Abstract
The effects of lysophospholipids (LPLs) on cancer microenvironment is a vast and growing field. These lipids are secreted physiologically by various cell types. They play highly important roles in the development, activation and regulation of the immune system. They are also secreted by cancerous cells and there is a strong association between LPLs and cancer. It is clear that these lipids and in particular sphingosine 1-phosphate (S1P) and lysophosphatidic acid (LPA) play major roles in regulating the growth of tumor cells, and in manipulating the immune system. These activities can be divided into two parts; the first involves the ability of S1P and LPA to either directly or through some of the enzymes that generate them such as sphingosine kinases or phospholipases, induce the motility and invasiveness of tumor cells. The second mechanism involves the recently discovered effects of these lipids on the anti-tumor effector natural killer (NK) cells. Whereas S1P and LPA induce the recruitment of these effector cells, they also inhibit their cytolysis of tumor cells. This may support the environment of cancer and the ability of cancer cells to grow, spread and metastasize. Consequently, LPLs or their receptors may be attractive targets for developing drugs in the treatment of cancer where LPLs or their receptors are up-regulated.
Keywords: Lysophospholipids, Cancer, Sphingosine 1-phosphate, Lysophosphatidic acid
Introduction
The progression of malignant diseases occurs through bilateral actions of cells and their microenvironment. Cells such as vascular endothelium, fibroblasts, immune cells and soluble factors comprise the microenvironment of cancer cells, affecting features of the disease such as angiogenesis, growth, metastasis and many more activities. Numerous agents with promising results from experimental models have failed to translate into prolonged survival of cancer patients as well as reductions in endpoints such as metastatic disease and tumor size. This has led to increased interest in the field of tumor microenvironment, as it bears promising possibilities for early prevention of cancer.
Lysophospholipids (LPLs) are derived from various cells including platelets, endothelium and red blood cells under physiological conditions, but are also secreted by cancer cells. These molecules were first discovered as constituents of cell membranes, and endothelium was later shown to exert multiple functions as a response to these growth factors, hence, their receptors were initially named endothelial differentiation gene (Edg), but were renamed as S1P1, S1P2, S1P3, S1P4, and S1P5, those that bind S1P. All these receptors are coupled to G proteins (GPCRs) [1]. The different receptors have been thoroughly reviewed and are beyond our scope [1–4]. In short, virtually all cells that engage in the immune response express LPL receptors, and antibodies to these receptors as well as receptor-null mice have provided us with insights into the importance of combined effects of the different receptors on various cellular activities.
After the detection of various receptors, research in the field of LPLs has been extensive, opening new doors to understanding the critical roles these compounds play in central processes of the cancer microenvironment, as they stimulate angiogenesis, are anti-apoptotic, and they modulate the immune response through extravasation and activation of leukocytes. It is thus clear that LPLs play a crucial role in shaping the environment around cancer cells and the development of cancer tissues. In this review, we will summarize the different roles of LPLs in the microenvironment of tumor cells. However, the review is not meant to discuss all aspects of LPLs in cancer, as a search in PubMed gives more than 700 hits for LPA and cancer, and more than 500 hits for S1P and cancer.
The two major classes of LPLs, lysoglycerophospholipids and lysosphingophospholipids are exemplified by lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), respectively [1–5]. As an example of the growth-regulated potentials, LPA is mitogenic or antimitogenic for different cells [6], and both S1P and LPA protect T cells from apoptosis [7]. LPLs are important regulators of most stages in cancer development as they affect ovarian cancer cells in terms of adhesion and migration [8], invasion [9] and metastasis [9, 10]. More than 10 years ago it was suggested that LPA may constitute a marker for ovarian cancer patients since it is highly increased in both the serum and ascitic fluids of women with this disease [11]. Recently it has been established that LPA levels measured by non-invasive method in ovarian cancer patients are associated with histological stages of the disease [12]. In addition, autotoxin (ATX) which produces LPA is increased in the plasma of patients with B-cell neoplasm, and in particular follicular lymphomas [13], suggesting that the levels of ATX and/or LPA could be used as a biomarker for this disease.
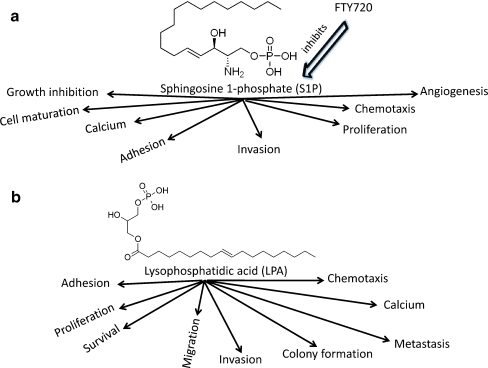
Many cell types produce S1P and LPA [14–16]. In the blood, erythrocytes, peripheral mononuclear cells and neutrophils contribute to S1P production in the resting state but yield little secretion after stimulation, while a large part of the platelet-derived S1P is secreted upon stimulation [17, 18]. Concerning LPA, it appears that it may act as an autocrine growth factor, as ovarian cancer cells (OCCs) express receptors for LPA qualitatively different from those detected on normal ovarian cells [19]. Both molecules are pleotropic and they modulate and/or activate/inhibit various cellular activities, as will be explained below (Fig. 1).
