Abstract
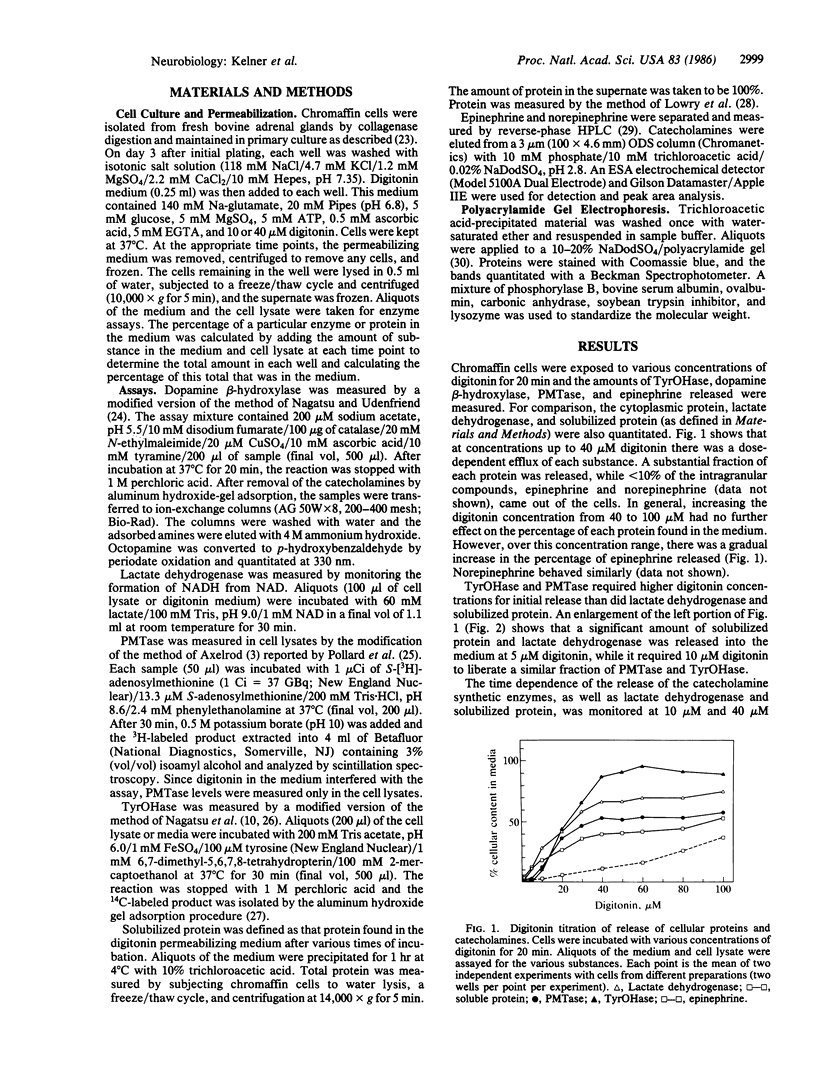
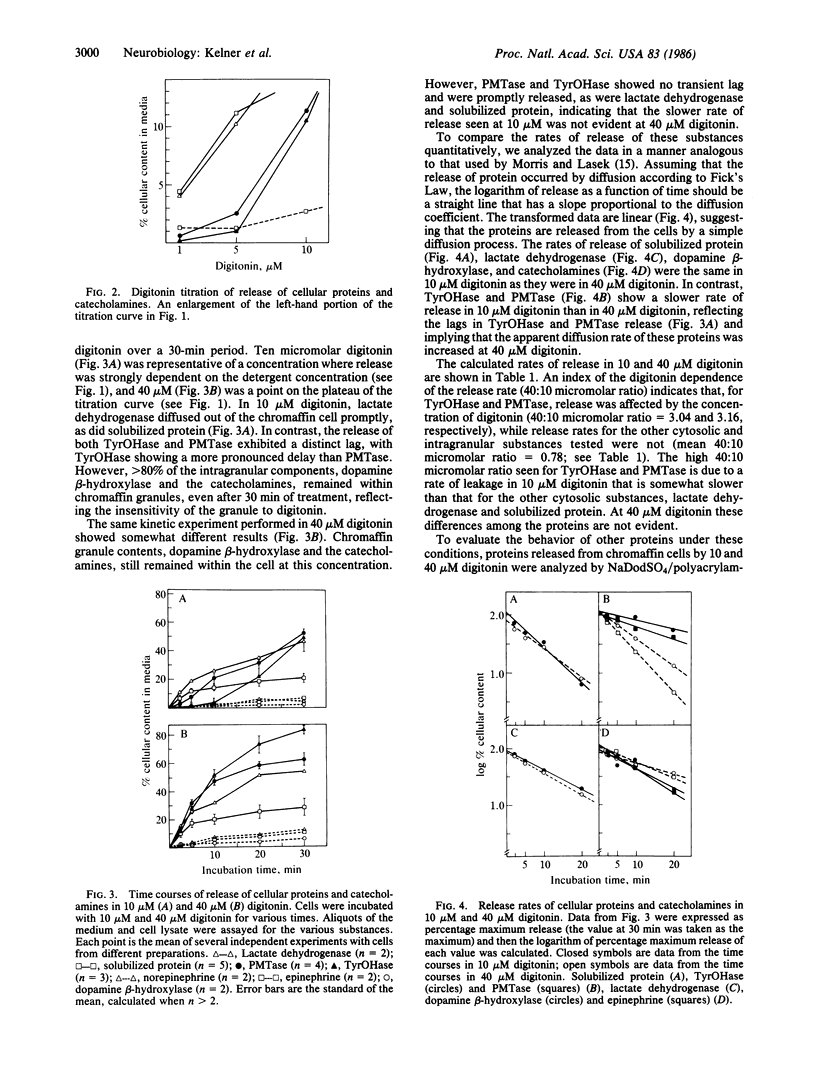
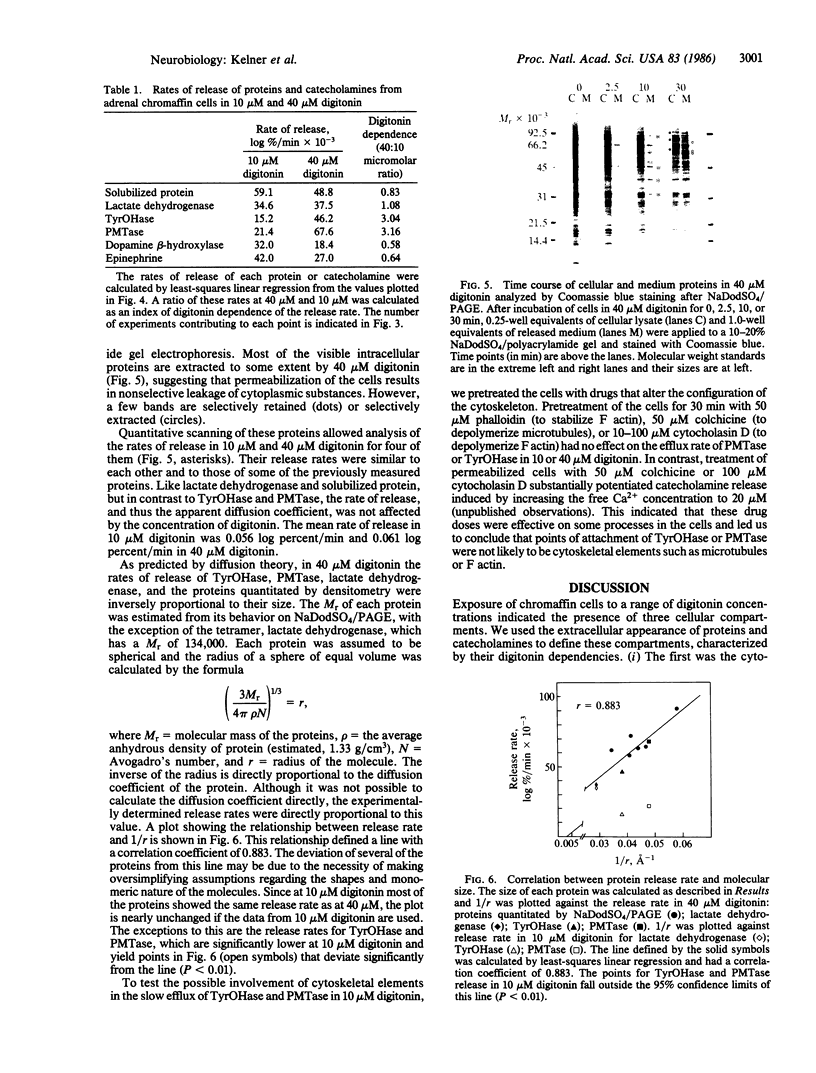
Tyrosine hydroxylase [TyrOHase; tyrosine 3-monooxygenase; L-tyrosine,tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2] and phenylethanolamine N-methyltransferase, EC 2.1.1.28) are involved in catecholamine biosynthesis and are considered soluble proteins. However, they may actually be localized on the surface of the chromaffin granule. We have used the detergent digitonin to permeabilize the plasma membrane of cultured adrenal chromaffin cells to investigate the subcellular localization of TyrOHase and PMTase. A digitonin titration of the release of proteins and catecholamines revealed the existence of at least three subcellular compartments that are distinguished by their digitonin sensitivity: (i) soluble proteins, which were released upon treatment of the cells with low digitonin concentrations (5 microM), (ii) a "digitonin-sensitive" cytoplasmic protein pool, which required higher concentrations of digitonin for release (10 microM) and included TyrOHase and PMTase, and (iii) the chromaffin granule, which was insensitive to digitonin. Analysis of the rates of release of all of these proteins revealed that the rate of TyrOHase and PMTase release was slower at 10 microM than at 40 microM digitonin, while the rates of release of the other proteins were similar at both concentrations and varied in proportion to their respective sizes. Treatment with cytoskeletal disrupting agents had no effect on TyrOHase or PMTase efflux. These data suggest that TyrOHase and PMTase are in a detergent-labile association in the cell. This is consistent with the concept that TyrOHase and PMTase may be localized on the surface of the chromaffin granule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J. Purification and properties of phenylethanolamine-N-methyl transferase. J Biol Chem. 1962 May;237:1657–1660. [PubMed] [Google Scholar]
