bone metastases examined in about 69% of prostate metastases and 29% of breast metastases [162]. HIF1-α also regulates the expression of other metastasis promoter factors such as adrenomedullin, CXCR4, and connective tissue growth factor (CTGF). [163, 164]. TGF-β potentiates HIF1-α signaling within the hypoxic bone microenvironment [165]. Hypoxia and HIF-1-α expression promotes the progression of bone metastases in breast cancer and leads to the development of osteolytic bone metastases by suppressing osteoblast differentiation and promoting osteoclastogenesis [166].
The acidic microenvironment of the bone also promotes bone metastases. Low pH results in increase in osteoclast mediated bone resorption and decrease in osteoblast mediated bone mineralization [167]. Acidosis alters cellular dynamics at the interface between the tumor and normal tissue, promoting apoptosis in adjacent normal cells and facilitating extracellular matrix degradation through the release of proteolytic enzymes [168]. Unlike normal cells, cancer cells have compensatory mechanisms to facilitate proliferation and metastasis even at low extracellular pH and thus are not susceptible to acid-induced apoptosis. Hypoxia further promotes acidosis within tumor cells through HIF-1-α mediated over expression of glycolytic enzymes and increased lactic acid production [169]. Together, hypoxia and pH regulatory mechanisms control survival and proliferation of tumor cell within the bone microenvironment.
Calcium is the primary inorganic component of the bone microenvironment. Active osteoclastic resorption can increase calcium levels from 1.1 to 1.3 mmol/L to rise up to 8 to 40 mmol/L [170]. Extracellular calcium-sensing receptor (CaSR), a G protein–coupled receptor, mediates the effect of calcium [171]. The CaSR is expressed in normal tissues and is over expressed in several types of cancer, including breast and prostate cancer where CaSR regulates the secretion of PTHrP a mechanism by which CaSR might promote skeletal metastasis [172, 173]. Knockdown of the CaSR by shRNA decreases PC3 cell proliferation in vitro and inhibited the formation of bone metastases in mice in vivo [174]. CaSR levels in breast cancer tumor samples positively correlates with the bone metastases. Thus CaSR may be a good potential marker for predicting bone metastases [175].
Cancer Cell and the HSC Niche
As discussed earlier in this review several molecules which play a role in homing of HSCs to the bone marrow are also used by tumor cells to metastasize and establish footholds in the marrow. Few of the common molecules which we discussed in this review are MMPs, CD26/DPPIV, CXCR4 or CXCR7/SDF-1 axis, integrins, Annexin2, CD44 and OPN (Table 1). HSC homing, quiescence and self-renewal in the bone marrow are now known to depend on a region termed the HSC niche [176, 177]. Recent study from our lab indicates that tumor cells specifically target the HSC niche during dissemination [178]. HSCs co-localize with prostate cancer cells to the endosteal bone surfaces both in vitro and in vivo suggesting a direct competition between prostate cancer cells and HSCs for niche occupancy. When the HSC niche is ablated, fewer prostate cancer cells go to the bone marrow. Conversely, increasing the number of HSC niches with PTH treatment, promotes tumor metastasis to the bone. Metastatic tumor cells just such as HSCs can be mobilized from the niche to the peripheral blood by using mobilizing agents such as G-CSF and AMD3100. Finally, disseminated prostate cancer cells reduce the number of HSCs in the bone marrow by driving HSCs into the progenitor pools and peripheral blood. All these observations strongly suggest that the HSC endosteal niche plays a significant role in establishing tumor metastasis in the bone marrow.
Table 1.
