Abstract
Dileptid and tracheliid ciliates have been traditionally classified within the subclass Haptoria of the class Litostomatea. However, their phylogenetic position among haptorians has been controversial and indicated that they may play a key role in understanding litostomatean evolution. In order to reconstruct the evolutionary history of dileptids and tracheliids, and to unravel their affinity to other haptorians, we have used a cladistic approach based on morphological evidence and a phylogenetic approach based on 18S rRNA gene sequences, including eight new ones. The molecular trees demonstrate that dileptids and tracheliids represent a separate subclass, Rhynchostomatia, that is sister to the subclasses Haptoria and Trichostomatia. The Rhynchostomatia are characterized by a ventrally located oral opening at the base of a proboscis that carries a complex oral ciliature. We have recognized two orders within Rhynchostomatia. The new order Tracheliida is monotypic, while the order Dileptida comprises two families: the new, typically bimacronucleate family Dimacrocaryonidae and the multimacronucleate family Dileptidae. The Haptoria evolved from the last common ancestor of the Litostomatea by polarization of the body, the oral opening locating more or less apically and the oral ciliature simplifying. The Trichostomatia originated from a microaerophylic haptorian by further simplification of the oral ciliature, possibly due to an endosymbiotic lifestyle.
Keywords: 18S rRNA gene, Apodileptus, Cladistics, Monomacrocaryon, Rhynchostomatia, Trachelius
Introduction
The first dileptid ciliate was discovered by Müller (1773) among duckweed (Lemna) in freshwater from Denmark. Sixty-eight years later, Dujardin (1841) assigned Müller's and two other nominal species to a new genus, Dileptus, defined by two gradually narrowing body ends representing a proboscis and a tail. The proboscis, the most characteristic feature of dileptids and tracheliids, has fascinated not only early protistologists (e.g., Claparède and Lachmann 1859; Ehrenberg 1838; Penard 1922; Schewiakoff 1896; Schrank 1803; Wrześniowski 1870), but also more recent researchers who have been particularly interested in its regeneration (e.g., Golińska 1974, 1978) and development (Golińska 1995; Vďačný and Foissner 2009). Moreover, the name-bearing type genus Dileptus has become a model organism in a number of studies on regulation of ciliary pattern (Golińska 1982, 1983), conjugation (Golińska and Afon’kin 1993; Vďačný and Foissner 2008a; Vinnikova 1974a, 1974b, 1976; Visscher 1927), ontogenesis (Golińska 1995; Vďačný and Foissner 2009), and food acquisition (Dragesco 1962; Dragesco and Métain 1948; Visscher 1923) in ciliates.
Kahl (1931) and Dragesco (1963) produced the first authoritative taxonomic studies on dileptids and tracheliids, recognizing about 50 species grouped in three genera, Dileptus, Paradileptus, and Trachelius. Based solely on the macronuclear pattern, Jankowski (1967) split the largest genus, Dileptus, into three subgenera: Dileptus with dispersed nodules; Dimacrocaryon with two nodules and a single micronucleus in between; and Monilicaryon with a moniliform macronucleus. Foissner (1984, 1997) and Foissner et al. (1999) redefined Jankowski's subgenera according to peculiarities of their ciliary patterns and oral structures, raising them to generic level and establishing three further genera, Pelagodileptus, Pseudomonilicaryon, and Rimaleptus.
Corliss (1979) assigned dileptids and tracheliids to the Haptoria which now belong to the class Litostomatea (Lynn 2008). Like all other haptorians, dileptids and tracheliids are predators with toxicysts that are used to immobilize and kill the prey (e.g., ciliates or microscopic metazoans, such as rotifers). Further, some of their ciliary rows are anteriorly differentiated into a so-called dorsal brush, a field of short and inflated cilia possibly with sensoric function (Golińska 1982). However, their oral ciliature is much more complex than in other haptorians, i.e., the right branch of the circumoral kinety is accompanied by a perioral kinety, while the left branch is associated with many short, oblique preoral kineties (e.g., Foissner 1984, 1997; Foissner et al. 2002; Golińska 1991; Grain and Golińska, 1969). Accordingly, Foissner and Foissner (1988) classified dileptids and tracheliids in a separate suborder, Dileptina, within the order Haptorida. Jankowski (1980) even suggested a separate subclass, Rhynchostomata, with a single order, Dileptida. On the other hand, Lynn and Small (2002) and Lynn (2008) remained conservative, assigning only a family rank to dileptids.
The phylogenetic position of dileptids within the subclass Haptoria became controversial when their first 18S rRNA gene sequence was published because it classified them basal to all other haptorians (Strüder-Kypke et al. 2006). Morphologists questioned this result due to morphological complexity of dileptids, suggesting that they originated from spathidiid haptorians by developing a proboscis (Vďačný and Foissner 2008a, 2009; Xu and Foissner 2005). In order to overcome this problem and reconstruct the evolutionary history of dileptids and tracheliids, we combine a traditional cladistic approach with a molecular phylogenetic approach based on eight new 18S rRNA gene sequences.
Material and Methods
Collection and sample processing
Eight species from all main rhynchostomatian lineages were sampled in order to reconstruct the 18S rRNA gene evolution of dileptids and tracheliids, and to reveal their phylogenetic position within the class Litostomatea (Table 1). Most species sequenced were collected from floodplain soils cultivated using the non-flooded Petri dish method described in Foissner et al. (2002). Trachelius ovum occurred in the periphyton of a pond at Salzburg University, Austria. Pelagodileptus trachelioides was found in the plankton of Lake Biwa in Japan. Species were identified using live observation and protargol impregnation (Foissner 1991). Between two and five hundred cells were picked with a micropipette, washed at least twice in water to remove contaminants, and transferred into 180 μl ATL buffer (Qiagen, Hildesheim, Germany). Samples were stored at +1 to +3 °C pending DNA extraction.
Table 1.
Origin and characterization of new 18S rRNA gene sequences of rhynchostomatians (arranged alphabetically).
| Taxon | Collection site | Culture conditiona | No. of cells picked | No. of clones sequenced | Average pairwise distance between clones (%) | Sequence length | GB accession number |
|---|---|---|---|---|---|---|---|
| Dileptus costaricanus Foissner, 1995 | Botswana, floodplain soil | NFP | 10 | 21 | 0.21 | 1641 | HM581679 |
| Dileptus cf. jonesib | Austria, Salzburg, ephemeral pond | NFP | 50 | 19 | 0.24 | 1640 | HM581678 |
| Monomacrocaryon terrenus (Foissner, 1981) comb. n. | Upper Austria, soil | NFP | 200 | 18 | 0.19 | 1639 | HM581674 |
| Rimaleptus microstomac (Vďačný and Foissner 2008b) comb. n. | USA, Idaho, Boise, floodplain soil | NFP | 30 | – | – | 1642 | HM581676 |
| Rimaleptus mucronatus (Penard, 1922) comb. n. | USA, Idaho, Boise, floodplain soil | NFP | 70 | 15 | 0.35 | 1639 | HM581675 |
| Pelagodileptus trachelioidesc (Zacharias, 1894) Foissner et al., 1999 | Japan, Shiga, Lake Biwa | ES | 5 | – | – | 1608d | AB558117 |
| Pseudomonilicaryon fraterculume | USA, Idaho, Boise, floodplain soil | NFP | 15 | 19 | 0.17 | 1640 | HM581677 |
| Trachelius ovum (Ehrenberg, 1831) Ehrenberg, 1833 | Austria, Salzburg, University pond | ES | 7 | 21 | 0.22 | 1636 | HM581673 |
ES – environmental sample, NFP – non-flooded Petri dish culture.