Fig. 1.
a Sphingosine 1-phosphate (S1P) is pleotropic lysophospholipid that exerts multiple activities on cancer cells as well as normal cells. Most of these activities are inhibited by the drug FTY720 which binds S1P1,3,4,5. b Similar to S1P, lysophospholipid (LPA) exerts different functions on normal and cancer cells
Effects of S1P
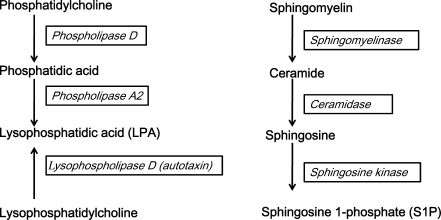
S1P was identified as a lipid metabolite that induces intracellular calcium rises and NIH3T3 cell proliferation acting intracellularly and extracellularly [20]. It is generated from sphingolipids, which are essential plasma membrane lipids concentrated in liquid-ordered domains, commonly known as lipid rafts [21]. It can be rapidly synthesized following the activation of an enzymatic cascade: sphingomyelin converted into ceramide by sphingomyelinase, ceramide into sphingosine by ceramidase and sphingosine into S1P by sphingosine kinase [22] (Fig. 2). This pathway has been denoted “the sphingomyelin cycle”, due to the fact that for all the steps of activation reverse reactions may take place catalyzed by specific enzymes such as S1P phosphatases, ceramide synthase and sphingomyelin synthase [23].
Fig. 2.
Generation of the lipids described in this article. These can be classified into lysosphingolipids (e.g. S1P) and lysoglycerolipids (e.g. LPA). The enzymes that catalyze each step in theirgenerations are shown in italics
Even though S1P is synthesized by most cells, the levels of this metabolite in tissues, including lymphoid tissues, are small due to irreversible degradation by intracellular S1P lyase or dephosphorylation by S1P phosphatases [24–27]. Inhibition of S1P lyase activity results in increased the level of S1P in tissues, thus ablating the concentration gradient between blood and tissues [28]. S1P in lymph is not derived from erythrocytes or other hematopoietic cells, but there is a reason to believe that lymph S1P and plasma S1P are regulated by the endothelium, as the physiological stimulus shear stress increased secretion of this lipid from these cells [29]. In contrast, neither platelets nor mast cells seem to play any role [29, 30]. The mechanisms for the constitutive secretion of S1P from erythrocytes and endothelium cells are unknown. It appears that one or more factors present in plasma might be required for the constitutive secretion of S1P from erythrocytes [31].
S1P in Cancer Development
S1P is considered to be a pro-survival lipid, because of its involvement in malignant transformation, cancer proliferation, inflammation, vasculogenesis and resistance to apoptotic cell death [32–34]. S1P is catalyzed by sphingosine kineases, of which there are two forms (SK1 and SK2). Over-expression of SK1 resulted in malignant transformation and tumor formation in 3T3 fibroblasts [35] whereas partial inhibition of expression resulted in apoptosis of MCF-7 human breast cancer cells [36]. An important role for the SK1/S1P pathway as a carcinogenic marker in a colon carcinogenesis model in rats has been shown, which was linked to the up-regulation of Cox-2 and PGE2 [37]. Hence, S1P is a target of research in cancer, due to the discovery that enhanced SK1 mRNA expression may promote the growth of solid tumors [38, 39].
S1P generation can also be catalyzed by SK2, and contrary to SK1, over expression of SK2 mediates apoptosis and suppressed proliferation. However, it was suggested that endogenous SK2 which is localized mainly to the nucleus, might act similar to SK1 providing pro-survival characteristics to cancer cells, as knock-down of SK2 sensitized cells to drug induced apoptosis [40]. Thus, available information supports the hypothesis that inhibition of SKs may enhance treatment of cancer, whereas selective elevation of S1P through nuclear SK2 in normal cells may provide protection against toxicity during therapy.
Role of S1P in Brain Cancer Development and Invasiveness
S1P enhances the motility and invasiveness of glioblastoma (GBM) cell lines. As an indication of the importance of this system in vivo, high expression of SK1 in GBM tissue correlated with more than three folds shorter survival time of GBM patients though expression of the receptors was not increased [41]. S1P also stimulated U-373MG cell migration, and in vitro invasion and adhesion in the absence of a concentration gradient indicating a chemokinetic response. Reports of high S1P levels in brain tissues and secretion of S1P from rat C6 astroglioma cells led to the idea of autocrine signaling [42].Thus, S1P could increase the invasiveness of glioblastoma cells in vivo by an autocrine or paracrine mechanism leading to enhanced cell motility, without the requirement for a chemotactic gradient.
S1P1 expression level is low in gliomas and glioblastomas compared to normal brain tissues, in which it is predominantly localized to the astrocytes [43]. In patients with glioblastoma, down-regulation of S1P1 was correlated with poor survival. S1P5 is present in oligodendrocytes of normal brain [44], however, only very low levels of S1P5 expression in a limited number of glioblastoma cases and several glioma cell lines were observed [45], suggesting that the expression of S1P5 is incompatible with malignant growth. S1P5 inhibited glioma cell proliferation when over-expressed in these cells which is in accordance with the observed inhibition of S1P1, 2 and 3—induced growth and invasion of glioma cells by S1P5 [45]. It also inhibited esophageal cancer cell proliferation [46]. Further, S1P5 over-expression decreased glioma cell adhesion, a response that may be related to the process retraction and cell survival seen in immature oligodendrocyte precursors [44].
Whereas overexpression of S1P1 enhanced B16F10 mouse melanoma cell migration and invasion through the activation of Rac, S1P2 inhibited migration through RhoA activation and Rac inhibition [45]. In addition, S1P2 potently enhanced expression of the matricellular protein CCN1/Cyr61, which has been implicated in tumor cell adhesion, and invasion as well as tumor angiogenesis [46]. Over-expression or knockout of S1P1 had a more potent effect on glioma cell proliferation than either S1P2 or S1P3. Glioma cell lines that did not express significant levels of S1P1 were mitogenically unresponsive to S1P, but uncoupling of S1P1 by pertussis toxin in cell lines highly expressing this receptor only partially inhibited proliferation indicating that pertussis toxin insensitive G proteins are activated [46].