Common molecular pathways used by tumor cells and hematopoietic stem and progenitor cells in their homing to the bone marrow
| Event | Molecules | Function |
|---|---|---|
| Degradation of the ECM | Matrix metalloproteinases | Large family of membrane bound or secreted proteases. Promotes skeletal metastasis in prostate and breast cancer cells and bone homing in CD34+ progenitor cells. |
| CD26/dipeptidylpeptidase IV | Membrane bound peptidase which cleaves SDF-1 and drives homing of prostate cancer cells and CD34+ stem progenitor cells | |
| Chemotaxis towards bone | CXCR4 or CXCR7/SDF-1 axis | Chemokine and their G protein coupled receptors. Involved in chemotaxis of solid tumors, leukemias and hematopoietic stem progenitor cells towards bone. |
| Cell to cell adhesion | Integrins such as VLA-4, VLA-5 and LFA-1 | Cell surface proteins which promote bone metastasis in solid tumors and HSC localization and retention in the bone marrow niche. |
| Annexin2/Annexin 2 receptor axis | Peripheral membrane protein which drive prostate cancer bone metastasis and HSC homing and engraftment. | |
| CD44 and hyaluronic acid | Promotes hematological malignancies and solid tumor bone metastasis and CD34+ progenitor cells homing and engraftment. | |
| Osteopontin | A multi-domain phosphorylated glycoprotein responsible for cell to extracelluar matrix adhesion. Plays a critical role in breast and prostate tumor bone metastasis and HSC homing and retention. | |
| Cadherin-11 | Calcium dependent binding proteins that promote bone metastasis and bone resorption in prostate cancer and breast cancer cells. Highly produced by the HSCs but its role in HSC homing is not clear. |
Studies also demonstrate that bone marrow-derived hematopoietic progenitor cells (HPCs) express VEGF receptor 1 (VEGFR1) and home to tumor-specific pre-metastatic sites and form cellular clusters before the arrival of tumor cells [179]. Removal of these clusters prevents bone metastasis of B16 melanoma cells. These VEGFR1 positive HPCs express VLA-4 and tumor-specific growth factors upregulate expression of VLA-4 ligand fibronectin on resident fibroblasts, thus providing a permissive niche for incoming tumor cells.
Therapeutics
At present there are only a few drugs which are being used clinically to prevent bone metastasis. The good news however, is that several drugs which targets different aspects of bone metastasis are currently being evaluated in preclinical models and in clinical trials. This brightens the future for patients with skeletal metastasis and provides hope for the development of therapeutics for skeletal metastasis. Following is a summary of many of those agents which are currently employed to treat skeletal related events or appear to hold great promise in ongoing trials.
Bisphosphonates are now routinely used in clinical practice to reduce complications related to bone metastasis. Bisphosphonates are stable synthetic analogues of pyrophosphate with a P-C-P backbone which can reduce osteolytic bone resorption. Bisphosphonates such as zoledronic acid and ibandronate have been shown to significantly reduce skeletal related events (SREs) across a number of tumor sites including breast prostate, lung and kidney cancers, as well as multiple myeloma and they can even significantly ameliorate bone pain [180]. Denosumab a fully humanised monoclonal antibody (IgG2) that binds with high affinity and high specificity to RANKL inhibits osteoclast formation and bone resorption. Phase II clinical trials with Denosumab proved very promising for inhibiting bone resorption in patients with prostate cancer, breast cancer and other neoplasms [181]. Interestingly in a recent phase III clinical trial Denosumab proved better that zoledronic acid in preventing SREs in breast cancer patients.
TGF-β has been proved to be a valuable target for reducing cancer homing. A small molecule inhibitor of TGF-β receptor TβRI, SD-208 significantly inhibits osteolytic bone metastatis in nude mice inoculated with melanoma cell line [182]. In mice with established bone metastases, the size of osteolytic lesions was significantly reduced after 4 weeks treatment with SD-208 compared with vehicle-treated mice. BMP-7 an antagonist of TGF-β was used to treat nude mice inoculated with prostate cancer. BMP-7 treatment decreased growth of prostate cancer cells in the bone and also inhibited EMT progression in these tumor cells [183].
Bone-derived placental growth factor (PlGF), a homologue of VEGF-A has been shown to play a role in driving osteolytic bone metastasis. A neutralizing antibody against PIGF prevents skeletal metastasis of breast cancer in a nude mice model and also prevents engraftment of tumor cells in the bone [184]. Endothelins (ETs) and their receptors have emerged as a potential target in prostate cancer bone metastasis. ET-1 antagonists such as atrasentan (ABT-627) and ZD4054 are currently being clinically evaluated as a biological therapy for prostate cancer bone metastasis [185]. PSK1404, an antagonist of α5β3 integrin significantly inhibits bone metastasis in animal models of metastatic breast and ovarian cancer [186]. In myeloma administration of anti-VLA-4 antibodies reduces bone destruction and the number of osteoclasts in a nude mice model [187].