Differs from D. jonesiDragesco, 1963 by the lack of ability to form a caudal mucous attachment thread.
PCR products sequenced directly.
Partial sequence.
The original description will be published in our monograph on dileptids in the Acta Protozoologica. To avoid nomenclatural problems we disclaim the name for nomenclatural purposes (Article 8.3 of the ICZN 1999).
DNA extraction, PCR amplification, and molecular cloning
Prior to DNA extraction, Proteinase K 20 μl (20 mg/ml) was added and the samples were incubated at 56 °C for 1 h. Genomic DNA was extracted using a DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA) or the modified chelex protocol (Strüder-Kypke and Lynn 2003). The 18S rRNA gene was amplified using the universal forward and reverse eukaryotic primers EukA and EukB (Medlin et al. 1988). The amplification reaction contained 10–20 ng of DNA template, 2.5 U HotStar Taq DNA polymerase (Qiagen, Valencia, CA, USA) in the manufacturer-provided reaction buffer, 200 μM of dNTP, and 0.5 μM of each oligonucleotide primer. The final volume was adjusted to 50 μl with sterile distilled water. The thermocycler program for 18S rRNA gene amplification consisted of an initial hot start incubation of 15 min at 95 °C followed by 30 identical amplification cycles (denaturing at 95 °C for 45 s, annealing at 55 °C for 1 min, and extension at 72 °C for 2.5 min), and a final extension at 72 °C for 10 min. Amplified DNA was checked for quality by agarose gel electrophoresis. The resulting PCR products were cloned into the vector plasmid (pCR 2.1) using the TOPO TA Cloning kit (Invitrogen, Carlsbad, CA, USA). Recombinant plasmids were sequenced bi-directionally using M13 forward and reverse primers supplied with the kit and an internal primer Euk528F (Elwood et al. 1985) at Beckman Coulter Genomics (Danvers, MA, USA) to obtain the full-length 18S rRNA gene sequences. The 18S rRNA gene PCR products for Rimaleptus microstoma were directly sequenced at Sequetech Corporation (Mountainview, CA, USA) using the amplification primers and two internal primers. Sequencing of two Pelagodileptus trachelioides specimens was performed on an ABIPRISM 310 Genetic Analyzer (for details, see Shimano et al. 2008).
Sequence processing and alignments
The sequence fragments were imported into Chromas ver. 2.33 (Technelysium Pty Ltd), checked for data quality and trimmed at the 5′ and 3′ ends. Trimmed sequences were assembled into contigs using BioEdit (Hall 1999). The consensus sequences, based on sequences from 15 to 21 clones, were created in BioEdit with an inclusion threshold frequency of 90% identity (Table 1). These consensus sequences were subsequently aligned to litostomatean 18S rRNA gene sequences available in the ARB-package (Ludwig et al. 2004). The alignment was manually corrected according to the secondary structural features of the 18S rRNA molecule. Ambiguously aligned and hyper variable regions were masked, using a sequence alignment filter created for the alignment in ARB.
Phylogenetic analyses
To determine the phylogenetic position of the newly sequenced species within the class Litostomatea, we analyzed an 18S rRNA gene sequence alignment containing 1442 unambiguously aligned nucleotide characters of 37 rhynchostomatian, haptorian and trichostomatian taxa. A second alignment comprised 1635 nucleotide characters of nine dileptid and tracheliid taxa and served to reveal in-group phylogenetic relationships. Modeltest (Posada and Crandall 1998) was employed to find the model of nucleotide substitution that best fit data. The general time reversible model with invariable sites and gamma distribution (GTR + I + Γ) was chosen for the first alignment, while the second transition model considering invariable sites and a gamma distributed substitution rate among sites (TIM2 + I + Γ) was found for the second alignment. Bayesian inference (BI) trees were computed in MrBayes (Ronquist and Huelsenbeck 2003), using the models suggested by Modeltest and the Markov Chain Monte Carlo (MCMC) algorithm. The reliability of branching pattern was assessed by four chains running 5,000,000 generations. Trees were sampled every 1000 generations. The first 25% of sampled trees were considered burn-in trees and were discarded prior to tree reconstruction. A 50% majority rule consensus of the remaining trees was used to calculate posterior probability (PP). The maximum likelihood (ML) analysis was implemented on the CIPRES Portal V 1.15 (http://www.phylo.org), using RAxML with settings as described in Stamatakis et al. (2008). The maximum parsimony (MP) and neighbour-joining (NJ) analyses were carried out in PAUP* ver. 4.0b8 with randomly added species and tree bisection-reconnection (TBR) branch-swapping algorithm in effect (Swofford 2003). A neighbour-joining (NJ) tree was constructed in ML distance with settings as suggested by Modeltest. Support for ML, MP and NJ analyses came from 1000 bootstrap replicates using heuristic searches.
Cladistic analyses
Morphological evolution of dileptids and tracheliids was analyzed using a Hennigian argumentation method (Hennig 1966) as well as the computer programs MrBayes and PAUP*. The genus Spathidium was chosen as the outgroup because it is morphologically nearest to dileptids and tracheliids, and belongs to the subclass Haptoria, which is a sister group of the Rhynchostomatia (Vďačný et al. 2011; Xu and Foissner 2005; present study). The characters and character states are summarized in Table 2, and their distribution in the taxa is given in Table 3. The computed trees were based on ordered states in the characters 8–11. Multistate characters were coded according to Lipscomb (1992). All characters were equally weighted. As concerns polymorphic characters (e.g., contractile vacuole pattern or number of dorsal brush rows), the “majority rule” was applied which codes a polymorphic genus as having the trait that is most common among its species (Wiens 2000). Nodal support in the Bayesian tree came from posterior probability using one million generations and trees sampled every 1000 generations. The reliability of internal branches in the MP cladogram was assessed using the bootstrap method with heuristic search including 100 replicates. Bremer indexes of the individual clades were calculated by PRAP (Müller 2004) in combination with PAUP* using parsimony ratcheting with default settings.
Table 2.
Characters, character states, and coding used for the cladogram shown in Fig. 3. For distribution of character states in the taxa, see Table 3.
| No. | Character | Plesiomorphic | Apomorphic |
|---|---|---|---|
| 1 | Structure of circumoral kinety | Dikinetidal (coded 0) | Hybrid (coded 1) |
| 2 | Preoral kineties | Oblique (coded 0) | Aligned to a perioral-like kinety (coded 1) |
| 3 | Number of perioral kineties | 1 (coded 0) | 2 (coded 1) |
| 4 | Localization of oral bulge opening | Ventral (coded 0) | Ventrolateral and inverted (coded 1) Apical (coded 2) |
| 5 | Shape of oral bulge opening | Roundish (coded 0) | Narrowly elliptical (coded 1) |
| 6 | Oral basket lined with granules | No (coded 0) | Yes (coded 1) |
| 7 | Shape of internal oral basket | Obconical (coded 0) | Club-shaped (coded 1) Bulbous (coded 2) |
| 8 | Number of macronuclear nodules | 1 (coded 0) | 2 (coded 1) ≥4 (coded 2) |
| 9 | Macronuclear pattern | Mononucleate (coded 0) | Binucleate (coded 1) Moniliform (coded 2) Multinucleate, scattered (coded 3) |
| 10 | Division mode of macronucleus | Ordinary mode (coded 0) |
Apodileptus mode (coded 1) Dileptus mode (coded 2) |
| 11 | Contractile vacuole pattern | One terminal vacuole (coded 0) | Dorsal stripe (coded 1) Many scattered vacuoles (coded 2) |
| 12 | Lateral fossa | Absent (coded 0) | Present (coded 1) |
| 13 | Dorsal brush pattern | Isoarchistichad (coded 0) | Anisoarchistichad (coded 1) |
| 14 | Number of dorsal brush rows | 3 (coded 000) | 1 (coded 011) 2 (coded 100) ≥4 (coded 010) |
| 15 | Habitat | Periphyton (coded 0) | Benthal (coded 1) Soil (coded 2) Pelagial (coded 3) |
Table 3.