Effect of S1P on Tumor Angiogenesis
In many ways S1P is associated with endothelial cell proliferation, migration, survival, and vascular morphogenesis [47] (Fig. 1a). Induction of chemotaxis in these cells leads to angiogenesis [48]. It is well established that S1P is able to promote endothelial cell barrier integrity through S1P1 receptor function. Deletion of S1P1 receptor in mice blocked vascular maturation, the phenomenon whereby smooth muscle cells and pericytes stabilize newly formed endothelial tubes [49]. On the other hand, S1P1 was increased in angiogenic tumor vessels in vivo and siRNAs targeting S1P1 in mouse suppressed growth of tumors by inhibiting the stabilization of new vessels [50]. The most dramatic abnormalities were seen in S1P1 knockout mice where vascular integrity was not established due to defective circumvascular migration and adherence of smooth muscle cells and pericytes. The resultant intraplacental hemorrhages led to embryonic lethality in 100% of homozygous S1P1 knockout mice [51]. In addition, treatment of mice with FTY720 or phosphorylated FTY720, a high affinity drug for S1P1,3,4,5 [52, 53], led to a strong inhibition of angiogenesis in the in vivo matrigel plug assay, reduced tumor size, and significantly inhibited metastatic spread of melanoma [54] indicating that blocking or down-regulation of S1P receptors by this drug has a beneficial effect for treating melanomas
S1P has atheroprotective effect, and it also confers cardio-protection in a mouse model for ischemia-reperfusion. S1P2,3 double knockout mice display significantly increased infarct size and compromised survival of endothelial cells and cardiomyocytes [55]. Furthermore, it has been reported that monoclonal antibody to S1P (Sphingomab™, which is being developed as a human therapeutic) blocked endothelial cell migration, capillary morphogenesis, and reduced tumor growth in murine xenograft models [56]. Collectively these studies suggest that cooperative and/or antagonistic signaling between S1P receptor subtypes influence pathological angiogenesis, permeability, wound healing, and other clinical syndromes associated with cancer, sepsis, stroke, and heart diseases.
Effect of S1P on Tumor Cell Migration
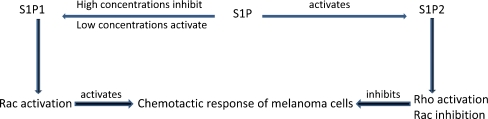
The main function of many of the S1P receptors is perhaps to facilitate cell migration. However, the chemotactic response of a cell depends on which S1P receptor is the predominant transducer. S1P1 and S1P3 signal chemotactic responses and amplify those exerted by other growth factors, whereas S1P2 signals inhibition of growth factor-evoked chemotaxis [45]. The principal difference is in the signaling pathways, namely stimulation of the small GTPase Rac by S1P1 and S1P3, in contrast to inhibiting Rac by S1P2 [57, 58]. Low concentrations of S1P promoted chemotaxis in a S1P1 dependent manner, whereas high concentrations seem to be inhibitory [59, 60]. This inhibitory effect might partly be due to the down-regulation of S1P1 by high concentrations of S1P, a mechanism that could prove relevant in vivo during the transit of cells in circulatory fluids, where the concentration of S1P is high (Fig. 3).
Fig. 3.
High concentrations of S1P inhibit the chemotactic response of cancer as well as untransformed cells, whereas low concentrations activate this process by binding S1P1 which activates Rac. In contrast, S1P binding to S1P2 inhibits Rac abut stimulates Rho, resulting in inhibiting melanoma cell chemotaxis
S1P is strongly chemotactic for endothelial cells [48, 61] as well as for immune cells [62] and cancer cells. It enhanced motility and invasiveness of glioblastoma cell lines [42] in a cooperative way with membrane type 1 matrix metalloproteinase [63], and migration in the absence of a concentration gradient indicates that S1P also evokes chemokinesis. It also induces the chemotaxis of OVCAR3 ovarian cancer cell migration [64]. In contrast, S1P-induced inhibition of B16-melanoma cells is due to activation of S1P2 [58], which prevents S1P-stimulated cell motility [65].
Effects of LPA
Generation of LPA
LPA production involves the activity of multiple highly regulated enzymes such as phospholipases (PLA1 and PLA2) and lysophospholipase D “lysoPLD, autotaxin” [66] (Fig. 1b). In short, multiple forms characterized by different patterns of saturatedness and plural ways of synthesis [67] are known to form both the inner and outher leaflet of the cell-membrane. LPA is known to regulate blood pressure, platelet aggregation, and cell proliferation by binding to multiple receptors, the most extensively studied are known as LPA1-5. These receptors are coupled to G proteins and mediate multiple downstream signaling pathways [2–4, 68].
Certain cancer cells produce LPA which acts as an autocrine growth factor. It is involved in multiple important stages of cancer development [69, 70], induces the release of angiogenic factors such as vascular endothelial growth factor [71], and is involved in neovascularization and tumor growth and survival. An inhibitor of LPA signaling pathway induced tumor regression and loss of vascularity in a model of tumor growth [72] Also, LPA2 as well as LPA1 and 3 expression is high in tumor cell lines [73, 74]. In contrast, LPA2 deficiency provides protection against cancer development [75], whereas LPA1 and LPA3 mRNA are elevated in epithelium derived from prostate cancers compared to benign glands [74]. This has led to suggesting that a switch from LPA1 to LPA3 may be in involved in cancer progression [74], while later expression of LPA1 might lead to metastatic development [73, 76]. Further, LPA1 is known to induce colony scattering of gastrointestinal cancer cells [73].