G-CSF and AMD3100, a small molecule inhibitor of CXCR4, both inhibit the SDF-1/CXCR4 axis and lead to mobilization of HSCs [188–190]. In animal models, AMD1300 treatment increases mobilization of myeloma cells into the circulation and increases their sensitivity to bortezomib [191]. Similarly in a mouse model of acute promyelocytic leukemia (APL), AMD3100 increases the number of APL cells in the blood and decreases tumor burden after treatment with chemotherapeutic drugs [192]. AMD3100 disrupts binding of small cell lung cancer cells (SCLC) to stromal cells and also increases sensitivity to the cytotoxic drug etopside [193]. Additionally, interferon gamma has been shown to reduce Annexin 2 expression in cells and thus limit the invasive capacity of prostate cancer cells [194].
Summary
The process of homing is complex and involves interplay of various factors. Specific features in the cancer cell and the bone microenvironment contribute to bone metastasis. Significant progress has been made in deciphering the mechanisms involved in homing of cancer cells to the bone. Yet much needs to be unveiled in this field particularly from the point of view of therapeutics. Apart from drug targets which target specific pathways, diagnosis and early detection of tumor metastasis may also reduce the rate of tumor metastasis. As discussed earlier in this review tumor cells share many common pathways with HSCs during homing and recent work in our lab indicates that prostate cancer cells and HSCs share the same niche in the bone marrow. This revelation will facilitate better understanding of the molecular events which leads to bone metastasis and thus lead to novel therapeutic opportunities for this fatal disease.
Acknowledgements
We thank Drs. Laurie K. McCauley and Evan T. Keller for scientific discussions. This work is directly supported by a Pediatric Oncology Research Fellowship (Y.S.). The National Cancer Institute (CA093900, K.J.P. and R.S.T., CA141426, R.S.T.), the Department of Defense (Y.S., K.J.P. and R.S.T) and the Prostate Cancer Foundation (K.J.P. and R.S.T.). K.J.P. receives support as an American Cancer Society Clinical Research Professor, NIH SPORE in prostate cancer grant P50 CA69568, and the Cancer Center support grant P30 CA46592.
References
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Mantyh PW, et al. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002;2(3):201–209. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006;5(16):1744–1750. doi: 10.4161/cc.5.16.2864. [DOI] [PubMed] [Google Scholar]
- 7.Morgan TM, et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15(2):677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun S, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 9.Cristofanilli M, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 10.Pierga JY, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14(21):7004–7010. doi: 10.1158/1078-0432.CCR-08-0030. [DOI] [PubMed] [Google Scholar]
- 11.Roudier M. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34(7):646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15(5):559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 14.Guise TA, et al. Basic Mechanisms Responsible for Osteolytic and Osteoblastic Bone Metastases. Clin Cancer Res. 2006;12(20):6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 1996;156(3):202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- 17.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 18.Zeisberg M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282(32):23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 19.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 20.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morel AP, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 23.Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 26.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76(1):69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6(7):506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 28.Kuperwasser C, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101(14):4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 31.Sternlicht MD, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98(2):137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein RH, et al. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010;70(24):10044–10050. doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20(3):237–250. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- 35.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7(1):14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 36.Wilson TJ, Singh RK. Proteases as modulators of tumor-stromal interaction: primary tumors to bone metastases. Biochim Biophys Acta. 2008;1785(2):85–95. doi: 10.1016/j.bbcan.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachmeier BE, et al. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 2001;21(6A):3821–3828. [PubMed] [Google Scholar]
- 38.Nakopoulou L, et al. MMP-2 protein in invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Res Treat. 2003;77(2):145–155. doi: 10.1023/a:1021371028777. [DOI] [PubMed] [Google Scholar]
