Abstract
In some settings increasing high density lipoprotein (HDL) levels has been associated with a reduction in experimental atherosclerosis. This has been most clearly seen in apolipoprotein A-I (apoA-I) transgenic mice or in animals infused with HDL or its apolipoproteins. A major mechanism by which these treatments are thought to delay progression or cause regression of atherosclerosis is by promoting efflux of cholesterol from macrophage foam cells. In addition, HDL has been described as having anti-inflammatory and other beneficial effects. Some recent research has linked anti-inflammatory effects to cholesterol efflux pathways but likely multiple mechanisms are involved. Macrophage cholesterol efflux may have a role in facilitating emigration of macrophages from lesions during regression. While macrophages can mediate cholesterol efflux by several pathways, studies in knockout mice or cells point to the importance of active efflux mediated by ATP binding cassette transporter (ABC) A1 and G1. In addition to traditional roles in macrophages, these transporters have been implicated in the control of hematopoietic stem cell proliferation, monocytosis and neutrophilia, as well as activation of monocytes and neutrophils. Thus, HDL and cholesterol efflux pathways may have important anti-atherogenic effects at all stages of the myeloid cell/monocyte/dendritic cell/macrophage lifecycle.
1. Introduction
Atherosclerosis is an indolent, macrophage dominated, focal inflammatory disease of the large arteries. This process is initiated by the deposition of ApoB containing lipoproteins on the arterial proteoglycan matrix in regions of disturbed blood flow, followed by their modification and uptake by macrophages [1–2]. Modified lipoproteins also activate combinatorial signaling by toll like receptors (TLR) and scavenger receptors (SR) on macrophages, and the effects of lipid loading and TLR/SR signaling lead to inflammatory and chemokine responses, ER stress, apoptosis and necrosis [3–5]. These latter events are thought to lead to the ultimate complications of plaque rupture and athero-thrombosis. Although traditionally viewed as having a key role in removing the mass of cholesterol from plaques in a process of reverse cholesterol transport, HDL is now seen as having key effects on macrophage inflammation, ER stress and apoptosis (Figure 1). Some of these effects are dependent on the fundamental ability of HDL and apoA-I to interact with the ATP binding cassette transporters on macrophages, ABCA1 and ABCG1, mediating efflux of cholesterol and oxidized lipids [6–8], but likely multiple mechanisms are involved. Recent studies also point to a role of HDL, ABCA1, ABCG1 in controlling monocyte activation, adhesiveness and inflammation [9–10], and in controlling the proliferation of the stem and progenitor cells [11] that give rise to monocytes and neutrophils that ultimately enter plaques.
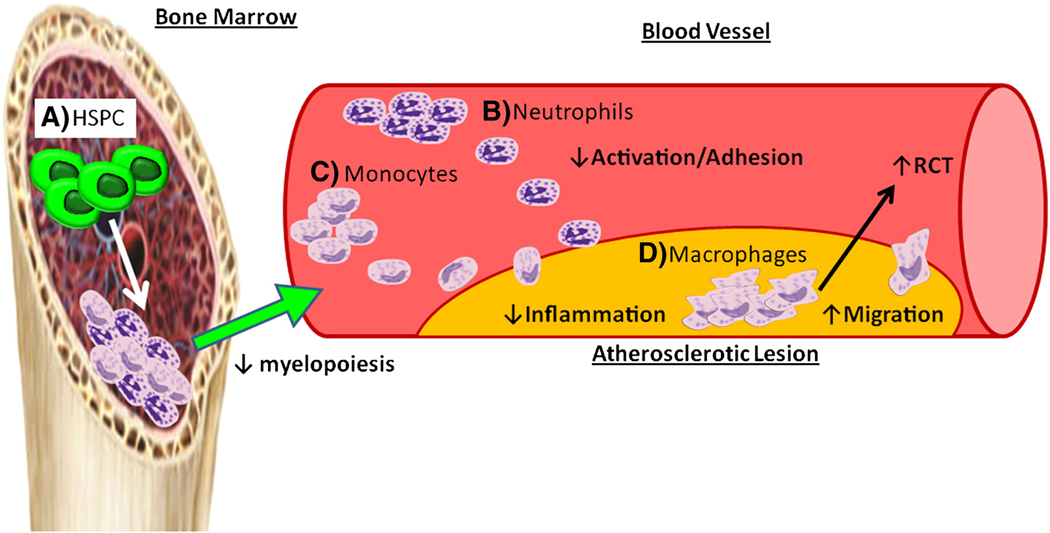
Figure 1. Anti-atherogenic functions of HDL - sites of action.
A) HDL interacts with ABCA1 and ABCG1 on the HSPCs to promote cholesterol efflux and inhibit their proliferation. This regulates the number of mature myeloid cells produced. B,C) HDL and apoA-I can act as an anti-inflammatory reducing monocyte and neutrophil activation. This leads to less recruitment of monocytes/neutrophils to the atherosclerotic lesion. D) HDL interacts with macrophages to regulate a number of cellular functions important to controlling atherosclerosis such as cholesterol efflux, reducing TLR-4 signaling, decreasing apoptosis during efferocytosis and modulating membrane lipid levels to aid in macrophage migration.
2. ABCA1 and ABCG1 are Key Mediators of Cholesterol Efflux
Francis and Oram made the seminal discovery that fibroblasts isolated from Tangier Disease (TD) subjects could not promote the efflux of cholesterol or phospholipids to lipid-free apoA-I [12–13]. Several groups discovered through the use of micro-arrays, genetic mapping and biochemical assays that Abca1 was the defective gene in Tangier Disease [14–18]. Through this discovery and using techniques to specifically knockdown the expression of Abca1 it was then demonstrated that ABCA1 exports cholesterol from cells to lipid-free apoA-I. Subsequently, it was shown that another transporter, ABCG1, promotes cholesterol efflux to mature HDL particles but not to lipid-poor apoA-I [19–20]. ABCA1 and ABCG1 are target genes of the nuclear receptors, liver X receptor (LXR) and are upregulated in response to sterol loading of macrophages and other cells. Recently it has been shown that ABCA1 (in mouse and human) and ABCG1 (in mouse) are regulated by microRNA-33 (miR-33) [21–22]. The sequence encoding miR-33 is embedded within the sterol response element binding protein-2 (Srebp2) gene, so that when cells are deprived of cholesterol, SREBP-2 is upregulated and miR-33 is produced. MiR-33 then binds to a site in the 3'-UTR of ABCA1 and ABCG1 down-regulating their mRNA and protein, thus shutting down cholesterol export. [21–25]
3. Cholesterol efflux pathways and immune cell production
3.1 HDL, ApoE, ABCA1 and ABCG1 regulate myelopoiesis and monocyte numbers
Hematopoiesis is hierarchical and ordered, and is initiated by long term self-renewing and multi-potent stem cells. Through a process of proliferation, lineage restriction and differentiation, HSPCs give rise to mature lineage committed cells, that ultimately form the mature blood cells. Production of blood cells in the steady state is tightly regulated by a number of well defined feedback loops. However, production can be increased when required, for instance in response to infection or blood loss. Emerging evidence suggests that cholesterol uptake and efflux can also regulate HSPC proliferation, providing a potential mechanism to explain the association between leukocytosis and atherosclerotic CVD [11].