Role of LPA in Ovarian Cancers
Elevated concentrations of LPA have been detected in lesional fluids of ovarian cancer cells, at least some are derived from the cancer cells [77]. In these cells a certain LPA receptor expression pattern is associated with malignancy [78], and LPA has the ability to stimulate cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer cells [70]. Analyses of mRNA encoding LPA receptors showed that LPA2 and LPA3 were the predominant receptors in ovarian cancer cells (OCCs) or ovarian cancer tissues, and were expressed at far higher levels in almost all human ovarian cancer tissue samples than in matching adjacent non involved ovarian tissues [78]. In contrast, mRNA encoding LPA1 was more abundant in ovarian epithelial cells (OSEs) than OCCs [78]. Western blot results support the findings of higher levels of LPA2 and LPA3 in OCCs than non malignant ovarian epithelial cells, and higher levels of LPA1 in OSE cells than OCCs has been reported. Thus, it is expected that OCCs would be more responsive functionally to LPA. Accordingly LPA stimulated the proliferation of the OV202 primary line of OCCs, but not ovarian epithelial cell line IOSE 29 cells. OV202 OCC generation of type II insulin-like growth factor (IGF-II), which is a potent mitogen for OCCs was increased to significant levels by addition of LPA [78].
Angiogenesis
In sharp contrast to S1P receptor deficient mice, LPA1-3 appear to be dispensable for mouse embryonic vascular cardiovascular development [79]. Of note, the effects of LPA4 and LPA5 on cancer development is not yet clear. Autotoxin deficient mice died at early embryonic development due to impaired blood vessel formation in both yolk sac and embryo [80], and LPA facilitated vascular network establishment of mouse allantois explants, suggesting functional importance both for LPA and its receptors in the mouse vascular system. Expression of LPA receptors is increased in ovarian cancer cells resulting in increased their ability of invasiveness, the size of tumors as well as the ascites volume, whereas receptor deficiency inhibited migration and invation [81], pointing to a role for LPA in neovascularization and spread of cancer cells. In addition, LPA induced the release of angiogenic factors such as vascular endothelial growth factor (VEGF) and stromal-derived factor-1α (SDF-1α)/CXCL12 [82]. VEGF acts back on endothelial cells by inducing autotaxin, PLA1 and LPA1 receptor expression [83]. Hence, LPA is involved in neovascularization and tumor growth and survival [84]. It acts directly on the endothelium of breast cancer cells to induce angiogenesis and expression of autotaxin and LPA receptors increased tumorigenesis and metastases of these cancers [85].
Effects of S1P and LPA on Immune Cells
The major effects of LPA and S1P on immune cells are growth-related, and cytoskeleton-related. Promoting the proliferation of many cells in a pertussis toxin (PTX) inhibitable manner defines most action of LPLs. Indirect effects such as increased autocrine secretion of growth factors, and increased expression of plasma membrane localized growth factors and their receptors contribute to the diversity of biological effects. LPA stimulates a range of responses in lymphocytes, eosinophils, macrophages, and mast cells in vitro and it was thus suggested that it has additional functions in vivo in the induction or amplification of immune or inflammatory responses [66]. Furthermore LPA induced the release of cytokines [86], as well as MMP-7 [87] and it was linked to inflammation by the induction of PLA2 leading to LPA synthesis by IL-1β [88].
Effects on T Cells
In earlier studies, LPA and S1P were shown to have striking effects on T cell susceptibility to apoptosis due to alterations in cellular levels of proteins of the Bcl-2 superfamily and of the caspases [34]. Both LPA and S1P protected Tsup-1 cells from apoptosis evoked by antibodies to surface proteins [21]. In contrast, S1P but not LPA suppressed apoptosis elicited by C6-ceramide. LPLs stimulation of CD4 T cell proliferation is augmented by both suppression of apoptosis and enhancement in expression of endogenous protein growth factors [89].
One report shows that invasion of T lymphoma cells is dependent on serum-borne S1P and LPA [90]. S1P concentrations in lymphoid organs (low S1P concentration) and circulation (high S1P concentration) control the mechanism of lymphocyte egress and immune surveillance [91]. On the receptor level, naïve mouse T cells express S1P1 but the receptor is lost during activation [92]. Further, mice with S1P1 over expressing T cells show increased T cell egress from the lymph nodes and attenuated humoral immunity response. This may be due to reduced numbers of antigen-stimulated T cells in draining lymph nodes, which is pertinent to provide help for B cells secreting immunoglobulins [93]. In accordance, blocking S1P1 receptor resulted in reduced naïve T cell release from the thymus and a transient reduction of T cells egress from lymph nodes [94].
Two approaches might explain the regulation of immune cell trafficking by S1P, namely in a fashion that is immune cell-centered or endothelial cell-centered [21, 91, 95]. The lymphocyte-centered model claims that expression of S1P1 enables sensing of a gradient of S1P from lymph nodes towards blood that directs cells out of lymph nodes while overriding CC-chemokine receptor 7 (CCR7)-mediated retention [60, 96]. Support was given to the lymphocyte-centered model as it was shown that lack of S1P rendered mice lymphocytes unable to egress into blood and lymph [30]. The endothelium-centered model suggests that lymphocyte egress proceeds constitutively from lymphoid tissues under physiological S1P concentrations but is blocked by agonism of S1P1 on endothelial cells. This is consistent with the induced block in lymphocyte egress by S1P1 agonists such as FTY720, and the reported failure of S1P1 antagonism to induce an egress block [97, 98]. FTY720 also reduced the infiltration of T helper 1 (Th1) and Th2 cells into airway inflammatory sites [99].
We examined the effect of lysophospholipids on T cells. Utilizing flow cytometric and RT-PCR analyses, we reported the expression of S1P1, S1P3, S1P4 and S1P5 in polyclonal T cells [100]. Activation of human T cells with anti-CD3 plus anti-CD28 down-regulated the expression of S1P1 and S1P4 in these cells. Further, S1P inhibited the proliferation of human T cells stimulated with anti-CD3 plus anti-CD28 or PMA plus ionomycin [100]. Mouse T cell proliferation was also inhibited by S1P upon activation with anti-CD3 plus anti-CD28 or IL-7 [60].