- 39.Eck SM, et al. Matrix metalloproteinase-1 promotes breast cancer angiogenesis and osteolysis in a novel in vivo model. Breast Cancer Res Treat. 2009;116(1):79–90. doi: 10.1007/s10549-008-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein A, et al. Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett. 2009;276(2):212–220. doi: 10.1016/j.canlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Dong Z, et al. Matrix metalloproteinase activity and osteoclasts in experimental prostate cancer bone metastasis tissue. Am J Pathol. 2005;166(4):1173–1186. doi: 10.1016/S0002-9440(10)62337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nabha SM, et al. Bone marrow stromal cells enhance prostate cancer cell invasion through type I collagen in an MMP-12 dependent manner. Int J Cancer. 2008;122(11):2482–2490. doi: 10.1002/ijc.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janowska-Wieczorek A, et al. Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Exp Hematol. 2000;28(11):1274–1285. doi: 10.1016/s0301-472x(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 44.Sloane BF. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990;1(2):137–152. [PubMed] [Google Scholar]
- 45.Sinha AA, et al. Ratio of cathepsin B to stefin A identifies heterogeneity within Gleason histologic scores for human prostate cancer. Prostate. 2001;48(4):274–284. doi: 10.1002/pros.1107. [DOI] [PubMed] [Google Scholar]
- 46.Brubaker KD, et al. Cathepsin K mRNA and protein expression in prostate cancer progression. J Bone Miner Res. 2003;18(2):222–230. doi: 10.1359/jbmr.2003.18.2.222. [DOI] [PubMed] [Google Scholar]
- 47.Testa JE, Quigley JP. The role of urokinase-type plasminogen activator in aggressive tumor cell behavior. Cancer Metastasis Rev. 1990;9(4):353–367. doi: 10.1007/BF00049524. [DOI] [PubMed] [Google Scholar]
- 48.Achbarou A, et al. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 1994;54(9):2372–2377. [PubMed] [Google Scholar]
- 49.Rabbani SA, et al. An amino-terminal fragment of urokinase isolated from a prostate cancer cell line (PC-3) is mitogenic for osteoblast-such as cells. Biochem Biophys Res Commun. 1990;173(3):1058–1064. doi: 10.1016/s0006-291x(05)80893-9. [DOI] [PubMed] [Google Scholar]
- 50.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169(12):7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 51.Sun YX, et al. CD26/dipeptidyl peptidase IV regulates prostate cancer metastasis by degrading SDF-1/CXCL12. Clin Exp Metastasis. 2008;25(7):765–776. doi: 10.1007/s10585-008-9188-9. [DOI] [PubMed] [Google Scholar]
- 52.Christopherson KW, 2nd, et al. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305(5686):1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 53.Cramer SD, Chen Z, Peehl DM. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-such as domain: inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J Urol. 1996;156(2 Pt 1):526–531. doi: 10.1097/00005392-199608000-00076. [DOI] [PubMed] [Google Scholar]
- 54.Dallas SL, et al. Dual role for the latent transforming growth factor-beta binding protein in storage of latent TGF-beta in the extracellular matrix and as a structural matrix protein. J Cell Biol. 1995;131(2):539–549. doi: 10.1083/jcb.131.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwamura M, et al. Alteration of the hormonal bioactivity of parathyroid hormone-related protein (PTHrP) as a result of limited proteolysis by prostate-specific antigen. Urology. 1996;48(2):317–325. doi: 10.1016/S0090-4295(96)00182-3. [DOI] [PubMed] [Google Scholar]
- 56.Killian CS, et al. Mitogenic response of osteoblast cells to prostate-specific antigen suggests an activation of latent TGF-beta and a proteolytic modulation of cell adhesion receptors. Biochem Biophys Res Commun. 1993;192(2):940–947. doi: 10.1006/bbrc.1993.1506. [DOI] [PubMed] [Google Scholar]
- 57.Nadiminty N, et al. Prostate-specific antigen modulates genes involved in bone remodeling and induces osteoblast differentiation of human osteosarcoma cell line SaOS-2. Clin Cancer Res. 2006;12(5):1420–1430. doi: 10.1158/1078-0432.CCR-05-1849. [DOI] [PubMed] [Google Scholar]
- 58.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10(3):179–185. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 59.Ponomaryov T, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106(11):1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loetscher M, et al. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269(1):232–237. [PubMed] [Google Scholar]
- 61.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 62.Kollet O, et al. Rapid and efficient homing of human CD34(+)CD38(−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97(10):3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 63.Peled A, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104(9):1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peled A, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95(11):3289–3296. [PubMed] [Google Scholar]
- 65.Wysoczynski M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105(1):40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 66.Taichman RS, et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–1837. [PubMed] [Google Scholar]