Recently, Yvan-Charvet et al [11] described an important role for HDL and cholesterol efflux pathways in the regulation of hematopoietic stem cell proliferation and myelopoiesis. The hematopoietic stem and multipotential progenitor cells (HSPCs) express relatively high levels of Abca1, Abcg1 and Apoe [26–29]. Mice deficient in Abca1 and Abcg1 develop a myeloproliferative disorder characterized by dramatic monocytosis and neutrophilia, and infiltration of the spleen, heart, liver, small intestine and other organs with macrophage foam cells and neutrophils. This occurs even in chow fed mice and resembles mouse models of chronic myeloid or myelo-monocytic leukemia. The underlying mechanism involves a marked 4 to 5-fold increase in the numbers and proliferation of the HSPC population. There is increased staining of the plasma membrane of HSPCs with cholera toxin B suggesting increased plasma membrane liquid ordered domains. The increased proliferative response of HSPCs reflects an increased responsiveness to the growth factor interleukin (IL) 3 caused by an increased amount of the common beta-subunit (CBS) of the IL-3/GM-CSF receptor in the cell surface of HSPCs (Figure 2). This mechanism may also explain why myeloid lineage cells such as common myeloid progenitors and granulocyte-macrophage progenitors were expanded and proliferating in these mice, reflecting increased GM-CSF responses. In contrast, lymphoid and megakaryocyte-erythroid progenitor populations were not expanded. Competitive bone marrow transplantation experiments showed that HSPC and myeloid expansion, monocytosis and neutrophilia occurred in a cell autonomous fashion in cells derived from ABCA1/G1 deficient bone marrow, indicating that HSPC expansion and myeloid proliferation did not require increased amounts of exogenous factors such as inflammatory cytokines. Gomes et al have also reported leukocytosis and thrombocytosis in WT mice after feeding the Paigen diet [30]. This was also due to an expansion of the BM progenitor cells. It was also shown that feeding an atherogenic diet results in BM stem cell mobilization by modulating the SDF-1:CXCR4 axis. While the LDLr, SR-BI and CD36 were found to be expressed in HSPCs in this study, incubation with LDL appeared to have little effect on HSPC proliferation. While our studies have emphasized the role of cholesterol efflux pathways in the regulation of HSPC proliferation, the mechanisms of cholesterol uptake and broader aspects of the regulation of cholesterol homeostasis in HSPCs are worth of further investigation.
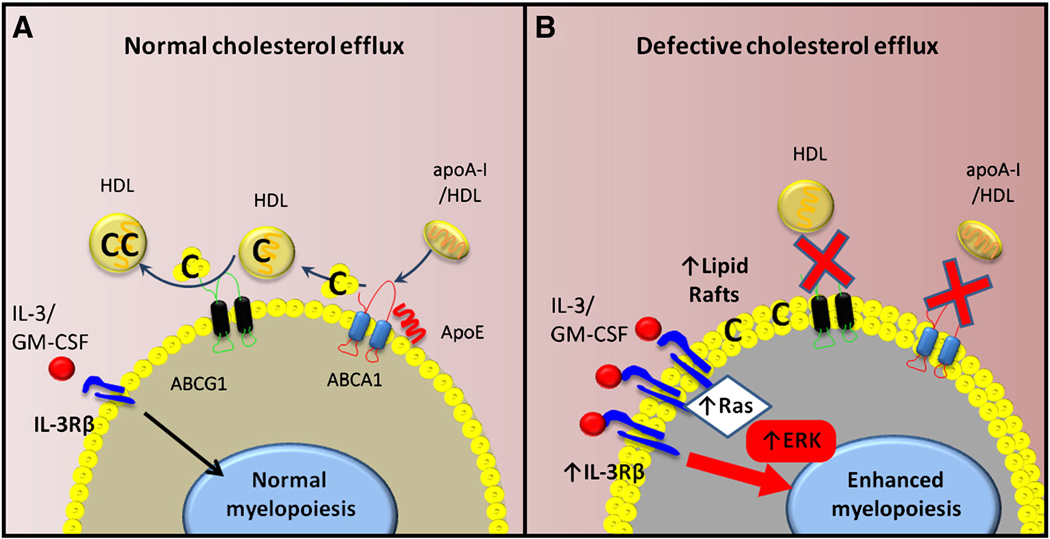
Figure 2. The importance of cholesterol efflux in regulating HSPC proliferation and myelopoiesis.
A) Under normal conditions HSPCs utilize ABCA1 and ABCG1 to efflux cholesterol to apoA-I/HDL and ApoE. This controls the amount of cholesterol in the cell membrane (lipid rafts) and may also ensure correct expression of proliferative cytokine receptors including IL-3Rβ. B) When cholesterol efflux pathways are disrupted the abundance of lipid rafts in the membrane of the HSPCs increases causing an increase in the expression of the IL-3Rβ. This results in the HSPC becoming more sensitive to cytokine induced proliferation and the pool of HSPCs begins to proliferate and expand. This ultimately causes enhanced myelopoiesis, more blood monocytes and this likely has an effect of accelerating atherosclerosis. C=cholesterol.
In Ldlr+/− mice transplanted with Abca1−/−Abcg1−/− bone marrow (BM) and fed a high fat, high cholesterol diet, atherosclerosis was markedly increased compared to WT or single KO bone marrow recipients, and there was a strong correlation between leukocyte numbers and atherosclerotic lesion size. In contrast an inflammatory marker, apoSAA while increased in DKO BM recipients, did not correlate with lesion area. This suggests a causal relationship between increased leukocytes and accelerated atherosclerosis. Moreover, when Abca1−/−Abcg1−/− BM was transplanted into a mice expressing a human Apoa-I transgene the expansion of the HSPCs was almost completely reversed, along with the increased lipid rafts and the expression of the CBS. The blood leukocyte levels were normalized, atherosclerosis and the myeloproliferative phenotype were markedly reduced in these mice [11]. Most likely, high levels of apoA-I and HDL were able to reverse the underlying defects, possibly reflecting the ability of HDL to mediate cholesterol efflux via alternative pathways, such as passive cholesterol efflux.