The expression of receptors for S1P in Th1 and Th2 cells has also been reported [101, 102]. S1P1, S1P3, S1P4, and S1P5 were found to be expressed in both cell types. S1P induced the chemotaxis of Th1 and Th2 cells, but the intracellular signaling pathways induced by this lipid are different in the two T helper cell subsets. For example, S1P enhanced Th1 cell chemotaxis through pertussis toxin (PTX)-insensitive, and PI3K-dependent pathways, whereas S1P-induced Th2 cell chemotaxis was mediated by PTX-sensitive G proteins, and PI3K-dependent pathway [101]. Finally, FTY720 inhibited T regulatory (Treg) cell proliferation in vitro and in vivo, suggesting that S1P may induce the proliferation and IL-2 expansion of these cells [102].
Effects on Dendritic Cells
Murine immature (i) dendritic cells (DCs), but not mature (m) DCs, migrated toward S1P in a pattern correlated with the upregulation of S1P1 and S1P3 during maturation [103]. This action is dependent on signaling through Rac/Cdc42 and Rho, as blocking of these small GTPases resulted in a complete failure to migrate. Also, iDCs migrated toward various chemokines and a combination of S1P with these chemokines had a synergistic effect on their migration, which in combination with a positive effect on iDC proliferation suggests a cummulative effect of S1P towards localizing these cells at the sites of antigen uptake [104]. Hence, part of the immune modulation accomplished by FTY720, in many instances working through antagonizing mechanisms on the S1P receptors, may be caused by impaired DC migration and proliferation.
Immature as well as mature DCs expressed LPA1, LPA2 or LPA3, and LPA stimulates a PTX-sensitive calcium mobilization, actin polymerization and chemotaxis responses in iDCs [105]. Moreover, ELISA experiments demonstrated that addition of LPA to immature DCs in the presence of LPS enhances the secretion of CXCL8/IL-8 and IL-6 [106]. Studies also showed that Gi protein and MAP-kinase pathways are involved in these cell responses. Corroborating these findings, LPA induced the phosphorylation of ERK1/2 in immature DCs but not in mature DCs [106]. These findings suggest that the effect of LPA on the release of CXCL8/IL-8 and IL-6 is rapid and occurs prior to the maturation of these cells.
Effects on NK Cells
The major functions of NK cells are tumor rejection and inhibition of virally infected cells [107, 108], but usually spare normal cells from killing. Although NK cells are blood-born and primarily found in the blood circulation and in the spleen, they migrate toward inflammatory or tumor growth sites in order to lyse infected or metastatic cells. To facilitate their distribution into these sites, NK cells express receptors for various chemoattractants such as chemokines [109]. We reported that resting as well as IL-2-activated NK cells express receptors for S1P, and they move chemotactically toward the concentration gradients of S1P [63]. It was recently shown that S1P inhibited NK cell lysis of human melanoma cell line Hs294T and the Burkitt’s lymphoma cell line RAJI [110]. In addition, S1P inhibited NK cells lysis of the human myleoid leukemia cell line K562 as well as blocking NK cell killing of immature DCs [111]. This is corroborated with increased expression of HLA-I and HLA-E on the surfaces of DCs making them resistant to NK cell lysis [111]. This effect of S1P was reversed by FTY720 and the S1P1 blocker SEW2871. Further, S1P reduced NK cell release of the inflammatory cytokines IL-17A and IFN-γ [111]. These results indicate that S1P may be an anti-inflammatory molecule suppressing the release of inflammatory cytokines, rendering NK cells unable to lyse target cells, and consequently, reducing inflammtion.
It was also reported that activated human NK cells express receptors for LPA, and that these cells are chemotaxed toward LPA [112]. This activity was inhibited by prior treatment of the cells with PTX, suggesting that heterotrimeric Gαi/o protein is involved in the chemotactic response. Furthermore, LPA induced the mobilization of intracellular calcium in NK cells, an effect that was partially inhibited by PTX, suggesting that [Ca2+]i is mediated by both PTX-sensitive and -insensitive G proteins [112]. The findings also determined that LPA1 is involved in both the chemotaxis and calcium mobilization, whereas LPA2 only induces the mobilization of intracellular calcium in activated NK cells. Similar to S1P, LPA inhibited NK cell lysis of human melanoma cell lines and Burkitt’s lymphoma cells [113], suggesting that this lipid may also act as an anti-inflammatory molecule.
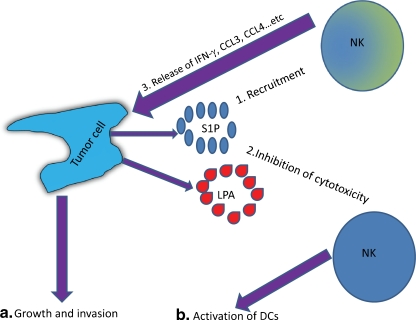
It is unclear at the present time why LPLs which are chemoattractants for NK cells inhibit their cytolytic activity. It remains a mystery why this strategy is followed with the anti-tumor effector cells NK cells. It is plausible that tumor cells might have developed the strategy of recruiting the anti-tumor effector cells NK cells (Fig. 4, step 1), and at the same time inhibit their cytolytic function (Fig. 4, step 2). These findings may support the concept indicating that the immune system initially attack cancer cells, but these cells develop immunosuppreive barrier that dampen immune attack [114]. Alternatively, this activity of LPLs might be a bystander effect as a result of LPLs anti-inflammatory effects, where the intention of these lipids is to subside inflammation. NK cells secrete inflammatory molecules such as IL-17 and IFN-γ as well as chemokines such as CCL3 and CCL4 [111, 115], which recruit various inflammatory cells, but these factors may also help promote the growth of tumor cells (Fig. 4, steps 3 and 4a). Alternatively, LPLs may down-regulate the cytoltic activity of NK cells and consequently, may facilitate these cells to activate rather than lyse DCs, and hence, potentiating the immune response (Fig. 4, step 4b). It is presently unclear which pathway may be predominant at the sites of tumor growth.
Fig. 4.