- 67.Sun YX, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20(2):318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 68.Sun YX, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 69.Arya M, et al. The importance of the CXCL12-CXCR4 chemokine ligand-receptor interaction in prostate cancer metastasis. J Exp Ther Oncol. 2004;4(4):291–303. [PubMed] [Google Scholar]
- 70.Wang J, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283(7):4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 71.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 72.Sloan EK, Anderson RL. Genes involved in breast cancer metastasis to bone. Cell Mol Life Sci. 2002;59(9):1491–1502. doi: 10.1007/s00018-002-8524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 74.Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006;238(1):30–41. doi: 10.1016/j.canlet.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 75.Shim H, et al. Lower expression of CXCR4 in lymph node metastases than in primary breast cancers: potential regulation by ligand-dependent degradation and HIF-1alpha. Biochem Biophys Res Commun. 2006;346(1):252–258. doi: 10.1016/j.bbrc.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 76.Salvucci O, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97(3):275–283. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 77.Bradstock KF, et al. Effects of the chemokine stromal cell-derived factor-1 on the migration and localization of precursor-B acute lymphoblastic leukemia cells within bone marrow stromal layers. Leukemia. 2000;14(5):882–888. doi: 10.1038/sj.leu.2401729. [DOI] [PubMed] [Google Scholar]
- 78.Bendall LJ, et al. Defective p38 mitogen-activated protein kinase signaling impairs chemotaxic but not proliferative responses to stromal-derived factor-1alpha in acute lymphoblastic leukemia. Cancer Res. 2005;65(8):3290–3298. doi: 10.1158/0008-5472.CAN-04-3402. [DOI] [PubMed] [Google Scholar]
- 79.Spiegel A, et al. Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103(8):2900–2907. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 80.Mohle R, et al. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91(12):4523–4530. [PubMed] [Google Scholar]
- 81.Voermans C, et al. Migratory behavior of leukemic cells from acute myeloid leukemia patients. Leukemia. 2002;16(4):650–657. doi: 10.1038/sj.leu.2402431. [DOI] [PubMed] [Google Scholar]
- 82.Burger JA, et al. CXCR4 chemokine receptors (CD184) and alpha4beta1 integrins mediate spontaneous migration of human CD34+ progenitors and acute myeloid leukaemia cells beneath marrow stromal cells (pseudoemperipolesis) Br J Haematol. 2003;122(4):579–589. doi: 10.1046/j.1365-2141.2003.04466.x. [DOI] [PubMed] [Google Scholar]
- 83.Tavor S, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004;64(8):2817–2824. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- 84.Monaco G, et al. Engraftment of acute myeloid leukemia in NOD/SCID mice is independent of CXCR4 and predicts poor patient survival. Stem Cells. 2004;22(2):188–201. doi: 10.1634/stemcells.22-2-188. [DOI] [PubMed] [Google Scholar]
- 85.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94(11):3658–3667. [PubMed] [Google Scholar]
- 86.Burger M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106(5):1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 87.Lu Y, et al. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol Cancer Res. 2008;6(4):546–554. doi: 10.1158/1541-7786.MCR-07-0277. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi S, et al. Cloning and identification of annexin II as an autocrine/paracrine factor that increases osteoclast formation and bone resorption. J Biol Chem. 1994;269(46):28696–28701. [PubMed] [Google Scholar]
- 89.Jung Y, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110(1):82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cole SP, et al. Elevated expression of annexin II (lipocortin II, p36) in a multidrug resistant small cell lung cancer cell line. Br J Cancer. 1992;65(4):498–502. doi: 10.1038/bjc.1992.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Emoto K, et al. Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer. 2001;92(6):1419–1426. doi: 10.1002/1097-0142(20010915)92:6<1419::aid-cncr1465>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 92.Roseman BJ, et al. Annexin II marks astrocytic brain tumors of high histologic grade. Oncol Res. 1994;6(12):561–567. [PubMed] [Google Scholar]
- 93.Vishwanatha JK, et al. Enhanced expression of annexin II in human pancreatic carcinoma cells and primary pancreatic cancers. Carcinogenesis. 1993;14(12):2575–2579. doi: 10.1093/carcin/14.12.2575. [DOI] [PubMed] [Google Scholar]