Apoe−/− mice fed a WTD have been shown to develop neutrophilia [31] and monocytosis [32–33]. The neutrophilia was shown to influence atherogenesis, while increased monocytes, particularly the Ly6-Chi inflammatory subset more readily entered the atherosclerotic lesion and formed lesional macrophages [31–34]. Our recent studies suggest that the monocytosis that develops in Apoe−/− mice fed high fat, high cholesterol diets also reflects increased proliferation of HSPCs and myeloid progenitors both in the bone marrow and spleen. It seems that ApoE is bound to the surface of HSCs by proteoglycans, and that this local concentrating effect favors interaction with ABCA1 and ABCG1, promoting cholesterol efflux, decreased cell surface CBS and limiting proliferative responses of HSCs [35]. We speculate that the normal role of these processes is to help to maintain HSCs in quiescence (Figure 2A). These pathways may be suppressed during acute infections, helping HSCs to emerge from quiescence and supporting myelopoiesis. However, in response to chronic hypercholesterolemia, especially in the setting of reduced HDL levels, the normal physiological regulation is overwhelmed. This leads to a condition of low-grade monocytosis and neutrophilia, enhancing chronic atherogenesis. The importance of monocyte numbers to the severity of atherosclerosis has been demonstrated in genetic mouse models [36–37]. Mice carrying the osteopetrotic (op/op) mutation, resulting in lower M-CSF display a gene dosage decrease in blood monocytes. The decrease in monocytes, despite an increase in plasma cholesterol, resulted in smaller atherosclerotic lesions that were also less complex. These studies suggest that independent of hypercholesterolemia, reducing monocytes can significantly affect atherosclerosis.
3.2 Lymphocyte proliferation is regulated by cellular cholesterol content
During an immune response when T-cell are activated this can lead to a proliferative response. Recent studies have shown the importance of cellular cholesterol levels in regulating T-cell proliferation [38–39]. T-cell activation resulted in increased SULT2B1, an oxysterol-metabolizing enzyme, followed by the suppression of LXRs. The suppression of the LXR pathway caused a decrease in cholesterol genes as the cells conserved their cholesterol and simultaneously induced the cholesterol synthesis pathway via SREBP [39]. The importance of LXRβ was shown in Lxrβ−/− mice enlarged spleen and lymph nodes that house increased number of T-cells compared to control mice. The LXR target gene induced to remove cholesterol and control proliferation was shown to be ABCG1 and not ABCA1. Adoptive transfer of Lxβr+/+ or Lxrβ−/− T-cells into Rag−/− mice confirmed this was cell intrinsic defect as Lxrβ−/− T-cells were more prevalent in the spleen. Further, inducing the LXR pathway in WT T-cells with an agonist before being transferred into Rag−/− mice in competition with vehicle treated T-cells resulted in a marked decrease in their abundance [39]. This suggests that modulating cholesterol levels may directly influence the proliferation of T-cells. This appears to be the case as incubating T-cells with soluble cholesterol increased the proliferation of WT T-cells [38]. However, a proliferative signal is still required. Abcg1−/− T-cells have increased lipid raft in the plasma membrane, however proliferation only occurred after activation via the T-cell receptor [38].
Disruption of the cholesterol efflux pathway by deletion of Apoa1 on an hypercholesterolemic background (Ldlr−/−) results in an autoimmune phenotype skin disease [40]. The skin layers of Apoa1−/− Ldlr−/− mice on a WTD had increased neutral lipid and more immune cell infiltrate [41]. T-cells were more activated and proliferated more in the draining lymph nodes of the skin. This was accompanied by an increase in CD11c+ dendritic-like cells, B-cells and macrophages in the lymph nodes. It is unknown if the increased numbers of T-cells is directly due to the loss of ApoA-I/HDL or fueled by cytokines in the inflammatory environment.
4. Anti-inflammatory Effects of HDL in the Innate Immune Response
In this review we have set out to detail the anti-atherosclerotic effects on myeloid cells. However it is of importance to note that in the setting of atherosclerosis and vascular inflammation that HDL also acts on the endothelial cells, the cells to which monocytes adhere to and use to migrate through to the atherosclerotic lesion. HDL plays a role in regulating vascular tone by stimulating endothelial cells to release nitric oxide (NO) by activating endothelial nitric oxide synthase (eNOS) when it engages SR-BI [42–44]. HDL has also been shown to inhibit the expression of key adhesion molecules on the endothelium, ICAM-1 and VCAM-1 [45]. This appears to be via a mechanism involving HDL binding to SR-BI eliciting a signaling cascade to produce NO inhibiting NF-κB (ref). Recently, HDL via SR-BI has also been shown to increase the expression of 3beta-hydroxysteroid-delta 24 reductase which interferes with the NF-κB pathway [46]. These in vitro findings have been confirmed in vivo models of vascular inflammation, suggesting the functional importance of these findings [47–48].
4.1. Monocytes
4.1.1. HDL Attenuates Monocyte Activation and Adhesion
HDL and apoA-I were found to reduce the expression of the adhesion molecule CD11b on the surface of human monocytes [49]. Experiments with cyclodextrin suggested that this decrease in monocyte activation was largely due to cholesterol removal from the cell, resulting in a reduced abundance of cholera toxin B staining plasma membrane lipid rafts. For apoA-I, ABCA1 was shown to have an essential role in this process, as shown using monocytes from subjects with Tangier Disease. Monocyte activation was also significantly attenuated in patients with peripheral vascular disease or type 2 diabetes after an infusion of rHDL, suggesting translational relevance of these observations [50–51].
The anti-inflammatory effect of HDL on monocytes was also shown in functional assays of key steps in the cell adhesion cascade. HDL inhibited monocyte adhesion and spreading on endothelial cells under shear-flow conditions and suppressed migration in response to the chemokine MCP-1. In a separate study HDL and apoA-I were also shown to inhibit M-CSF induced monocyte spreading by decreasing levels of Cdc42, a key GTPase involved in cytoskeletal organization [52]. HDL has also been linked to decreased f-actin content in monocytes [49]. Taken together these findings suggest that HDL prevents the cytoskeletal reorganization of monocytes that is required for migration towards a chemotactic signal. HDL may also suppress the expression of a number of key chemotactic molecules and receptors on monocytes, macrophages and endothelial cells. Infusions of apoA-I into Apoe−/− mice reduced the expression of the chemokine receptors CCR2 and CX3CR1 in atherosclerotic plaques, and plasma levels of CCL2 (MCP-1) and CCL5 (RANTES) [53]. The underlying mechanisms were thought to involve modulation of PPAR-γ and NF-κB activities. Overall these findings suggest that HDL has the potential to acts at multiple levels of the cell adhesion cascade to inhibit the adhesion and migration of monocytes into the atherosclerotic lesion. However, this has not yet been directly demonstrated.