Distribution of characters and their coding in the taxa for the computer programs MrBayes and PAUP*. For characters and character states, see Table 2. Explanations: ? = not known, – = not applicable.
| Taxa | Characters |
||||
|---|---|---|---|---|---|
| 1–7 | 8–10 | 11 | 12–14 | 15 | |
| Trachelius | 0000001 | 000 | 2 | 10000 | 0 |
| Monomacrocaryon | 1000000 | 000 | 2 | 01010 | 2 |
| Dimacrocaryon | 100011? | 110 | 1 | 01100 | 2 |
| Rimaleptus | 1000002 | 110 | 1 | 01100 | 2 |
| Monilicaryon | 1100000 | 220 | 1 | 01011 | 1 |
| Pseudomonilicaryon | 1000000 | 220 | 1 | 01010 | 2 |
| Paradileptus | 1011000 | 220 | 2 | 01010 | 3 |
| Pelagodileptus | 1010100 | 220 | 2 | 01010 | 3 |
| Apodileptus | 1000000 | 231 | 1 | 01010 | 1 |
| Dileptus | 1000000 | 232 | 2 | 01010 | 1 |
| Spathidium (outgroup) | 0– –2000 | 000 | 0 | 00000 | 2 |
Results and Discussion
Characters and character states
The cladistic analyses are based on five groups of diagnostic and phylogenetically informative characters in dileptids and tracheliids: the morphology of the oral apparatus (characters 1–7), pattern and division mode of the nuclear apparatus (characters 8–10), contractile vacuole pattern (character 11), patterns of the somatic ciliature (characters 12–14), and habitat (character 15). The characters and character states are summarized in Table 2, and their distribution is given in Table 3.
Character 1: Structure of circumoral kinety. The outgroup has a circumoral kinety composed exclusively of dikinetids. This state is maintained in only a single rhynchostomatian genus, Trachelius. All other rhynchostomatians display a unique, highly derived state, i.e., a hybrid circumoral kinety composed of dikinetids in the proboscis and oral monokinetids associated with nematodesmata around the oral bulge opening (e.g., Golińska 1991, 1995). Interestingly, nematodesmata-bearing monokinetids (so-called “oralized somatic monokinetids”) also occur in the acropisthiids and enchelyine haptorians (Foissner and Foissner 1985, 1988), where they are not part of a circumoral kinety, but are localized in the anterior portion of the somatic ciliary rows and bear nematodesmata forming the external oral basket.
Character 2: Preoral kineties. The left branch of the circumoral kinety is associated with many short, oblique preoral kineties in all rhynchostomatian genera (Fig. 1g), Monilicaryon being an exception, displaying instead a single perioral-like kinety (Foissner 1997). However, this kinety very likely originates from a linear arrangement of many short preoral kineties. We base this assumption on the pattern of several “typical” dileptids in which the preoral kineties are so strongly oblique that they almost form a single, perioral-like kinety, e.g., in Rimaleptus microstoma (Vďačný and Foissner 2008b).
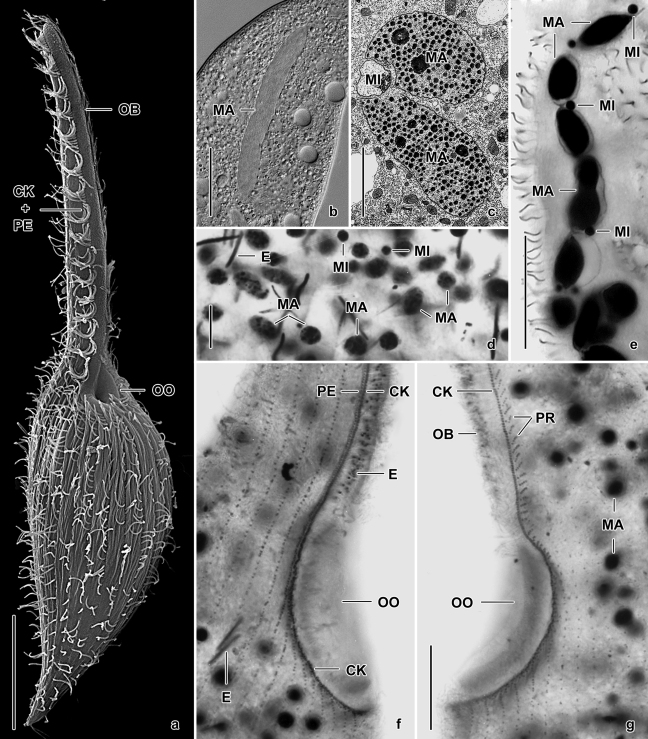
Fig. 1.
(a–g) Main features of rhynchostomatians in vivo (b), after protargol impregnation (d–g), and in the scanning (a) and transmission (c) electron microscope. From Foissner et al., 1995 (d, f, g) and originals (a–c, e). (a) Monomacrocaryon terrenus, overview showing general body organization. (b–e) There are four basic nuclear patterns: a cylindroidal macronucleus with a single micronucleus in Monomacrocaryon (b); two macronuclear nodules with a single micronucleus in between in Dimacrocaryon (c); many macronuclear nodules and micronuclei scattered throughout cytoplasm in Dileptus (d); and a moniliform macronuclear strand with several micronuclei in Pseudomonilicaryon (e). (f, g) Dileptus margaritifer, right and left side view of oral ciliary pattern. CK – circumoral kinety, E – extrusomes, MA – macronucleus (nodules), MI – micronucleus, OB – oral bulge, OO – oral bulge opening, PE – perioral kinety, PR – preoral kineties. Scale bars: 5 μm (c, d), 20 μm (e–g), and 30 μm (a, b).
Character 3: Perioral kinety. In almost all rhynchostomatian genera, the right branch of the circumoral kinety is accompanied by a single, densely ciliated perioral kinety (Fig. 1f). Two perioral kineties side by side, in addition to the circumoral kinety, represent a derived state occurring only in a specialized group of planktonic dileptids, viz., Paradileptus and Pelagodileptus (Foissner et al. 1999), where many densely spaced cilia might increase the efficiency of food acquisition.
Character 4: Localization of oral bulge opening. The oral bulge opening is on the ventral surface at the base of the proboscis in most rhynchostomatians (Fig. 1a). Only in Paradileptus is the opening on the left side and rotated by approximately 180° (Wenrich 1929). Obviously, this is a derived state.