- 94.Shiozawa Y, et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105(2):370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shiozawa Y, Pedersen EA, Taichman RS. GAS6/Mer axis regulates the homing and survival of the E2A/PBX1-positive B-cell precursor acute lymphoblastic leukemia in the bone marrow niche. Exp Hematol. 2010;38(2):132–140. doi: 10.1016/j.exphem.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gjerdrum C, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA. 2010;107(3):1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koorstra JB, et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009;8(7):618–626. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rankin EB, et al. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010;70(19):7570–7579. doi: 10.1158/0008-5472.CAN-10-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hector A, et al. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther. 2010;10(10):1009–1018. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gustafsson A, et al. Gas6 and the receptor tyrosine kinase Axl in clear cell renal cell carcinoma. PLoS One. 2009;4(10):e7575. doi: 10.1371/journal.pone.0007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiozawa Y, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12(2):116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hill A, et al. The emerging role of CD44 in regulating skeletal micrometastasis. Cancer Lett. 2006;237(1):1–9. doi: 10.1016/j.canlet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Avigdor A, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103(8):2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 104.Nilsson SK, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 105.Fedarko NS, et al. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7(12):4060–4066. [PubMed] [Google Scholar]
- 106.Rudland PS, et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62(12):3417–3427. [PubMed] [Google Scholar]
- 107.Thalmann GN, et al. Osteopontin: possible role in prostate cancer progression. Clin Cancer Res. 1999;5(8):2271–2277. [PubMed] [Google Scholar]
- 108.Tuck AB, et al. Osteopontin and p53 expression are associated with tumor progression in a case of synchronous, bilateral, invasive mammary carcinomas. Arch Pathol Lab Med. 1997;121(6):578–584. [PubMed] [Google Scholar]
- 109.Tuck AB, et al. Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene. 1999;18(29):4237–4246. doi: 10.1038/sj.onc.1202799. [DOI] [PubMed] [Google Scholar]
- 110.Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res. 2004;121(2):228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 111.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 112.Furger KA, et al. Beta(3) integrin expression increases breast carcinoma cell responsiveness to the malignancy-enhancing effects of osteopontin. Mol Cancer Res. 2003;1(11):810–819. [PubMed] [Google Scholar]
- 113.Sun YX, et al. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67(1):61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 114.Sethi T, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5(6):662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 115.Hodkinson PS, et al. ECM overrides DNA damage-induced cell cycle arrest and apoptosis in small-cell lung cancer cells through beta1 integrin-dependent activation of PI3-kinase. Cell Death Differ. 2006;13(10):1776–1788. doi: 10.1038/sj.cdd.4401849. [DOI] [PubMed] [Google Scholar]
- 116.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20(36):4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 117.Matsunaga T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9(9):1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 118.Verfaillie CM. Adhesion receptors as regulators of the hematopoietic process. Blood. 1998;92(8):2609–2612. [PubMed] [Google Scholar]
- 119.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105(7):2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 120.Chu K, et al. Cadherin-11 promotes the metastasis of prostate cancer cells to bone. Mol Cancer Res. 2008;6(8):1259–1267. doi: 10.1158/1541-7786.MCR-08-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang CF, et al. Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res. 2010;70(11):4580–4589. doi: 10.1158/0008-5472.CAN-09-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tamura D, et al. Cadherin-11-mediated interactions with bone marrow stromal/osteoblastic cells support selective colonization of breast cancer cells in bone. Int J Oncol. 2008;33(1):17–24. [PubMed] [Google Scholar]
- 123.Wein F, et al. N-cadherin is expressed on human hematopoietic progenitor cells and mediates interaction with human mesenchymal stromal cells. Stem Cell Res. 2010;4(2):129–139. doi: 10.1016/j.scr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 124.Kiel MJ, et al. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4(2):170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fidler IJ, Kim S-J, Langley RR. The role of the organ microenvironment in the biology and therapy of cancer metastasis. J Cell Biochem. 2007;101(4):927–936. doi: 10.1002/jcb.21148. [DOI] [PubMed] [Google Scholar]
- 126.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328(3):679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 127.Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 128.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 129.Simonet WS, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 130.Grimaud E, et al. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol. 2003;163(5):2021–2031. doi: 10.1016/s0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brown JM, et al. Osteoprotegerin and rank ligand expression in prostate cancer. Urology. 2001;57(4):611–616. doi: 10.1016/s0090-4295(00)01122-5. [DOI] [PubMed] [Google Scholar]