4.2 Neutrophils
Increased blood neutrophils are correlated with atherosclerosis and acute coronary events [54–55] and have been identified in atherosclerotic lesions [56]. Neutrophils can become activated in hyperlipidemia and severity directly correlates with CD11b expression and superoxide release [57–59]. Moreover, cholesterol loading of neutrophils causes activation [60]. A recent study has described a clear role for neutrophils in atherogenesis. In Apoe−/− mice fed a WTD neutrophils were discovered in the atherosclerotic lesion for the first 4 weeks and the numbers of neutrophils correlated with lesion severity [31]. Reduction of neutrophils led to a decrease in plaque size, demonstrating a clear and important role for neutrophils in atherogenesis [31, 61].
4.2.1. Neutrophil Activation is Inhibited by HDL
ApoA-I through ABCA1 rapidly inhibits neutrophil activation (CD11b expression) while HDL requires a longer incubation [62]. The decrease in membrane lipid rafts by apoA-I and HDL was likely a key event as lipid raft abundance correlated with CD11b activation. Loading neutrophils with cholesterol has been shown to prime them for activation and increase their adhesiveness to the endothelium [60]. A number of studies have also described the importance of lipid rafts in the activation of neutrophils and the release of inflammatory molecules [63–66]. Although the role of neutrophils in plaque development is still not completely clear, this represents another potential site of beneficial action by HDL.
4.2.2. ApoA-I and HDL Modulate Neutrophil Recruitment to Inflamed Tissue
It has also been shown in a different in vivo model of vascular inflammation in rabbits, that infusion of HDL or apoA-I can significantly attenuate neutrophil infiltration into the intima [47–48]. The initial study by Nicholls [47] and colleagues described a role for pre-infusion of rHDL in attenuating inflammation and was attributed to down regulation of endothelial adhesion molecules, vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1) through a well defined process [46]. The subsequent study by Puranik and co-workers [48] sought to determine if infusion of lipid-free apoA-I could rescue the inflammation. Infusion of lipid-free apoA-I either 3 or 9 h after the cuff was applied could still reduce the infiltration of neutrophils. This was suggested to be due to the effects of the apoA-I on the endothelium but is also likely to be due to the anti-inflammatory effects of apoA-I on the circulating neutrophils [62].
ApoA-I and HDL were also reported to attenuate neutrophil adhesion and spreading to activated platelet monolayers and migration to fMLP [62]. This in vitro finding was then examined in an in vivo model of acute inflammation using intravital microscopy. Stimulating mice with TNF-α results in the activation of leukocytes and endothelial cells and induces neutrophil recruitment [67–68]. Infusion of apoA-I in mice pre-stimulated with TNF-α lead to a decrease in the stationary adhesion of neutrophils to the vasculature, while increasing the rolling velocity suggesting a down regulation of adhesion molecules. In line with the proposed mechanism, CD11b expression was also attenuated on neutrophils. The decrease in adhesion of the neutrophils also appeared to be to a direct effect of apoA-I attenuating neutrophil activation. While these studies may be relevant to a variety of inflammatory diseases, relevance to chronic atherosclerosis remains uncertain. Thus, expression of the human apoA-I transgene in Apoe−/− mice was associated with markedly reduced atherosclerosis, but this did not appear to involve a decrease in the expression of VCAM-1 or ICAM-1 in endothelial cells [69].
4.3. Macrophages
4.3.1. The Role of Cholesterol Efflux in TLR Signaling
The studies performed in Abca1−/− Abcg1−/− mice have shed light on a number of new roles for the HDL pathway [8, 11, 70–73]. The deletion of these two transporters perturbs a number of the normal functions of the macrophage, the most obvious being cholesterol efflux [8]. Macrophages deficient in Abca1 have an enhanced response to LPS, due to increased activation of the NF-κB and MAPK pathways dependent on MyD88 [73]. We observed that Abcg1−/− and Abca1−/−Abcg1−/− macrophages express more TLR-4/MD2 complex suggesting they are primed for activation in response to bacterial signals or other TLR-4 ligands (Figure 3.A). The increased TLR-4 expression also correlated with increased lipid rafts and TLR-4 is known to localize in rafts when activated and complexes with MD2 [74–75]. Reducing raft abundance with cyclodextrin normalized the response to LPS suggesting the dependence on cellular cholesterol levels [73, 76]. Incubation of primary human monocyte-derived macrophages with apoA-I or the apoA-I mimetic peptide 4F also attenuated the expression of TLR-4, CD14 and lipid rafts [77]. This led to an abolition of the inflammatory gene response to LPS while boosting levels of IL-10. Another study examined the effect of HDL on inhibiting the inflammatory response of macrophages to the TLR4 ligand LPS and found that it was the TRAM/TRIF arm of the TLR4 signaling branch that was largely suppressed [78]. This was independent of the cholesterol removal by ABCA1 or ABCG1 or passive efflux pathways as HDL somehow removed this complex from the inner-cell membrane to intracellular compartments where it could not be activated (Figure 3.B). This suggests that HDL can inhibit both the MyD88 and TRAM/TRIF actions of TLR4 activation.
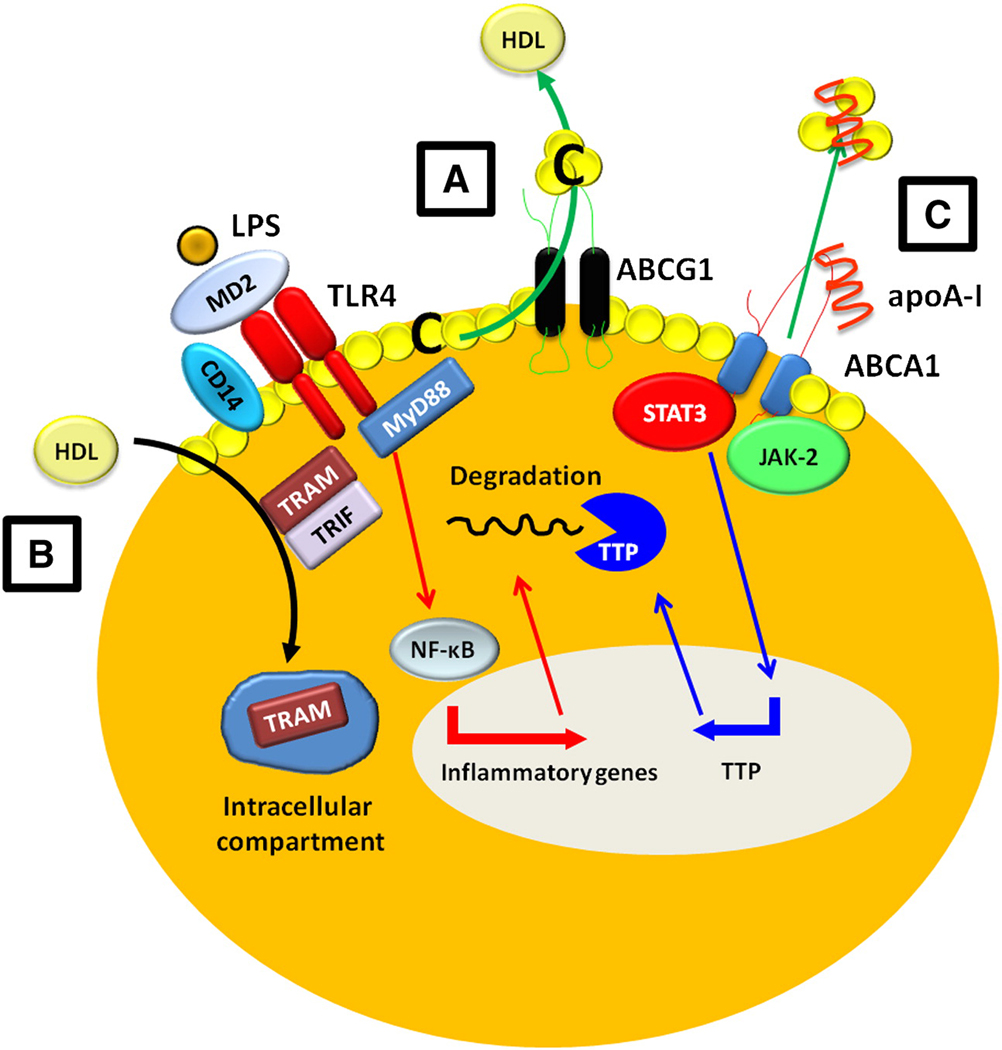
Figure 3. Anti-inflammatory functions of HDL on the macrophage.