- Akerman K. E., Nicholls D. G. Intrasynaptosomal compartmentation of calcium during depolarization-induced calcium uptake across the plasma membrane. Biochim Biophys Acta. 1981 Jul 6;645(1):41–48. doi: 10.1016/0005-2736(81)90509-5. [DOI] [PubMed] [Google Scholar]
- BURN G. P., FIELD E. O. A colorimetric method for the diagnosis of phaeochromocytoma. Br Med J. 1956 Nov 17;2(5002):1152–1154. doi: 10.1136/bmj.2.5002.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannata J. J., Valle E., Docampo R., Cazzulo J. J. Subcellular localization of phosphoenolpyruvate carboxykinase in the trypanosomatids Trypanosoma cruzi and Crithidia fasciculata. Mol Biochem Parasitol. 1982 Sep;6(3):151–160. doi: 10.1016/0166-6851(82)90074-3. [DOI] [PubMed] [Google Scholar]
- Dunn L. A., Holz R. W. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4989–4993. [PubMed] [Google Scholar]
- Joh T. H., Goldstein M. Isolation and characterization of multiple forms of phenylethanolamine N-methyltransferase. Mol Pharmacol. 1973 Jan;9(1):117–129. [PubMed] [Google Scholar]
- Kelner K. L., Pollard H. B. Glucocorticoid receptors and regulation of phenylethanolamine-N-methyltransferase activity in cultured chromaffin cells. J Neurosci. 1985 Aug;5(8):2161–2168. doi: 10.1523/JNEUROSCI.05-08-02161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laduron P. M. Evidence for a localization of dopamine-beta-hydroxylase within the chromaffin granules. FEBS Lett. 1975 Mar 15;52(1):132–134. doi: 10.1016/0014-5793(75)80654-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leake D. S., Heald B., Peters T. J. Properties and subcellular localization of acid phosphatase activity in cultured arterial smooth muscle cells. Eur J Biochem. 1982 Nov 15;128(2-3):557–563. doi: 10.1111/j.1432-1033.1982.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Masters C. Interactions between glycolytic enzymes and components of the cytomatrix. J Cell Biol. 1984 Jul;99(1 Pt 2):222s–225s. doi: 10.1083/jcb.99.1.222s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A. M., Keith A. D. Diffusion in the aqueous compartment. J Cell Biol. 1984 Jul;99(1 Pt 2):180s–187s. doi: 10.1083/jcb.99.1.180s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. R., Lasek R. J. Monomer-polymer equilibria in the axon: direct measurement of tubulin and actin as polymer and monomer in axoplasm. J Cell Biol. 1984 Jun;98(6):2064–2076. doi: 10.1083/jcb.98.