- 132.Lynch CC, et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7(5):485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 133.Sordillo EM, Pearse RN. RANK-Fc: a therapeutic antagonist for RANK-L in myeloma. Cancer. 2003;97(3 Suppl):802–812. doi: 10.1002/cncr.11134. [DOI] [PubMed] [Google Scholar]
- 134.Jones DH, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 135.Miller J, et al. The core-binding factor beta subunit is required for bone formation and hematopoietic maturation. Nat Genet. 2002;32(4):645–649. doi: 10.1038/ng1049. [DOI] [PubMed] [Google Scholar]
- 136.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 137.Barnes GL, et al. Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003;63(10):2631–2637. [PubMed] [Google Scholar]
- 138.Enomoto H, et al. Induction of osteoclast differentiation by Runx2 through receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin regulation and partial rescue of osteoclastogenesis in Runx2-/- mice by RANKL transgene. J Biol Chem. 2003;278(26):23971–23977. doi: 10.1074/jbc.M302457200. [DOI] [PubMed] [Google Scholar]
- 139.Pratap J, et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25(19):8581–8591. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pratap J, et al. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 2006;25(4):589–600. doi: 10.1007/s10555-006-9032-0. [DOI] [PubMed] [Google Scholar]
- 141.Akech J, et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29(6):811–821. doi: 10.1038/onc.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baniwal SK, et al. Runx2 transcriptome of prostate cancer cells: insights into invasiveness and bone metastasis. Mol Cancer. 2010;9:258. doi: 10.1186/1476-4598-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23(9):2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 144.Mohammad KS, et al. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One. 2009;4(4):e5275. doi: 10.1371/journal.pone.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 146.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 147.Dallas SL, et al. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J Biol Chem. 2002;277(24):21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 148.Kakonen SM, et al. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem. 2002;277(27):24571–24578. doi: 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- 149.Kitazawa S, Kitazawa R. RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J Pathol. 2002;198(2):228–236. doi: 10.1002/path.1199. [DOI] [PubMed] [Google Scholar]
- 150.Kondo H, Guo J, Bringhurst FR. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J Bone Miner Res. 2002;17(9):1667–1679. doi: 10.1359/jbmr.2002.17.9.1667. [DOI] [PubMed] [Google Scholar]
- 151.Gallwitz WE, Guise TA, Mundy GR. Guanosine nucleotides inhibit different syndromes of PTHrP excess caused by human cancers in vivo. J Clin Invest. 2002;110(10):1559–1572. doi: 10.1172/JCI11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Javelaud D, et al. Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Res. 2007;67(5):2317–2324. doi: 10.1158/0008-5472.CAN-06-3950. [DOI] [PubMed] [Google Scholar]
- 153.Alexaki VI, et al. GLI2-mediated melanoma invasion and metastasis. J Natl Cancer Inst. 2010;102(15):1148–1159. doi: 10.1093/jnci/djq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7(1–2):51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 155.Katsuno Y, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27(49):6322–6333. doi: 10.1038/onc.2008.232. [DOI] [PubMed] [Google Scholar]
- 156.Lai TH, et al. Osteoblasts-derived BMP-2 enhances the motility of prostate cancer cells via activation of integrins. Prostate. 2008;68(12):1341–1353. doi: 10.1002/pros.20799. [DOI] [PubMed] [Google Scholar]
- 157.Virk MS, et al. Influence of simultaneous targeting of the bone morphogenetic protein pathway and RANK/RANKL axis in osteolytic prostate cancer lesion in bone. Bone. 2009;44(1):160–167. doi: 10.1016/j.bone.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hauschka PV, et al. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem. 1986;261(27):12665–12674. [PubMed] [Google Scholar]
- 159.Goya M, et al. Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice by a ligand-specific antibody to human insulin-such as growth factors. Cancer Res. 2004;64(17):6252–6258. doi: 10.1158/0008-5472.CAN-04-0919. [DOI] [PubMed] [Google Scholar]
- 160.Golen CM, et al. Insulin-such as growth factor-I receptor expression regulates neuroblastoma metastasis to bone. Cancer Res. 2006;66(13):6570–6578. doi: 10.1158/0008-5472.CAN-05-1448. [DOI] [PubMed] [Google Scholar]
- 161.Kingsley LA, et al. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6(10):2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 162.Zhong H, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5835. [PubMed] [Google Scholar]