Tumor cell editing of NK cells. In step 1, cancer cells such as ovarian cancer cells secrete lysophospholipids (S1P and LPA, among others) which recruit NK cells. At the same time, these LPLs inhibit NK cell-mediated cytotoxicity (Step 2). In Step 3, NK cells secrete growth factors (CCL3 and CCL4, among others) which may facilitate the growth of tumor cells (Step 4a). Alternatively, the lipids may preclude DCs lysis by NK cells which may result in stimulating the immune response (Step 4b)
Conclusions
We have briefly touched on a vast and growing field regarding the effects of LPLs on the cancer microenvironment. These lipids are secreted physiologically by platelets and other cell types, play highly important roles on the development, activation and regulation of the immune system. Notably, they are also secreted by cancerous cells and there is a strong association between LPLs and cancer. It is clear that these lipids and in particular S1P and LPA play major roles in regulating the growth of tumor cells, and in manipulating the immune system. Consequently, LPLs or their receptors may be attractive targets for developing drugs to treat cancer and other diseases.
Acknowledgments
The work in the authors’ laboratory is supported by grants from the Norwegian Cancer Society, University of Oslo, UNIFOR, and Forskerlinjen fellowships.
References
- 1.Chun J, Goetzl EJ, Hla T, et al. International union of pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2008;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol. 2008;160:25–46. doi: 10.1007/112_0507. [DOI] [PubMed] [Google Scholar]
- 3.Choi JW, Herr DR, Noguchi K, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 4.Maghazachi AA. Insights into seven and single transmembrane-spanning domain receptors and their signaling pathways in human natural killer cells. Pharmacol Rev. 2005;57:339–357. doi: 10.1124/pr.57.3.5. [DOI] [PubMed] [Google Scholar]
- 5.Cinque B, Di ML, Centi C, et al. Sphingolipids and the immune system. Pharmacol Res. 2003;47:421–437. doi: 10.1016/s1043-6618(03)00051-3. [DOI] [PubMed] [Google Scholar]
- 6.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 7.Tigyi G, Dyer DL, Miledi R. Lysophosphatidic acid possesses dual action in cell proliferation. Proc Natl Acad Sci USA. 1994;91:1908–1912. doi: 10.1073/pnas.91.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengupta S, Xiao YJ, Xu Y. A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. FASEB J. 2003;17:1570–1572. doi: 10.1096/fj.02-1145fje. [DOI] [PubMed] [Google Scholar]
- 9.Sengupta S, Kim KS, Berk MP, et al. Lysophosphatidic acid downregulates tissue inhibitor of metalloproteinases, which are negatively involved in lysophosphatidic acid-induced cell invasion. Oncogene. 2007;26:2894–2901. doi: 10.1038/sj.onc.1210093. [DOI] [PubMed] [Google Scholar]
- 10.Kim KS, Sengupta S, Berk M, et al. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006;66:7983–7990. doi: 10.1158/0008-5472.CAN-05-4381. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Shen Z, Wiper DW, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 12.Sedláková I, Vávrová J, Tošner J, Hanousek L. Lysophosphatidic acid in patients with ovarian cancer. Clin Ovarian Cancer. 2010;3:41–46. [Google Scholar]
- 13.Masuda A, Nakamura K, Izutsu K, et al. Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br J Haematol. 2008;143:60–70. doi: 10.1111/j.1365-2141.2008.07325.x. [DOI] [PubMed] [Google Scholar]
- 14.Sano T, Baker D, Virag T, Wada A, et al. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 15.Yatomi Y, Ohmori T, Rile G, et al. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2009;96:3431–3438. [PubMed] [Google Scholar]
- 16.Yatomi Y, Ozaki Y, Ohmori T, Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Yatomi Y, Miura Y, et al. Metabolism and functional effects of sphingolipids in blood cells. Br J Haematol. 1999;107:282–293. doi: 10.1046/j.1365-2141.1999.01697.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast cancer cells. Biochem J. 1995;309:933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel S, Milstien S. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 2004;476:55–57. doi: 10.1016/s0014-5793(00)01670-7. [DOI] [PubMed] [Google Scholar]
- 21.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- 24.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 25.Mechtcheriakova D, Wlachos A, Sobanov J, et al. Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell Signal. 2007;19:748–760. doi: 10.1016/j.cellsig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Peest U, Sensken SC, Andreani P, et al. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J Cell Biochem. 2008;104:756–772. doi: 10.1002/jcb.21665. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Kalari SK, Usatyuk PV, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 29.Venkataraman K, Lee YM, Michaud J, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 31.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 32.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- 34.Goetzl EJ, Kong Y, Mei B. Lysophosphatidic acid and sphingosine 1-phosphate protection of T cells from apoptosis in association with suppression of Bax. J Immunol. 1999;162:2049–2056. [PubMed] [Google Scholar]
- 35.Xia P, Gamble JR, Wang L, et al. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 36.Taha TA, Kitatani K, El-Alwani M, et al. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- 37.Kawamori T, Osta W, Johnson KR, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 38.French KJ, Upson JJ, Keller SN, et al. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 39.French KJ, Schrecengost RS, Lee BD, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 40.Sankala HM, Hait NC, Paugh SW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 41.Brocklyn JR, Jackson CA, Pearl DK, et al. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 42.Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi: 10.1016/s0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida Y, Nakada M, Sugimoto N, et al. Sphingosine-1-phosphate receptor type 1 regulates glioma cell proliferation and correlates with patient survival. Int J Cancer. 2010;126:2341–2352. doi: 10.1002/ijc.24933. [DOI] [PubMed] [Google Scholar]