Character 5: Shape of oral bulge opening. Three variants can be distinguished. Roundish openings occur in many haptorians and most rhynchostomatians, suggestive of a plesiomorphic state. In the genus Paradileptus, the roundish oral bulge opening became inverted, i.e., the posterior end of the opening faces the anterior end of the cell (see above). When the roundish opening is stretched, a more or less elliptical pattern is formed as found in the genera Pelagodileptus and Dimacrocaryon, but also in Pseudomonilicaryon angustistoma (Foissner 1984; Foissner et al. 1999, 2002). The occurrence of elliptical to very narrowly elliptical oral openings in several, possibly fairly distantly related genera indicates that this feature evolved convergently several times.
Character 6: Oral basket. Nine out of the ten rhynchostomatian genera have a distinct oral basket composed of long rods (nematodesmata) like in many other haptorians. However, in Dimacrocaryon, the basket rods are so fine that they are recognizable only with TEM (unpubl. observations). Additionally, the oral basket is lined with highly refractive granules, thus appearing in vivo as a conspicuous oral sac (Foissner 1984). This state is considered an apomorphy.
Character 7: Shape of internal oral basket. The oral apparatus is composed, inter alia, of an internal and external basket. The nematodesmata of the external basket originate from the basal bodies around the oral bulge opening and form a conical structure. The internal basket is formed by laminar transverse microtubule arrays embedded in fibrillar material (Grain and Golińska, 1969). Three shape variants of the internal oral basket evolved: obconical, club-like, and bulbous. The obconical internal basket is common in haptorians and most rhynchostomatians, indicating it as the ancestral state. Trachelius has a strongly developed, long, club-shaped internal basket, while that of most Rimaleptus species is short and bulbous.
Characters 8 and 9: Nuclear pattern. Jankowski (1967) first recognized the number and arrangement of the macronuclear nodules as a very stable feature of high cladistic significance in dileptids. The molecular data from several heterotrichs, such as Blepharisma and Stentor, suggest the monomacronucleate state is ancestral (Schmidt et al. 2007; Thamm et al. 2010). This is sustained during the ontogenesis where a monomacronucleate pattern occurs transiently even in species with two or several macronuclear nodules (Golińska 1965; Penard 1922; Vďačný and Foissner 2009). When Haeckel's ontogenetic principle is applied, the Monomacrocaryon pattern should be considered to be the plesiomorphic state. Further, the Monomacrocaryon pattern is quite common in haptorids in general and in the outgroup in particular.
Four macronuclear patterns can be distinguished, each considered to define a distinct genus. The number and pattern of the micronuclei is correlated with the macronucleus, and thus will not be used in the cladistic analysis.
Monomacrocaryon pattern (Fig. 1b): The macronucleus has a more or less long rod shape, sometimes slightly to markedly constricted in the middle. This pattern occurs only in Trachelius and Monomacrocaryon.
Dimacrocaryon pattern (Fig. 1c): Two oblong nodules with a single micronucleus in between occur in two dileptid genera, viz., Dimacrocaryon and Rimaleptus. Rarely, Monomacrocaryon has a rather pronounced constriction in the mid-portion of the macronucleus with a single micronucleus close to it, showing how the binucleate state may have evolved.
Monilicaryon pattern (Fig. 1e): At least four serially arranged nodules form a moniliform or distinctly nodulated strand. This pattern has been found in four possibly closely related genera, viz., Monilicaryon, Pseudomonilicaryon, Pelagodileptus, and Paradileptus. A moniliform macronucleus very likely evolved from the Dimacrocaryon pattern by doubling the nodule number. Thus, in the first step a chain of four nodules was generated, still present in some species (e.g., Pseudomonilicaryon edaphoni, P. aculeatum) and highly characteristic for early exconjugants (Vďačný and Foissner 2008a; Vinnikova 1974a; Visscher 1927). Later, further divisions added more nodules.
Dileptus pattern (Fig. 1d): Usually more than 50 small, oblong nodules scattered in the trunk. This pattern is typical for two genera, viz., Dileptus and Apodileptus gen. n. The Dileptus pattern very likely evolved from the Monilicaryon pattern by fragmentation of the moniliform macronuclear strand. This hypothesis is supported by the conjugation data. In ex-conjugants of Dileptus margaritifer, four macronuclear anlagen transiently form a Monilicaryon pattern. Later, the anlagen divide amitotically generating hundreds of scattered nodules (Visscher 1927).
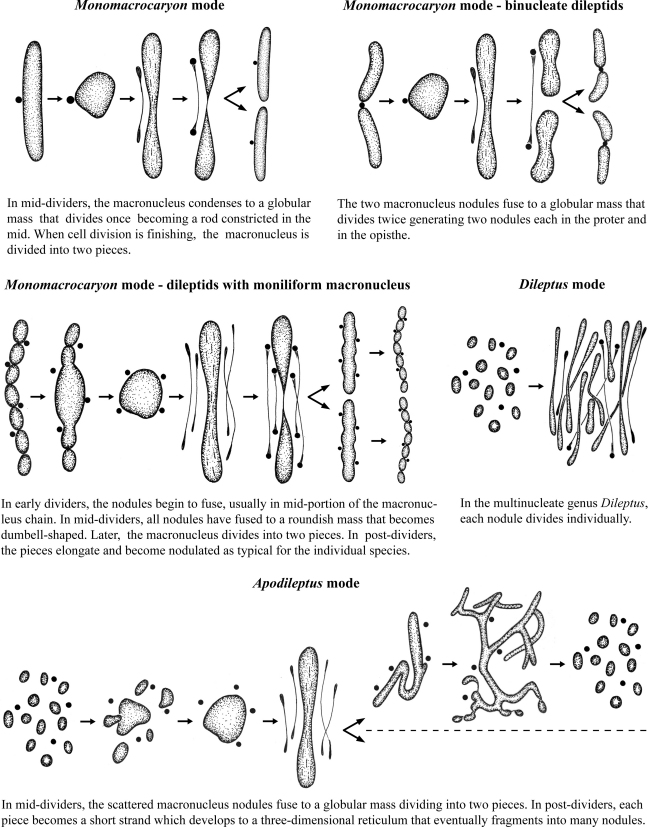
Character 10: Division mode of macronucleus (Fig. 2). Three division modes occur, the first mode being most widespread in ciliates and thus considered as plesiomorphic (Raikov 1996).
Fig. 2.
Division modes of macronucleus in rhynchostomatians.
Monomacrocaryon mode: In mid-dividers, the macronucleus condenses to a globular mass that becomes a long rod that divides into two oblong pieces (Vďačný and Foissner 2009). Two modifications of this mode evolved: (i) in the binucleate dileptids, the condensed mass divides twice generating two nodules each in the proter and opisthe, while (ii) in dileptids with moniliform macronucleus, the mass divides once into two oblong pieces which elongate and become nodulated (Golińska 1965).
Apodileptus mode: In mid-dividers, the many scattered macronuclear nodules fuse to a globular mass that divides into two pieces. In post-dividers, each piece becomes a short strand and later a three-dimensional reticulum that fragments into many nodules (unpubl. observations), as in multinucleate spathidiids and fuscheriids (Foissner et al. 2002; Gabilondo and Foissner 2009).
Dileptus mode: In the multinucleate genus Dileptus, each nodule divides individually (Golińska 1971; Hayes 1938; Jones 1951). This is a rare mode occasionally found also in multinucleate hypotrichs, specifically, in the genus Pseudokeronopsis, where this character is considered apomorphic and defines the subfamily Pseudokeronopsinae (Berger 2006).