ABCA1 and ABCG1 promote cholesterol efflux from macrophages to apoA-I/HDL. This interaction can attenuate macrophage inflammation via a number of pathways. A) The removal of cholesterol from lipid rafts can decrease TLR-4/CD14 expression on the surface of the macrophage desensitizing signaling by LPS. B) HDL can inhibit LPS stimulated type I INF response independent of sterol metabolism via a mechanism involving removal of the TRAM signaling molecule away from the cell membrane and into intracellular compartments. C) Binding of apoA-I to ABCA1 causes the activation of JAK-2 which then allows STAT-3 to directly bind and become activated by ABCA1. This results in the transcription of TTP which targets and degrades the inflammatory mRNAs transcribed by NF-κB from LPS signaling. C=Cholesterol
4.3.2. HDL Attenuates LPS Signaling: Mechanisms Involving CD14
The interaction between cholesterol efflux and TLR signaling is not the only avenue that HDL takes to reduce LPS signaling. A number of studies have shown that HDL can bind, sequester and neutralize LPS preventing the activation of monocytes and macrophages. LPS bound to soluble CD14 can be shuttled to HDL in a process involving LPS-binding protein (LPS-BP) and neutralized [79–80]. HDL can also neutralize LPS activity by promoting its release from the surface of monocytes and macrophages [81]. CD14 is essential for MyD88-independent LPS signaling through TLR4 [82]. Non-insulin dependent diabetic subjects with CVD have increased levels of CD14 on the surface of CD14+CD16- monocytes compared to healthy subjects [83]. HDL levels were inversely correlated with CD14 levels. Interestingly, HDL and apoA-I can attenuate the surface expression of CD14 on monocytes [77, 84].
Infusion of rHDL in healthy individuals protects from the inflammatory events caused by LPS [84]. Subjects with higher HDL levels have also been reported to tolerate an endotoxin challenge better than subjects with low HDL [85]. Infusion of rHDL also lowered the number of monocytes and neutrophils over a 24h period compared to placebo [84]. This could reflect a decrease in the production of monocytes and neutrophils by the mechanisms we have recently described in mice [11]. Infusion of rHDL also prevents monocyte activation as assessed by CD11b levels, possibly by acting on the monocyte to down regulate CD14 expression [84]. Pre-incubation of monocytes with HDL followed by addition of LPS to monocytes where HDL had been removed also results in protection and may reflect changes in TLR4 and/or CD14 expression [49]. Therefore it would appear that HDL, via a number of mechanisms, acts as a negative regulator of LPS induced inflammation.
4.3.4. ABC Transporters modulate the response to Efferocytosis
Efferocytosis is the process in which apoptotic cells are cleared by phagocytic cells. Efferocytosing Abca1−/−Abcg1−/− macrophages are more prone to apoptosis [7]. While transporter deficient cells displayed no difference in the ability to phagocytose apoptotic cells, this led to in dramatic apoptosis of the macrophages. Oxidized phospholipids were shown to cause the increased apoptosis which could be attenuated by incubating the macrophages with HDL. There was also a decrease in apoptosis of the macrophages after phagocytosis when macrophages were pre-incubated with cyclodextrin to remove cholesterol. Macrophages from Abca1−/− Abcg1−/− mice had an increased oxidative burst response compared to WT. The enhanced oxidative burst reflected increased assembly of NOX2 and the apoptosis was secondary to early activation of and sustained signaling through the c-Jun N-terminal kinase (JNK) pathway, likely due to increases ROS formation inactivating JNK phosphatases [86]. Apoptosis could be suppressed by the addition of anti-oxidants, HDL or cyclodextrin. Increased TLR-4 expression as observed in the previous study [7], was also a contributor to enhanced NOX2 mediated ROS production and apoptosis. In a different study ABCG1 was identified as an important transporter of inflammatory oxysterols from the cell. Formed on LDL particles or consumed in the diet, oxysterols are toxic to cells and are believed to have a pivotal role in atherogenesis. Macrophages deficient in ABCG1 accumulate 7-ketocholesterol which results in apoptosis due to oxysterol-induced cytotoxicity [6]. These findings suggest that uptake of cholesterol-rich apoptotic cells or necrotic debris by macrophages requires functional ABCA1 and ABCG1 in order to preserve their viability and to suppress inflammatory responses.
4.3.5. ApoA-I Binding to ABCA1 in macrophages Induces signaling via JAK2/STAT3 pathway
Oram and colleagues have shown that the interaction of apoA-I with ABCA1 leads to activation of Janus Kinase 2 (JAK2). JAK2 activation further increased binding of apoA-I to ABCA1 and increased lipid efflux. JAK-2 activation appeared to recruit signal transducer and activator of transcription-3 (STAT-3) to ABCA1, leading to its phosphorylation [87]. The cytoplasmic domain of ABCA1 contains two candidate STAT3 docking sites that conform to the consensus sequence and are highly conserved across a number of different species from chimpanzees to platypi [88]. Mutating these sites reduced p-STAT3 but had no effect on cholesterol efflux, suggesting a divergent signaling pathway. The JAK/STAT pathway is commonly associated with pro-inflammatory and oncogenic gene expression [89]. However, in macrophages STAT3 appeared to be functioning as an anti-inflammatory molecule [90–91]. Activation of the JAK-2/STAT3 pathway in macrophages by apoA-I suppressed LPS-induced pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6. Activation of STAT3 by apoA-I/ABCA1 interactions led to its translocation to the nucleus and increased the expression of the mRNA-destabilizing protein tristetraprolin (TTP) [92]. TTP targets AU-rich elements (AREs) in the 3'untranslated regions of the mRNA of pro-inflammatory cytokines and degrades the mRNA thus inhibiting translation. Disrupting the JAK/STAT pathway did not completely reverse the ability of apoA-I to suppress the LPS-induced gene expression, consistent with involvement of other pathways such as those noted above.