- 44.Jaillard C, Harrison S, Stankoff B, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young N, Brocklyn JR. Roles of sphingosine-1-phosphate (S1P) receptors in malignant behavior of glioma cells. Differential effects of S1P2 on cell migration and invasiveness. Exp Cell Res. 2007;313:1615–1627. doi: 10.1016/j.yexcr.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brocklyn JR, Letterle C, Snyder P, Prior T. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: role of ERK MAP kinase and phosphatidylinositol 3-kinase beta. Cancer Lett. 2002;181:195–204. doi: 10.1016/s0304-3835(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 47.Morris AJ, Panchatcharam M, Cheng HY, et al. Regulation of blood and vascular cell function by bioactive lysophospholipids. J Thrombosis and Haemostasis. 2009;7(Suppl):43. doi: 10.1111/j.1538-7836.2009.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee OH, Kim YM, Lee YM, et al. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;264:743–750. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- 49.Paik JH, Skoura A. Chae SS et al Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114:1082–1089. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kono M, Mi Y, Liu Y, Sasaki T, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 52.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 54.LaMontagne K, Littlewood-Evans A, Schnell C, et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–231. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- 55.Theilmeier G, Schmidt C, Herrmann J, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 56.Visentin B, Vekich JA, Sibbald BJ, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi H, Kitayama J, Takuwa N, et al. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem J. 2003;374:715–722. doi: 10.1042/BJ20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arikawa K, Takuwa N, Yamaguchi H, et al. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J Biol Chem. 2003;278:32841–32851. doi: 10.1074/jbc.M305024200. [DOI] [PubMed] [Google Scholar]
- 59.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 60.Dorsam G, Graeler MH, Seroogy C, et al. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J Immunol. 2003;171:3500–3507. doi: 10.4049/jimmunol.171.7.3500. [DOI] [PubMed] [Google Scholar]
- 61.English D, Kovala AT, Welch Z, et al. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res. 1999;8:627–634. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- 62.Kveberg L, Bryceson Y, Inngjerdingen M, et al. Sphingosine 1 phosphate induces the chemotaxis of human natural killer cells. Role for heterotrimeric G proteins and phosphoinositide 3 kinases. Eur J Immunol. 2003;32:1856–1864. doi: 10.1002/1521-4141(200207)32:7<1856::AID-IMMU1856>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 63.Annabi B, Lachambre MP, Plouffe K, et al. Modulation of invasive properties of CD133 (+) glioblastoma stem cells: a role for MT1-MMP in bioactive lysophospholipid signaling. Mol Carcinogenesis. 2009;48:910–919. doi: 10.1002/mc.20541. [DOI] [PubMed] [Google Scholar]
- 64.Park KS, Kim MK, Lee HY, et al. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochem Biophys Res Commun. 2007;356:239–244. doi: 10.1016/j.bbrc.2007.02.112. [DOI] [PubMed] [Google Scholar]
- 65.Okamoto H, Takuwa N, Yokomizo T, et al. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin DA, Boyce JA. Lysophospholipids as mediators of immunity. Adv Immunol. 2006;89:141–167. doi: 10.1016/S0065-2776(05)89004-2. [DOI] [PubMed] [Google Scholar]
- 67.Tigyi G. Aiming drug discovery at lysophosphatidic acid targets. Br J Pharmacol. 2010;161:241–270. doi: 10.1111/j.1476-5381.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radeff-Huang J, Seasholtz TM, Matteo RG, Brown JH. G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. J Cell Biochem. 2004;92:949–966. doi: 10.1002/jcb.20094. [DOI] [PubMed] [Google Scholar]
- 69.Liu S, Umezu-Goto M, Murph M, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15:539–550. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H, Wang D, Zhang H, Kirmani K, et al. Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Mol Cancer Ther. 2009;8:1692–1701. doi: 10.1158/1535-7163.MCT-08-1106. [DOI] [PubMed] [Google Scholar]
- 71.Hu YL, Tee MK, Goetzl EJ, et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93:762–768. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- 72.Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer. 2010;116:1739–1750. doi: 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin KJ, Kim YL, Lee S, Kim D, et al. Lysophosphatidic acid signaling through LPA receptor subtype 1 induces colony scattering of gastrointestinal cancer cells. J Cancer Res Clin Oncol. 2009;135:45–52. doi: 10.1007/s00432-008-0441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng Y, Kakehi Y, Nouh MA, et al. Gene expression profiles of lysophosphatidic acid-related molecules in the prostate: relevance to prostate cancer and benign hyperplasia. Prostate. 2009;69:283–292. doi: 10.1002/pros.20879. [DOI] [PubMed] [Google Scholar]
- 75.Lin S, Wang D, Iyer S, Ghaleb AM, et al. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shida D, Kitayama J, Yamaguchi H, et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–1711. [PubMed] [Google Scholar]
- 77.Fang X, Schummer M, Mao M, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 78.Goetzl EJ, Dolezalova H, Kong Y, et al. Distinctive expression and functions of the type 4 endothelial differentiation gene-encoded G protein-coupled receptor for lysophosphatidic acid in ovarian cancer. Cancer Res. 1999;59:5370–5375. [PubMed] [Google Scholar]