Character 11: Contractile vacuole pattern. Most haptorids and spathidiids possess a single, terminal contractile vacuole, while all described rhynchostomatians have at least two, one each in the anterior and posterior half of the trunk. However, there is a second dorsal vacuole in some spathidiids, e.g., Arcuospathidium bulli (Foissner 2000) and Spathidium faurefremieti (Foissner 2003), suggesting a bi- or multivacuolate ancestor of rhynchostomatians. We suppose that the first derived state is a dorsal row (stripe) of vacuoles and the second derived state is a stripe of vacuoles each in the dorsal and ventral side of the cell. The cladogram shows that various contractile vacuole patterns evolved convergently in dileptids, and thus the feature is of significance mainly at species level.
Character 12: Right side fossa. This concavity, surrounded and lined by narrowly spaced kineties, is present only in Trachelius (Ehrenberg 1838; Foissner 1997).
Character 13: Dorsal brush pattern. In the outgroup and in Trachelius, the brush rows start at the same level anteriorly (i.e., isoarchistichad type), while all other rhynchostomatians have a staggered brush with the rows gradually shortened anteriorly from left to right (i.e., anisoarchistichad type). The anisoarchistichad type is not caused by simple spatial constraints since there is sufficient space available for full-length brush rows in both the large species and in the small ones with a two-rowed brush. Thus, the staggered brush is considered as an apomorphy.
Character 14: Number of dorsal brush rows. In many haptorids and most spathidiids, the dorsal brush is composed of three rows, a pattern found in only one rhynchostomatian genus, viz., Trachelius (Foissner 1997; Song and Wilbert 1989). All other rhynchostomatians have one, two or many rows. In the two-rowed species, such as Dimacrocaryon amphileptoides, a third row may be present in some specimens (Foissner 1984), possibly a vestige of the ancestral state. In accordance with Foissner et al. (2002), we consider a lower or higher number than three brush rows as apomorphic.
Character 15: Habitat. Several rhynchostomatian genera show a distinct habitat preference. For instance, Trachelius occurs mainly in the periphyton, where it feeds predominantly on peritrichs, while Pelagodileptus and Paradileptus are restricted to the pelagial (Foissner et al. 1999); Monilicaryon inhabits benthic mud (Foissner 1997; Foissner et al. 1995), while Dimacrocaryon and Rimaleptus prefer terrestrial biotopes (Foissner 1998). Periphyton-inhabiting protists have a number of features (e.g., pronounced flexibility of the body) pre-adapting them for the exploitation of soil (Schönborn 1966). Schönborn's model also shows that a variety of freshwater niches may be colonized from the periphyton, which is thus supposed to be the ancestral habitat, from which rhynchostomatians spread into the benthic, pelagic and soil environments.
Characters and genera not considered: The following features were not included in the cladistic approach because they are known only in a small portion of the species or are merely relevant to intrageneric evolution: the presence/absence of a tail, the somatic ciliary pattern of the right and left side of the proboscis, ontogenetic peculiarities (e.g., presence/absence of a transient indentation in the prospective fission area), the fate of the parental degenerating macronucleus during conjugation (e.g., nodules fusing into a mass that degenerates or the nodules degenerating individually), the fate of the macronucleus during encystment (e.g., fusing or not fusing), and resting cyst characteristics (e.g., cyst wall structure and presence/absence of escape apparatus).
Two genera, Teuthophrys and Branchioecetes, traditionally assigned to the dileptids, were excluded. Teuthophrys belongs to the spathidiids (Foissner et al. 1999; Strüder-Kypke et al. 2006), and Branchioecetes is still very poorly known (Kahl 1931) and invalid because no type species was fixed (Article 13.3 of the ICZN 1999).
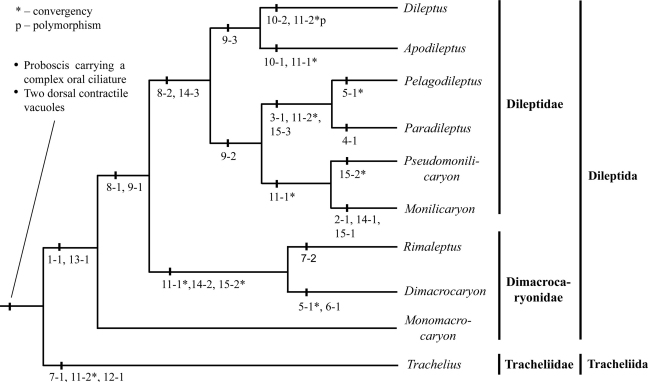
Hennigian argumentation and morphological trees
This is the first attempt to elucidate the morphological evolution of dileptids and tracheliids using both Hennig's traditional (i.e., manual) method (Fig. 3) and computer-based statistical methods, including Bayesian inference (BI) and maximum parsimony (MP) algorithm (Fig. 4). As expected, the cladograms generated by each approach are similar because they are based on the same characters. The Hennigian argumentation tree provides better resolution among the multinucleate family Dileptidae. The family Dimacrocaryonidae is paraphyletic in cladograms generated by both approaches since we have been unable to identify a morphological synapomorphy for Monomacrocaryon and the Dimacrocaryon-Rimaleptus lineage.
Fig. 3.
Cladogram of ten rhynchostomatian genera generated by traditional Hennigian argumentation. For character coding, see Table 2 and section on character states. Only apomorhpies are shown.
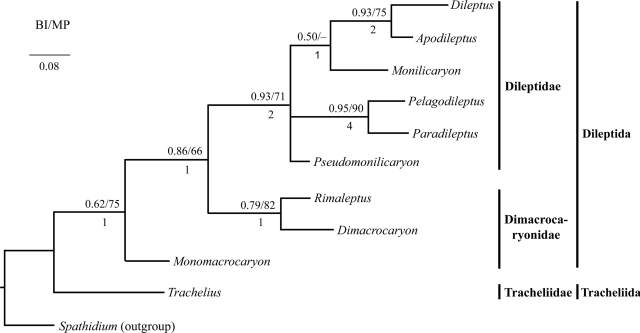
Fig. 4.
Phylogenetic tree of ten rhynchostomatian genera inferred from 15 characters using the genus Spathidium as the outgroup. Two methods (Bayesian inference and maximum parsimony) were used to construct the tree, both resulting in the same topology. Nodal supports are indicated by posterior probabilities for the Bayesian inference (BI) and bootstrap values for the maximum-parsimony (MP) analysis shown above and by Bremer indexes shown below each node. For character coding and distribution of characters among taxa, see Tables 2 and 3. The scale bar indicates the fraction of substitutions per site.
Dileptids and tracheliids share the following synapomorphies: (i) a proboscis with a complex oral ciliature and (ii) at least two dorsal contractile vacuoles. Based on the Hennigian argumentation, we propose that their last common ancestor inherited the following plesiomorphies from the last common progenitor of the class Litostomatea: (i) a dikinetidal circumoral kinety; (ii) an oblong, unsegmented macronucleus; and (iii) a three-rowed, isoarchistichad dorsal brush. The proboscis of the ancestor was most likely immobile and short, i.e., resembling that of Trachelius. This is also consistent with the maturation processes of the proboscis (Vďačný and Foissner 2009).
The cladogram is based on 15 characters dividing rhynchostomatians into two major lineages: (i) the monotypic Tracheliida ord. n. containing the family Tracheliidae and (ii) the order Dileptida uniting the Dimacrocaryonidae fam. n. and the family Dileptidae. This deep split into two orders is moderately to poorly supported with a posterior probability (PP) of 0.62 and MP bootstrap values of 75% (Fig. 4).