- 163.Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23(3–4):293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 164.Garayoa M, et al. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol Endocrinol. 2000;14(6):848–862. doi: 10.1210/mend.14.6.0473. [DOI] [PubMed] [Google Scholar]
- 165.McMahon S, et al. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281(34):24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 166.Hiraga T, et al. Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res. 2007;67(9):4157–4163. doi: 10.1158/0008-5472.CAN-06-2355. [DOI] [PubMed] [Google Scholar]
- 167.Brandao-Burch A, et al. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005;77(3):167–174. doi: 10.1007/s00223-004-0285-8. [DOI] [PubMed] [Google Scholar]
- 168.Webb SD, Sherratt JA, Fish RG. Alterations in proteolytic activity at low pH and its association with invasion: a theoretical model. Clin Exp Metastasis. 1999;17(5):397–407. doi: 10.1023/a:1006667303583. [DOI] [PubMed] [Google Scholar]
- 169.Shannon AM, et al. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29(4):297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 170.Berger CE, et al. Scanning electrochemical microscopy at the surface of bone-resorbing osteoclasts: evidence for steady-state disposal and intracellular functional compartmentalization of calcium. J Bone Miner Res. 2001;16(11):2092–2102. doi: 10.1359/jbmr.2001.16.11.2092. [DOI] [PubMed] [Google Scholar]
- 171.Chattopadhyay N. Effects of calcium-sensing receptor on the secretion of parathyroid hormone-related peptide and its impact on humoral hypercalcemia of malignancy. Am J Physiol Endocrinol Metab. 2006;290(5):E761–E770. doi: 10.1152/ajpendo.00350.2005. [DOI] [PubMed] [Google Scholar]
- 172.Sanders JL, et al. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology. 2000;141(12):4357–4364. doi: 10.1210/endo.141.12.7849. [DOI] [PubMed] [Google Scholar]
- 173.Sanders JL, et al. Ca(2+)-sensing receptor expression and PTHrP secretion in PC-3 human prostate cancer cells. Am J Physiol Endocrinol Metab. 2001;281(6):E1267–E1274. doi: 10.1152/ajpendo.2001.281.6.E1267. [DOI] [PubMed] [Google Scholar]
- 174.Liao J, et al. Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 2006;66(18):9065–9073. doi: 10.1158/0008-5472.CAN-06-0317. [DOI] [PubMed] [Google Scholar]
- 175.Mihai R, et al. Expression of the calcium receptor in human breast cancer–a potential new marker predicting the risk of bone metastases. Eur J Surg Oncol. 2006;32(5):511–515. doi: 10.1016/j.ejso.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 176.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 177.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116(5):1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shiozawa Y, et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121(4):1298–1312 [DOI] [PMC free article] [PubMed]
- 179.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roelofs AJ, et al. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12(20 Pt 2):6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 181.Fizazi K, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 182.Mohammad KS, et al. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011;71(1):175–184. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Buijs JT, et al. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol. 2007;171(3):1047–1057. doi: 10.2353/ajpath.2007.070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Coenegrachts L, et al. Anti-placental growth factor reduces bone metastasis by blocking tumor cell engraftment and osteoclast differentiation. Cancer Res. 2010;70(16):6537–6547. doi: 10.1158/0008-5472.CAN-09-4092. [DOI] [PubMed] [Google Scholar]
- 185.Carducci MA, Jimeno A. Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin Cancer Res. 2006;12(20 Pt 2):6296s–6300s. doi: 10.1158/1078-0432.CCR-06-0929. [DOI] [PubMed] [Google Scholar]
- 186.Zhao Y, et al. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67(12):5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 187.Mori Y, et al. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104(7):2149–2154. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 188.Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 189.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 190.Abkowitz JL, et al. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102(4):1249–1253. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 191.Azab AK, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nervi B, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113(24):6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Burger JA, Stewart DJ. CXCR4 chemokine receptor antagonists: perspectives in SCLC. Expert Opin Investig Drugs. 2009;18(4):481–490. doi: 10.1517/13543780902804249. [DOI] [PubMed] [Google Scholar]
- 194.Hastie C, et al. Interferon-gamma reduces cell surface expression of annexin 2 and suppresses the invasive capacity of prostate cancer cells. J Biol Chem. 2008;283(18):12595–12603. doi: 10.1074/jbc.M800189200. [DOI] [PMC free article] [PubMed] [Google Scholar]