- 79.Ye X, Hama K, Contos JJ, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meeteren LA, Ruurs P, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu S, Murph MM, Lu Y, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeon ES, Heo SC, Lee IH, et al. Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 from human mesenchymal stem cells. Exp Mol Med. 2010;42:280–293. doi: 10.3858/emm.2010.42.4.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ptaszynska MM, Pendrak ML, Stracke ML, Roberts DD. Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor-induced endothelial cell migration. Mol Cancer Res. 2010;8:309–321. doi: 10.1158/1541-7786.MCR-09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin CI, Chen CN, Huang MT, et al. Lysophosphatidic acid upregulates vascular endothelial growth factor-C and tube formation in human endothelial cells through LPA(1/3), COX-2, and NF-kappaB activation- and EGFR transactivation-dependent mechanisms. Cell Signal. 2008;20:1804–1814. doi: 10.1016/j.cellsig.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 85.Boucharaba A, Guillet B, Menaa F, et al. Bioactive lipids lysophosphatidic acid and sphingosine 1-phosphate mediate breast cancer cell biological functions through distinct mechanisms. Oncol Res. 2009;18:173–184. doi: 10.3727/096504009790217399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fang X, Yu S, Bast RC, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 87.Wang FQ, Ariztia EV, Boyd LR, et al. Lysophosphatidic acid (LPA) effects on endometrial carcinoma in vitro proliferation, invasion, and matrix metalloproteinase activity. Gynecol Oncol. 2010;117:88–95. doi: 10.1016/j.ygyno.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 88.Degousee N, Stefanski E, Lindsay TF, et al. p38 MAPK regulates group IIa phospholipase A2 expression in interleukin-1beta -stimulated rat neonatal cardiomyocytes. J Biol Chem. 2001;276:43842–43849. doi: 10.1074/jbc.M101516200. [DOI] [PubMed] [Google Scholar]
- 89.Goetzl EJ, Graeler M, Huang MC, Shankar G. Lysophospholipid growth factors and their G protein-coupled receptors in immunity, coronary artery disease, and cancer. ScientificWorldJournal. 2002;2:324–338. doi: 10.1100/tsw.2002.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stam JC, Michiels F, Kammen RA, et al. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 92.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 93.Chi H, Flavell RA. Regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J Immunol. 2005;174:2485–2488. doi: 10.4049/jimmunol.174.5.2485. [DOI] [PubMed] [Google Scholar]
- 94.Morris MA, Gibb DR, Picard F, et al. Transient T cell accumulation in lymph nodes and sustained lymphopenia in mice treated with FTY720. Eur J Immunol. 2005;35:3570–3580. doi: 10.1002/eji.200526218. [DOI] [PubMed] [Google Scholar]
- 95.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 97.Wei SH, Rosen H, Matheu MP, et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- 98.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 99.Sawicka E, Zuany-Amorim C, Manlius C, et al. Inhibition of Th1- and Th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J Immunol. 2003;171:6206–6214. doi: 10.4049/jimmunol.171.11.6206. [DOI] [PubMed] [Google Scholar]
- 100.Jin Y, Knudsen E, Wang L, Bryceson Y, et al. Sphingosine 1-phosphate is a novel inhibitor of T-cell proliferation. Blood. 2003;101:4909–4915. doi: 10.1182/blood-2002-09-2962. [DOI] [PubMed] [Google Scholar]
- 101.Wang L, Knudsen E, Jin Y, Gessani S, Maghazachi AA. Lysophospholipids and chemokines activate distinct signal transduction pathways in T helper 1 and T helper 2 cells. Cell Signal. 2004;16:991–1000. doi: 10.1016/j.cellsig.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Wolf AM, Eller K, Zeiser R, et al. The sphingosine 1-phosphate agonist FTY720 potently inhibits regulatory T cell proliferation in vitro and in vivo. J Immunol. 2009;183:3751–3760. doi: 10.4049/jimmunol.0901011. [DOI] [PubMed] [Google Scholar]
- 103.Idzko M, Panther E, Corinti S, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 2002;16:625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 104.Eigenbrod S, Derwand R, Jakl V, et al. Sphingosine kinase and sphingosine-1-phosphate regulate migration, endocytosis and apoptosis of dendritic cells. Immunol Invest. 2006;35:149–165. doi: 10.1080/08820130600616490. [DOI] [PubMed] [Google Scholar]
- 105.Panther E, Idzko M, Corinti S, et al. The influence of lysophosphatidic acid on the functions of human dendritic cells. J Immunol. 2002;169:4129–4135. doi: 10.4049/jimmunol.169.8.4129. [DOI] [PubMed] [Google Scholar]
- 106.Oz-Arslan D, Ruscher W, Myrtek D, et al. IL-6 and IL-8 release is mediated via multiple signaling pathways after stimulating dendritic cells with lysophospholipids. J Leukoc Biol. 2006;80:287–297. doi: 10.1189/jlb.1205751. [DOI] [PubMed] [Google Scholar]
- 107.Albertsson PA, Basse PH, Hokland M, et al. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603–609. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 108.Maghazachi AA, Al-Aoukaty A. Chemokines activate natural killer cells through heterotrimeric G-proteins: implications for the treatment of AIDS and cancer. FASEB J. 1998;12:913–924. doi: 10.1096/fasebj.12.11.913. [DOI] [PubMed] [Google Scholar]
- 109.Maghazachi AA. Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol. 2010;341:37–58. doi: 10.1007/82_2010_20. [DOI] [PubMed] [Google Scholar]
- 110.Lagadari M, Lehmann K, Ziemer M, et al. Sphingosine-1-phosphate inhibits the cytotoxic activity of NK cells via Gs protein-mediated signalling. Int J Oncol. 2009;34:287–294. [PubMed] [Google Scholar]
- 111.Rolin J, Sand KL, Knudsen E, Maghazachi AA. FTY720 and SEW2871 reverse the inhibitory effect of S1P on natural killer cell mediated lysis of K562 tumor cells and dendritic cells but not on cytokine release. Cancer Immunol Immunother. 2010;59:575–586. doi: 10.1007/s00262-009-0775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jin Y, Knudsen E, Wang L, Maghazachi AA. Lysophosphatidic acid induces human natural killer cell chemotaxis and intracellular calcium mobilization. Eur J Immunol. 2003;33:2083–2089. doi: 10.1002/eji.200323711. [DOI] [PubMed] [Google Scholar]
- 113.Lagadari M, Truta-Feles K, Lehmann K, et al. Lysophosphatidic acid inhibits the cytotoxic activity of NK cells: involvement of Gs protein-mediated signaling. Int Immunol. 2009;21:667–677. doi: 10.1093/intimm/dxp035. [DOI] [PubMed] [Google Scholar]
- 114.Shankaran V, Ikeda H, Bruce AT, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1071–111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 115.Sand KL, Knudsen E, Rolin J, et al. Modulation of natural killer cell cytotoxicity and cytokine release by the drug glatiramer acetate. Cell Mol Life Sci. 2009;66:1446–1456. doi: 10.1007/s00018-009-8726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]