Order Tracheliida. This order contains a single species, Trachelius ovum, which is defined by three apomorphies: (i) a club-shaped internal oral basket; (ii) a lateral fossa with specialized ciliature; and (iii) many scattered contractile vacuoles. The latter feature evolved at least two times convergently, viz., in the Pelagodileptus–Paradileptus clade and in some species of the genus Dileptus. Trachelius displays several old plesiomorphies inherited from the last common ancestor of the class Litostomatea: (i) a dikinetidal circumoral kinety; (ii) a three-rowed, isoarchistichad dorsal brush; and (iii) a short, immobile proboscis. Thus, this new order differs from the order Dileptida by several important morphological traits, i.e., by the unique fossa, the structure of the circumoral kinety (dikinetidal vs. hybrid), and the dorsal brush pattern (isoarchistichad vs. anisoarchistichad).
Order Dileptida. This order unites nine genera, sharing the following strong synapomorphies: (i) a hybrid circumoral kinety; (ii) an anisoarchistichad dorsal brush, and possibly in connection, (iii) a stripe barren of basal bodies on the left side of the proboscis. In both the Hennigian argumentation scheme and the morphological trees generated from statistical methods, there are three lineages within this order (Figs 3, 4): (i) Monomacrocaryon with an unsegmented macronucleus; (ii) Dimacrocaryon and Rimaleptus with two macronuclear nodules; and (iii) a large clade comprising six genera having many macronuclear nodules. The first two lineages are united into the family Dimacrocaryonidae, while the third clade represents the family Dileptidae. A sister relationship of the Dimacrocaryon–Rimaleptus clade and the family Dileptidae is indicated by the segmented macronucleus consisting of at least two nodules. Further, this relationship is supported by a posterior probability of 0.86 and 66% MP bootstrap. However, in the absence of a recognized morphological synapomorphy for the genus Monomacrocaryon and the Dimacrocaryon–Rimaleptus clade, we cannot exclude the possibility that the relationship between the Dimacrocaryon–Rimaleptus clade and the family Dileptidae is an artefact.
Family Dimacrocaryonidae. This family is paraphyletic, containing three genera (Dimacrocaryon, Monomacrocaryon and Rimaleptus), in both the Hennigian argumentation scheme and the computer-generated cladograms (see above). The classification of Monomacrocaryon within the family Dimacrocaryonidae is based on the similarity of the nuclear pattern. The two macronuclear nodules of Dimacrocaryon and most Rimaleptus species are usually so close together that they appear as a single, oblong structure resembling the macronucleus of Monomacrocaryon. Further, only these three genera have a single micronucleus which is, however, very likely a plesiomorphic feature.
The genera Dimacrocaryon and Rimaleptus likely descend from a common ancestor inhabiting terrestrial habitats (Figs 3, 4). This is also the most parsimonious explanation for the pronounced similarities in the nuclear apparatus (two macronuclear nodules with a single micronucleus in between) and the oral as well as the somatic ciliature (dileptid oral ciliary pattern and a two-rowed dorsal brush). However, the oral apparatus of the genus Dimacrocaryon deviates not only from that of Rimaleptus but also from that of all other dileptids, in having (i) an oral basket lined with highly refractive granules; (ii) a hardly protruding oral bulge; and (iii) a narrowly elliptical oral bulge opening. The latter feature probably evolved convergently as explained in the description of the characters. The cladograms suggest that the peculiarities of the oral apparatus of Dimacrocaryon arose relatively recently. The single apomorphy of Rimaleptus is the short, bulbous internal oral basket.
Family Dileptidae. The monophyly of the family Dileptidae is supported by two apomorphies: (i) more than three dorsal brush rows and (ii) four or more macronuclear nodules. Further, it is moderately supported by Bayesian interference (0.93 PP) and by the 71% MP bootstraps (Fig. 4). However, the internal relationships of the family Dileptidae were rather poorly resolved in the computer-generated trees. Therefore, we refer here mainly to the cladogram created by traditional Hennigian argumentation, where we recognized two separate branches: the Monilicaryon and the Dileptus branch (Fig. 3).
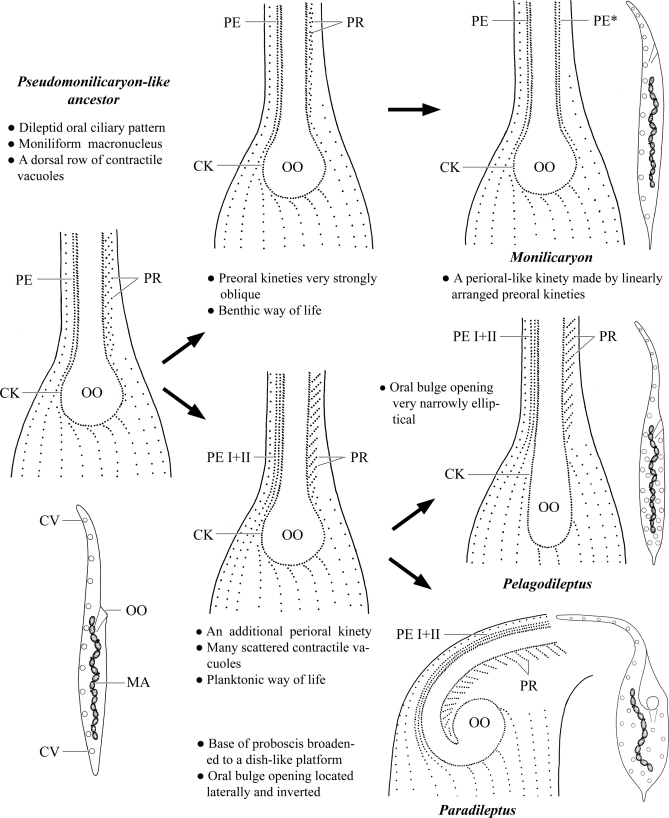
The Monilicaryon branch unites four genera, exhibiting a moniliform macronuclear strand of at least four nodules. They form two clades which differ mainly in the oral ciliary pattern: Pseudomonilicaryon and Monilicaryon maintained the plesiomorphic state, while Paradileptus and Pelagodileptus each evolved an additional perioral kinety. The monilicaryonid and paradileptid pattern can be derived from that of Pseudomonilicaryon (Fig. 5). The single apomorphy of the first clade is the dorsal stripe of contractile vacuoles that very likely evolved convergently, for instance, in the Dimacrocaryonidae. The important cladistic characteristics of the mud-inhabiting Monilicaryon are: (i) a perioral-like kinety left of the oral bulge formed by linearly arranged preoral kineties and (ii) a dorsal brush composed of several kineties which appear as a single, fragmented row. In the computer-generated trees, Monilicaryon was sister to the Dileptus branch. However, this node is only very poorly supported (0.50 PP). The genus Pseudomonilicaryon lacks a distinct apomorphy, except possibly the preferred soil environment. The Paradileptus–Pelagodileptus clade is strongly supported by three apomorphies: (i) right branch of the circumoral kinety accompanied by two perioral kineties side by side; (ii) many scattered contractile vacuoles and (iii) a planktonic way of life. Further, this clade is strongly supported by all statistical analyses (0.95 PP, 90% MP). Pelagodileptus evolved a very narrowly elliptical oral bulge opening, while Paradileptus broadened the left half of the proboscis base to a dish-like platform taking along the oral bulge and the bulge opening which became inverted and laterally located (Fig. 5).
Fig. 5.