4.3.6. Cholesterol Efflux and Macrophage Migration
Macrophage emigration from the atherosclerotic lesion and the role this plays lesion regression is currently under active investigation. Using a aortic transplantation model, established lesions from Apoe−/− mice were transplanted into WT, Apoa-I−/−, Apoe−/− or Apoe−/−Apoa-I transgenic mice. Transplantation into WT mice lead to lesion regression highlighted by a depletion of macrophage-foam cells, whereas no regression was observed in lesions transplanted into Apoa-I−/− or Apoe−/− mice [93]. This finding was thought to be due to a decrease in emigration of monocyte/macrophages from the lesion after conducting bead labeling studies. Monocyte entry was not measured in these studies. The presence of the human Apoa-I transgene was able to reverse the defective regression observed in the Apoe−/− mice suggesting the importance of cholesterol efflux pathways. A similar finding was observed using a genetic switch to lower cholesterol levels in mice on an Ldlr−/− background (Reversa mice) [94]. The egress of the macrophages appeared to involve CCR7 which may be induced by LXRs and somewhat paradoxically also by cholesterol removal from cells [93–95].
Loading macrophages with cholesterol causes membrane ruffling and spreading of the cell with reversal by cyclodextrin [96–97]. Cholesterol loading resulted in increased Rac-1 activity, while decreasing RhoA and myosin light chain activation and reducing migratory responses [98]. This prevented polarization of the macrophage, where under normal conditions a leading edge would extend and anchor while the trailing edge detaches and allow the cell to move. The migratory defect in Abca1−/− Abcg1−/− macrophages was recently shown to be caused by increased cholesterol content on the inner leaflet of the plasma membrane and is associated with Rac-1 localization [99]. Abca1−/− Abcg1−/− macrophages are unable to move sterol from the inner leaflet to the outer leaflet of the plasma membrane and this likely prevents the cycling of the small GTPases such as Rac-1 to and from the plasma membrane and holds them in their activate GTP state [99].
The importance of macrophage egress in the regression of atherosclerosis has recently been questioned, albeit in a different model to that using aortic transplantation. This study employed an adenovirus to restore Apoe expression (Ad-Apoe) in the liver of Apoe−/− mice with established lesions, which led to a 4-fold increase in HDL in the active treatment group. Using bead labeling to track monocytes it was shown that fewer monocytes entered the lesion after ApoE re-expression, while disappearance of bead labeled cells proceeded at a low basal rate that was not different between mice infused with Apoe or control virus. A reduction of neutral lipid staining in plaques preceded the decrease in macrophages in the lesions of Ad-Apoe compared to Ad-empty infused Apoe−/− mice [100]. A significant reduction in macrophage content in the lesions of Ad-Apoe mice was only observed after 2 weeks. It is possible that the decrease in lipids observed after the first week changed the phenotype of the macrophage from pro-inflammatory to anti-inflammatory, in association with cholesterol efflux. Thus macrophages would no longer be secreting high amounts of chemokines such as MCP-1, resulting in decreased monocyte recruitment. Re-expression of Apoe also resulted in a decrease in monocyte activation also possibly due increased cholesterol efflux [49].
5. Conclusions and Future Directions
There appears to be a discordance between the relative lack of success of HDL raising strategies in the clinic, and the plethora of beneficial actions of HDL that have been demonstrated in cell culture and animal models. It is clear that not all strategies for raising HDL are likely to be beneficial. There is a need for more critical evaluation of the different proposed functionalities of HDL, as outlined here and elsewhere [101–104] both in animal models and in the clinic. Our thesis is that the central beneficial effects of HDL relate to its ability to promote cholesterol efflux via ABCA1/G1 and by passive mechanisms, with many secondary effects including reduced inflammatory signaling in macrophages, decreased proliferation of HSPCs and reduced monocytosis, and increase eNOS activity in endothelium. However, there are specific signaling events such as eNOS activation [42] and decreased adhesion molecule expression [46] on endothelial cells along with increased glucose uptake in skeletal muscle cells and insulin secretion from pancreatic β-cells [105–106] that appear not to be dependent on cholesterol efflux mechanisms. The relative importance of these different pathways and mechanisms in various cell types including hematopoietic stem cells, different kinds of myeloid cells and endothelial cells remains to be determined.
Abbreviations
- HDL
high Density Lipoprotein
- apo
apolipoprotein
- ABC
ATP Binding cassette transporter
- TLR
toll like receptor
- HSPC
hematopoietic stem and multipotent progenitor cell
- IL
interleukin
- CBS
IL-3 receptor common beta-subunit
- GM-CSF
granulocyte macrophage-colony stimulating factor
- BM
bone marrow
- Ldlr
low density lipoprotein receptor
- JAK-2
Janus Kinase 2
- STAT-3
signal transducer and activator of transcription-3
References
- 1.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KJ, Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr Opin Lipidol. 1998;9:471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terasaka N, Wang N, Yvan-Charvet L, Tall AR. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc Natl Acad Sci U S A. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yvan-Charvet L, Pagler TA, Seimon TA, Thorp E, Welch CL, Witztum JL, Tabas I, Tall AR. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res. 2010;106:1861–1869. doi: 10.1161/CIRCRESAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy AJ, Chin-Dusting JP, Sviridov D, Woollard KJ. The anti inflammatory effects of high density lipoproteins. Curr Med Chem. 2009;16:667–675. doi: 10.2174/092986709787458425. [DOI] [PubMed] [Google Scholar]
- 10.Murphy AJ, Woollard KJ. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol. 2010;37:710–718. doi: 10.1111/j.1440-1681.2009.05338.x. [DOI] [PubMed] [Google Scholar]
- 11.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oram JF, Mendez AJ, Lymp J, Kavanagh TJ, Halbert CL. Reduction in apolipoprotein-mediated removal of cellular lipids by immortalization of human fibroblasts and its reversion by cAMP: lack of effect with Tangier disease cells. J Lipid Res. 1999;40:1769–1781. [PubMed] [Google Scholar]