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Nagatsu I., Karasawa N., Kondo Y., Inagaki S. Immunocytochemical localization of tyrosine hydroxylase, dopamine-beta-hydroxylase and phenylethanolamine-N-methyltransferase in the adrenal glands of the frog and rat by a peroxidase-antiperoxidase method. Histochemistry. 1979 Nov;64(2):131–144. doi: 10.1007/BF00490094. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Levitt M., Udenfriend S. Conversion of L-tyrosine to 3,4-dihydroxyphenylalanine by cell-free preparations of brain and sympathetically innervated tissues. Biochem Biophys Res Commun. 1964;14:543–549. doi: 10.1016/0006-291x(64)90266-9. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Udenfriend S. Photometric assay of dopamine- -hydroxylase activity in human blood. Clin Chem. 1972 Sep;18(9):980–983. [PubMed] [Google Scholar]
- Paine P. L. Diffusive and nondiffusive proteins in vivo. J Cell Biol. 1984 Jul;99(1 Pt 2):188s–195s. doi: 10.1083/jcb.99.1.188s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. M., Pedersen P. L. Intracellular localization and properties of particulate hexokinase in the Novikoff ascites tumor. Evidence for an outer mitochondrial membrane location. J Biol Chem. 1983 Sep 25;258(18):10904–10912. [PubMed] [Google Scholar]
- Petrack B., Sheppy F., Fetzer V. Studies on tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1968 Feb 25;243(4):743–748. [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Reis D. J. Monoamine-synthesizing enzymes in central dopaminergic, noradrenergic and serotonergic neurons. Immunocytochemical localization by light and electron microscopy. J Histochem Cytochem. 1976 Jul;24(7):792–306. doi: 10.1177/24.7.8567. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Stopak S. S., Pazoles C. J., Creutz C. E. A simplified, one-step method for radiometric analysis of phenylethanolamine-N-methyltransferase in adrenal chromaffin cells. Anal Biochem. 1979 Nov 1;99(2):281–282. doi: 10.1016/s0003-2697(79)80007-x. [DOI] [PubMed] [Google Scholar]