Supposed evolution of the oral ciliary patterns and body shapes from a Pseudomonilicaryon-like ancestor. CK – circumoral kinety, CV – contractile vacuoles, MA –moniliform macronuclear strand, OO – oral bulge opening, PE (I + II) – perioral kinety (1 and 2), PE* – perioral-like kinety, PR – preoral kineties.
The apomorphy of the Dileptus branch is the nuclear pattern, i.e., many macronuclear nodules and several micronuclei scattered throughout the cytoplasm. The monophyly of this clade is strongly to moderately supported by Bayesian inference (0.93 PP) and by the 75% MP bootstraps (Fig. 4). This clade comprises two genera: Dileptus and Apodileptus, which are distinguishable only during binary fission. In Dileptus each macronuclear nodule divides individually, while in Apodileptus the multinucleate condition results from fragmentation of an extensive reticulum into multiple nodules after division (Fig. 2). The latter mode evolved convergently in several distantly related haptorians, for instance, in Spathidium turgitorum (Foissner et al. 2002) and Fuscheria uluruensis (Gabilondo and Foissner 2009).
Small subunit rRNA gene sequences
The 18S rRNA gene of eight species from all main rhynchostomatian lineages is only about 1640 nucleotides long, significantly shorter than that of other ciliates, because of deletions in the helices 23-1, 23-8, 23-9, and deletion of the entire helix 23-5. This is also typical for all other litostomatean sequences (Leipe et al. 1994; Strüder-Kypke et al. 2006; Vďačný et al. 2011; Wright and Lynn 1997a, 1997b; Wright et al. 1997). The level of intraspecies sequence variation is relatively low with an average of 0.23% (Table 1). We assume that this small difference results from intraspecific variation and sequencing errors. The most dissimilar is Trachelius ovum, showing an average pairwise difference of 4.5% to the dileptids. The average pairwise difference among representatives from the order Dileptida is 2.1%.
Molecular phylogeny
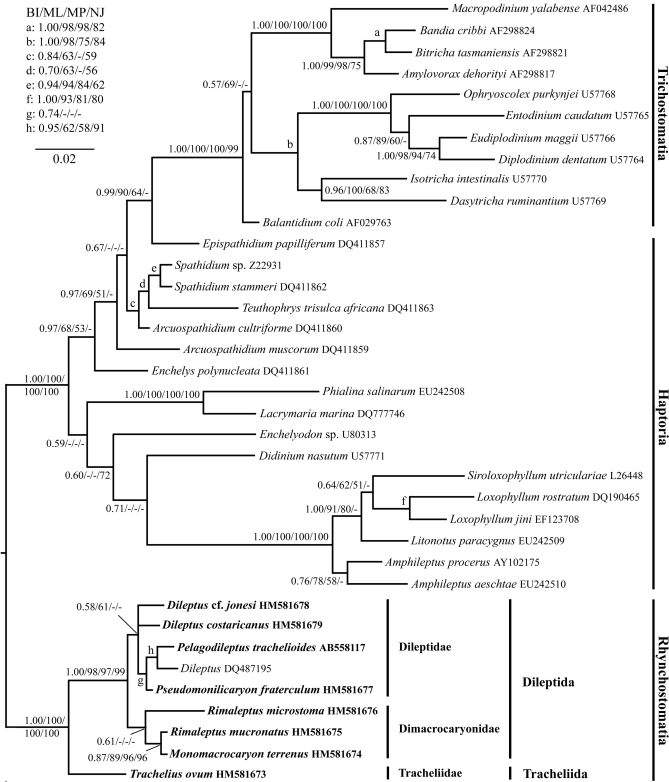
The monophyly of the class Litostomatea is fully supported in all analyses performed. Dileptids and tracheliids consistently form a clade, the subclass Rhynchostomatia, as already suggested by Jankowski (1980). The Rhynchostomatia are sister to the subclass Haptoria including the Trichostomatia. This deep split within the Litostomatea is fully sustained by all four methods (Fig. 6).
Fig. 6.
Small subunit rRNA gene phylogeny based on 1442 nucleotide characters of 37 litostomatean taxa. The three was constructed using four methods (Bayesian inference, maximum likelihood, maximum parsimony, and neighbour-joining) with GTR + I + Γ substitution model and the variable-site gamma distribution shape parameter at 0.4900, the proportion of invariable sites at 0.6460, and a rate matrix for the model as suggested by Modeltest. Posterior probabilities (PP) for the Bayesian inference and bootstrap values for the maximum-likelihood (ML), maximum-parsimony (MP), and neighbour-joining (NJ) analyses are shown at nodes (a dash indicates values below 0.50 or 50%, respectively). Sequences in bold were obtained during this study. The scale bar indicates two substitutions per one hundred nucleotide positions.
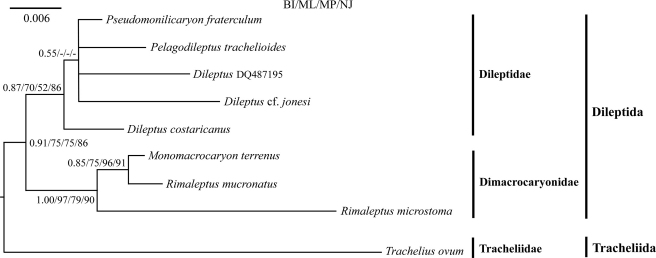
In all analyses, Trachelius ovum is sister to all other rhynchostomatians, i.e., dileptids. This node is fully supported by Bayesian inference and strongly supported by the 98% ML, 97% MP and 99% NJ bootstrap values (Fig. 6), justifying, together with three strong morphological apomorphies (see “Morphological section”), the establishment of a new order, Tracheliida. Such high ranking is also suggested by various morphological peculiarities described in the morphological section. All other rhynchostomatians form a monophylum, the order Dileptida (Figs 6, 7). All analyses consistently depict two clusters within this order, viz., the family Dimacrocaryonidae (1.00 PP, 97% ML, 79% MP, and 90% NJ) with one or two macronuclear nodules and a single micronucleus, and the family Dileptidae (0.87 PP, 70% ML, 52% MP, and 86% NJ) with at least four macronuclear nodules and many micronuclei (Fig. 7). As concerns the family Dimacrocaryonidae, the binucleate genus Rimaleptus seems to be paraphyletic because R. microstoma does not cluster together with R. mucronatus, which forms a clade together with Monomacrocaryon terrenus instead (Figs 6, 7). The internal relationships of the family Dileptidae are very poorly resolved in all analyses, as there is a basal polytomy that is suggestive of a radiation event (Figs 6, 7).
Fig. 7.
Small subunit rRNA gene phylogeny based on 1635 nucleotide characters of nine rhynchostomatian taxa. Four methods (Bayesian inference, maximum likelihood, maximum parsimony, and neighbour-joining) were used to construct the tree, all resulting in a very similar topology. Posterior probabilities (PP) for the Bayesian inference and bootstrap values for the maximum-likelihood (ML), maximum-parsimony (MP), and neighbour-joining (NJ) analyses are shown at nodes (a dash indicates values below 0.50 or 50%, respectively). The scale bar indicates six substitutions per one thousand nucleotide positions.