- 14.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 15.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 16.Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104:R25–R31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remaley AT, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, Peterson KM, Koch C, Arnould I, Prades C, Duverger N, Funke H, Assman G, Dinger M, Dean M, Chimini G, Santamarina-Fojo S, Fredrickson DS, Denefle P, Brewer HB., Jr Human ATP-binding cassette transporter 1 (ABC1): genomic organization and identification of the genetic defect in the original Tangier disease kindred. Proc Natl Acad Sci U S A. 1999;96:12685–12690. doi: 10.1073/pnas.96.22.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 24.Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, Hasegawa K, Yokode M, Kimura T, Kita T. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci U S A. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Grouw EP, Raaijmakers MH, Boezeman JB, van der Reijden BA, van de Locht LT, de Witte TJ, Jansen JH, Raymakers RA. Preferential expression of a high number of ATP binding cassette transporters in both normal and leukemic CD34+CD38− cells. Leukemia. 2006;20:750–754. doi: 10.1038/sj.leu.2404131. [DOI] [PubMed] [Google Scholar]
- 27.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeters SD, van der Kolk DM, de Haan G, Bystrykh L, Kuipers F, de Vries EG, Vellenga E. Selective expression of cholesterol metabolism genes in normal CD34+CD38− cells with a heterogeneous expression pattern in AML cells. Exp Hematol. 2006;34:622–630. doi: 10.1016/j.exphem.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Tang L, Bergevoet SM, Gilissen C, de Witte T, Jansen JH, van der Reijden BA, Raymakers RA. Hematopoietic stem cells exhibit a specific ABC transporter gene expression profile clearly distinct from other stem cells. BMC Pharmacol. 2010;10:12. doi: 10.1186/1471-2210-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes AL, Carvalho T, Serpa J, Torre C, Dias S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood. 2010;115:3886–3894. doi: 10.1182/blood-2009-08-240580. [DOI] [PubMed] [Google Scholar]
- 31.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 32.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy AJ, Pagler T, Akhtari M, Tall AR. Abstract 19263: Enhancing Cholesterol Efflux Pathways Suppresses Hematopoietic Stem Cell Proliferation and Monocytosis in apoE−/− Mice. Circulation. 2010;122:A19263. [Google Scholar]
- 36.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, Lusis AJ, Rajavashisth TB. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 37.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong AJ, Gebre AK, Parks JS, Hedrick CC. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J Immunol. 2010;184:173–183. doi: 10.4049/jimmunol.0902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Owen J, Bharadwaj M, Walzem R, Chan L, Oka K, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler Thromb Vasc Biol. 2009;29:843–849. doi: 10.1161/ATVBAHA.108.183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, Bhat S, Gibbs DP, Jr, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr−/−, ApoA-I−/− mice. J Biol Chem. 2010;285:36158–36169. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci U S A. 2004;101:6999–7004. doi: 10.1073/pnas.0306266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong M, Wilson M, Kelly T, Su W, Dressman J, Kincer J, Matveev SV, Guo L, Guerin T, Li XA, Zhu W, Uittenbogaard A, Smart EJ. HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J Clin Invest. 2003;111:1579–1587. doi: 10.1172/JCI16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 45.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 46.McGrath KC, Li XH, Puranik R, Liong EC, Tan JT, Dy VM, DiBartolo BA, Barter PJ, Rye KA, Heather AK. Role of 3beta-hydroxysteroid-delta 24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:877–882. doi: 10.1161/ATVBAHA.109.184663. [DOI] [PubMed] [Google Scholar]
- 47.Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, Rye KA, Barter PJ. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–1550. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 48.Puranik R, Bao S, Nobecourt E, Nicholls SJ, Dusting GJ, Barter PJ, Celermajer DS, Rye KA. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis. 2008;196:240–247. doi: 10.1016/j.atherosclerosis.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 50.Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol. 2009;53:962–971. doi: 10.1016/j.jacc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 52.Diederich W, Orso E, Drobnik W, Schmitz G. Apolipoprotein AI and HDL(3) inhibit spreading of primary human monocytes through a mechanism that involves cholesterol depletion and regulation of CDC42. Atherosclerosis. 2001;159:313–324. doi: 10.1016/s0021-9150(01)00518-4. [DOI] [PubMed] [Google Scholar]
- 53.Bursill CA, Castro ML, Beattie DT, Nakhla S, van der Vorst E, Heather AK, Barter PJ, Rye KA. High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2010;30:1773–1778. doi: 10.1161/ATVBAHA.110.211342. [DOI] [PubMed] [Google Scholar]
- 54.Soehnlein O. An elegant defense: how neutrophils shape the immune response. Trends Immunol. 2009 doi: 10.1016/j.it.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Sweetnam PM, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol. 1997;145:416–421. doi: 10.1093/oxfordjournals.aje.a009123. [DOI] [PubMed] [Google Scholar]
- 56.Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Björkström NK, Malmberg KJ, Lindbom L, Eriksson EE. Distinct infiltration of neutrophils in lesion shoulders in ApoE−/− mice. Am J Pathol. 2010;177:493–500. doi: 10.2353/ajpath.2010.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Araujo FB, Barbosa DS, Hsin CY, Maranhao RC, Abdalla DS. Evaluation of oxidative stress in patients with hyperlipidemia. Atherosclerosis. 1995;117:61–71. doi: 10.1016/0021-9150(94)05558-z. [DOI] [PubMed] [Google Scholar]
- 58.Mazor R, Shurtz-Swirski R, Farah R, Kristal B, Shapiro G, Dorlechter F, Cohen-Mazor M, Meilin E, Tamara S, Sela S. Primed polymorphonuclear leukocytes constitute a possible link between inflammation and oxidative stress in hyperlipidemic patients. Atherosclerosis. 2008;197:937–943. doi: 10.1016/j.atherosclerosis.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 60.Oh H, Mohler ER, 3rd, Tian A, Baumgart T, Diamond SL. Membrane cholesterol is a biomechanical regulator of neutrophil adhesion. Arterioscler Thromb Vasc Biol. 2009;29:1290–1297. doi: 10.1161/ATVBAHA.109.189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, Krohn R, Schober A, Sperandio M, Soehnlein O, Bornemann J, Tacke F, Biessen EA, Weber C. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 62.Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D, Chin-Dusting JP. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein a-I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- 63.Itoh S, Susuki C, Takeshita K, Nagata K, Tsuji T. Redistribution of P-selectin glycoprotein ligand-1 (PSGL-1) in chemokine-treated neutrophils: a role of lipid microdomains. J Leukoc Biol. 2007;81:1414–1421. doi: 10.1189/jlb.0606398. [DOI] [PubMed] [Google Scholar]