- Rijksen G., Staal G. E., Beks P. J., Streefkerk M., Akkerman J. W. Compartmentation of hexokinase in human blood cells. Characterization of soluble and particulate enzymes. Biochim Biophys Acta. 1982 Dec 17;719(3):431–437. doi: 10.1016/0304-4165(82)90230-6. [DOI] [PubMed] [Google Scholar]
- Sabban E. L., Goldstein M. Subcellular site of biosynthesis of the catecholamine biosynthetic enzymes in bovine adrenal medulla. J Neurochem. 1984 Dec;43(6):1663–1668. doi: 10.1111/j.1471-4159.1984.tb06093.x. [DOI] [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- Stephens J. K., Masserano J. M., Vulliet P. R., Weiner N., Nakane P. K. Immunocytochemical localization of tyrosine hydroxylase in rat adrenal medulla by the peroxidase labeled antibody method: effects of enzyme activation on ultrastructural distribution of the enzyme. Brain Res. 1981 Mar 30;209(2):339–354. doi: 10.1016/0006-8993(81)90158-x. [DOI] [PubMed] [Google Scholar]
- Treiman M., Weber W., Gratzl M. 3',5'-cyclic adenosine monophosphate- and Ca2+-calmodulin-dependent endogenous protein phosphorylation activity in membranes of the bovine chromaffin secretory vesicles: identification of two phosphorylated components as tyrosine hydroxylase and protein kinase regulatory subunit type II. J Neurochem. 1983 Mar;40(3):661–669. doi: 10.1111/j.1471-4159.1983.tb08031.x. [DOI] [PubMed] [Google Scholar]
- Van Orden L. S., Burke J. P., Redick J. A., Rybarczyk K. E., Van Orden D. E., Baker H. A., Hartman B. K. Immunocytochemical evidence for particulate localization of phenylethanolamine-N-methyltransferase in adrenal medulla. Neuropharmacology. 1977 Feb;16(2):129–133. doi: 10.1016/0028-3908(77)90060-0. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4994–5000. [PubMed] [Google Scholar]
- Wurzburger R. J., Musacchio J. M. Subcellular distribution and aggregation of bovine adrenal tyrosine hydroxylase. J Pharmacol Exp Ther. 1971 Apr;177(1):155–168. [PubMed] [Google Scholar]