- 64.Kannan KB, Barlos D, Hauser CJ. Free cholesterol alters lipid raft structure and function regulating neutrophil Ca2+ entry and respiratory burst: correlations with calcium channel raft trafficking. J Immunol. 2007;178:5253–5261. doi: 10.4049/jimmunol.178.8.5253. [DOI] [PubMed] [Google Scholar]
- 65.Pierini LM, Eddy RJ, Fuortes M, Seveau S, Casulo C, Maxfield FR. Membrane lipid organization is critical for human neutrophil polarization. J Biol Chem. 2003;278:10831–10841. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- 66.Shao D, Segal AW, Dekker LV. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- 67.Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods. 2007;4:219–222. doi: 10.1038/nmeth1018. [DOI] [PubMed] [Google Scholar]
- 68.Woollard KJ, Suhartoyo A, Harris EE, Eisenhardt SU, Jackson SP, Peter K, Dart AM, Hickey MJ, Chin-Dusting JP. Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ Res. 2008;103:1128–1138. doi: 10.1161/CIRCRESAHA.108.180273. [DOI] [PubMed] [Google Scholar]
- 69.Dansky HM, Charlton SA, Barlow CB, Tamminen M, Smith JD, Frank JS, Breslow JL. Apo A-I inhibits foam cell formation in Apo E-deficient mice after monocyte adherence to endothelium. J Clin Invest. 1999;104:31–39. doi: 10.1172/JCI6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Out R, Jessup W, Le Goff W, Hoekstra M, Gelissen IC, Zhao Y, Kritharides L, Chimini G, Kuiper J, Chapman MJ, Huby T, Van Berkel TJ, Van Eck M. Coexistence of foam cells and hypocholesterolemia in mice lacking the ABC transporters A1 and G1. Circ Res. 2008;102:113–120. doi: 10.1161/CIRCRESAHA.107.161711. [DOI] [PubMed] [Google Scholar]
- 71.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 75.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 76.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M, Kurundkar AR, Datta G. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. Am J Physiol Cell Physiol. 2010;298:C1538–C1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki M, Pritchard DK, Becker L, Hoofnagle AN, Tanimura N, Bammler TK, Beyer RP, Bumgarner R, Vaisar T, de Beer MC, de Beer FC, Miyake K, Oram JF, Heinecke JW. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122:1919–1927. doi: 10.1161/CIRCULATIONAHA.110.961193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park CT, Wright SD. Plasma lipopolysaccharide-binding protein is found associated with a particle containing apolipoprotein A-I, phospholipid, and factor H-related proteins. J Biol Chem. 1996;271:18054–18060. doi: 10.1074/jbc.271.30.18054. [DOI] [PubMed] [Google Scholar]
- 80.Wurfel MM, Hailman E, Wright SD. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitchens RL, Wolfbauer G, Albers JJ, Munford RS. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J Biol Chem. 1999;274:34116–34122. doi: 10.1074/jbc.274.48.34116. [DOI] [PubMed] [Google Scholar]
- 82.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 83.Patino R, Ibarra J, Rodriguez A, Yague MR, Pintor E, Fernandez-Cruz A, Figueredo A. Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. Am J Cardiol. 2000;85:1288–1291. doi: 10.1016/s0002-9149(00)00757-8. [DOI] [PubMed] [Google Scholar]
- 84.Pajkrt D, Doran JE, Koster F, Lerch PG, Arnet B, van der Poll T, ten Cate JW, van Deventer SJ. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birjmohun RS, van Leuven SI, Levels JH, van 't Veer C, Kuivenhoven JA, Meijers JC, Levi M, Kastelein JJ, van der Poll T, Stroes ES. High-density lipoprotein attenuates inflammation and coagulation response on endotoxin challenge in humans. Arterioscler Thromb Vasc Biol. 2007;27:1153–1158. doi: 10.1161/ATVBAHA.106.136325. [DOI] [PubMed] [Google Scholar]
- 86.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 87.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaughan AM, Tang C, Oram JF. ABCA1 mutants reveal an interdependency between lipid export function, apoA-I binding activity, and Janus kinase 2 activation. J Lipid Res. 2009;50:285–292. doi: 10.1194/jlr.M800366-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams LM, Sarma U, Willets K, Smallie T, Brennan F, Foxwell BM. Expression of constitutively active STAT3 can replicate the cytokine-suppressive activity of interleukin-10 in human primary macrophages. J Biol Chem. 2007;282:6965–6975. doi: 10.1074/jbc.M609101200. [DOI] [PubMed] [Google Scholar]
- 90.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165:1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 91.O'Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–1018. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin K, Deng X, Mo ZC, Zhao GJ, Jiang J, Cui LB, Tan CZ, Wen GB, Fu Y, Tang CK. Tristetraprolin-dependent Post-transcriptional Regulation of Inflammatory Cytokine mRNA Expression by Apolipoprotein A-I: ROLE OF ATP-BINDING MEMBRANE CASSETTE TRANSPORTER A1 AND SIGNAL TRANSDUCER AND ACTIVATOR OF TRANSCRIPTION 3. J Biol Chem. 2011;286:13834–13845. doi: 10.1074/jbc.M110.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van Rooijen N, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaus K, Kritharides L, Schmitz G, Boettcher A, Drobnik W, Langmann T, Quinn CM, Death A, Dean RT, Jessup W. Apolipoprotein A-1 interaction with plasma membrane lipid rafts controls cholesterol export from macrophages. FASEB J. 2004;18:574–576. doi: 10.1096/fj.03-0486fje. [DOI] [PubMed] [Google Scholar]
- 97.Qin C, Nagao T, Grosheva I, Maxfield FR, Pierini LM. Elevated plasma membrane cholesterol content alters macrophage signaling and function. Arterioscler Thromb Vasc Biol. 2006;26:372–378. doi: 10.1161/01.ATV.0000197848.67999.e1. [DOI] [PubMed] [Google Scholar]
- 98.Nagao T, Qin C, Grosheva I, Maxfield FR, Pierini LM. Elevated cholesterol levels in the plasma membranes of macrophages inhibit migration by disrupting RhoA regulation. Arterioscler Thromb Vasc Biol. 2007;27:1596–1602. doi: 10.1161/ATVBAHA.107.145086. [DOI] [PubMed] [Google Scholar]
- 99.Pagler TA, Wang M, Mondal M, Murphy AJ, Westerterp M, Moore KJ, Maxfield FR, Tall AR. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ Res. 2011;108:194–200. doi: 10.1161/CIRCRESAHA.110.228619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy AJ, Remaley AT, Sviridov D. HDL therapy: two kinds of right? Curr Pharm Des. 2010;16:4134–4147. doi: 10.2174/138161210794519228. [DOI] [PubMed] [Google Scholar]
- 103.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rye KA, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2009;50 Suppl:S195–S200. doi: 10.1194/jlr.R800034-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, Thomas WG, Mukhamedova N, de Courten B, Forbes JM, Yap FY, Kaye DM, van Hall G, Febbraio MA, Kemp BE, Sviridov D, Steinberg GR, Kingwell BA. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–2111. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 106.Fryirs MA, Barter PJ, Appavoo M, Tuch BE, Tabet F, Heather AK, Rye KA. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler Thromb Vasc Biol. 2010;30:1642–1648. doi: 10.1161/ATVBAHA.110.207373. [DOI] [PubMed] [Google Scholar]