The subclass Haptoria including the endocommensal Trichostomatia is monophyletic in all analyses, consistent with Gao et al. (2008), Pan et al. (2010), Strüder-Kypke et al. (2006, 2007), and Vďačný et al. (2010, 2011). The branching pattern within the haptorians is not well resolved and most nodes are only poorly supported, which may be due to undersampling of haptorian genera. However, monophylies of the order Lacrymariida (Lacrymaria marina and Phialina salinarum) and the order Pleurostomatida (Amphileptus spp., Litonotus paracygnus, Loxophyllum spp., and Siroloxophyllum utriculariae) are fully supported in all trees. Enchelyodon sp. and Didinium nasutum branch basal to the pleurostomatid clade. The order Spathidiida, including here Arcuospathidium muscorum, A. cultriforme, Spathidium spp., Epispathidium papilliferum, and Teuthophrys trisulca africana, gains very strong support in the Bayesian tree (0.97 PP), while is only poorly supported in the maximum likelihood and maximum parsimony analyses (Fig. 6).
The subclass Trichostomatia is monophyletic with full support from three methods (1.00 PP, 100% ML and 100% MP) and very strong bootstrap support from the distance analysis (99% NJ). The trichostomatians branch rather deeply within the subclass Haptoria where they cluster together with the haptorian Epispathidium papilliferum in the BI, ML, and MP trees, while they form a separate lineage within the basal polytomy in the NJ analysis (Fig. 6). Balantidium coli is sister to all other trichostomatians which are classified into three distinct groups, viz., the orders Macropodiniida (Macropodinium yalabense, Bitricha tasmaniensis, Bandia cribbi, and Amylovorax dehorityi), Entodiniomorphida (Diplodinium dentatum, Entodinium caudatum, Eudiplodinium maggii, and Ophryoscolex purkynjei), and Vestibuliferida (Isotricha intestinalis and Dasytricha ruminantium).
Comparison of morphological and molecular trees
The morphological trees are basically congruent with those based on the 18S rRNA gene sequences, especially in that the genus Trachelius is sister to dileptids, and the order Dileptida as well as the multinucleate family Dileptidae are monophyletic. The family Dimacrocaryonidae is monophyletic in the molecular trees, while paraphyletic in the morphological analyses. This discrepancy is caused by the lack of morphological synapomorphies for the genus Monomacrocaryon and the Dimacrocaryon–Rimaleptus clade. Accordingly, the basal position of Monomacrocaryon within the order Dileptida in the morphological trees is very likely artificial. The molecular data indicate that the unsegmented macronucleus of Monomacrocaryon terrenus evolved from a binucleate state by fusion of the macronuclear nodules and not vice versa as suggested by morphological phylogenies. The cladistic classification of the Dimacrocaryonidae is rather uncertain, but the “typical” nuclear pattern and the usually only two-rowed dorsal brush, are a tempting indication that such dileptids form a distinct evolutionary lineage. Further, dimacrocaryonid dileptids consistently cluster outside the Dileptus/Pseudomonilicaryon/Pelagodileptus clade in morphological cladograms and molecular trees. Thus, the establishment of the Dimacrocaryonidae is justified when evolutionary classification is used, as recommended by Hörandl (2006) and Hörandl and Stuessy (2010).
Resolution at the base of the class Litostomatea
According to Lynn (2008), the class Litostomatea includes two morphologically and ecologically different subclasses. The subclass Haptoria Corliss, 1974 comprises free-living predators with toxicysts, while the subclass Trichostomatia Bütschli, 1889 unites endosymbionts without toxicysts. Dileptids and tracheliids were traditionally assigned to the Haptoria because their overall morphology and way of life are similar to many other members of this subclass, especially, to spathidiids (Corliss 1979; Foissner and Foissner 1988; Lynn 2008). Moreover, dileptids were considered as morphologically highly derived and possibly originating from a spathidiid ancestor by development of a proboscis with a complex ciliature (Xu and Foissner 2005). This assumption was also corroborated by the formation of various spathidiid body shapes and ciliary patterns during ontogenesis and conjugation of dileptids (Vďačný and Foissner 2008a, 2009). Thus, it was surprising when molecular phylogenies suggested a sister relationship between rhynchostomatians (i.e, dileptids and tracheliids) and all other litostomateans (i.e., haptorians and trichostomatians). This deep bifurcation of the Litostomatea and morphological apomorphies of main litostomatean lineages have been extensively discussed by Vďačný et al. (2010, 2011).
Our phylogenetic analyses show that there are not two but three subclasses within the Litostomatea: Rhynchostomatia, Haptoria, and Trichostomatia. The subclass Rhynchostomatia unites Tracheliida and Dileptida, all free-living predators with a proboscis that carries a complex oral ciliature comprising a circumoral kinety, a perioral kinety and preoral kineties. The oral opening of rhynchostomatians is located ventrally, that is, at the base of the proboscis. The subclass Haptoria maintained the ancestral predatory way of life and evolved from the last common ancestor of the Litostomatea by polarization (apicalization) of the body, causing the oral opening to become located more or less apically or dorsally and the oral ciliature to be simplified, i.e., the preoral kineties were lost (Vďačný et al. 2010, 2011; Xu and Foissner 2005). The subclass Trichostomatia originated from a microaerophylic haptorian by further simplification of the oral ciliature, possibly associated with their endosymbiotic lifestyle. Thus, the subclasses Haptoria and Trichostomatia display obvious trends towards simplification of oral structures, which even resulted in a loss of the dikinetidal circumoral kinety in enchelyine haptorids and in all trichostomatians (Vďačný et al. 2010, 2011).
Taxonomic Summary
We redefine the subclass Rhynchostomatia, the order Dileptida, and the family Dileptidae. Further, we establish a new order Tracheliida, a new family Dimacrocaryonidae, and two new genera, Apodileptus and Monomacrocaryon. Dileptus microstoma Vďačný and Foissner, 2008b and D. mucronatus Penard, 1922 are transferred to the genus Rimaleptus Foissner, 1984 because of the two macronuclear nodules and the well developed oral basket lacking highly refractive granules (Table 1).
Subclass Rhynchostomatia Jankowski, 1980
Improved diagnosis: Litostomatea with body partitioned into proboscis and trunk with or without tail. At least two dorsal contractile vacuoles. Oral bulge opening ventral at base of proboscis. Oral ciliary pattern complex, i.e., right branch of circumoral kinety accompanied by at least one perioral kinety, left branch by many oblique preoral kineties or a single perioral-like kinety.
Type order: Dileptida Jankowski, 1978.
Etymology: Not given in original description. Derived from the Greek noun rhynchos (proboscis) and the Latin noun stoma (mouth), obviously referring to the oral bulge opening at the base of the proboscis.
Remarks: Jankowski (1980) originally described this subclass under the name Rhynchostomata. Here, we resurrect this name and change its suffix to -ia, as usual for ciliate subclasses (Lynn 2008). Based on both morphological and molecular data, we recognized two orders within the subclass Rhynchostomatia: Tracheliida ord. n. and Dileptida Jankowski, 1978. Tracheliids are easily distinguished from dileptids in vivo by body shape (broadly ovoidal vs. narrow to rod-like) and the presence (vs. absence) of a lateral fossa. Further, the proboscis of the tracheliids is immobile and short, and thus less conspicuous than that of the dileptids.
Tracheliida ord. n.
Diagnosis: Body broadly dileptid. Proboscis immobile or only slightly mobile, with dorsal side distinctly shorter than ventral one. Distinct groove (fossa) on right side containing and surrounded by condensed somatic ciliature. Dorsal brush three- to four-rowed and isoarchistichad. Circumoral kinety dikinetidal throughout. Internal oral basket clavate.
Type family: Tracheliidae Ehrenberg, 1838.
Etymology: Composite of the stem of the generic name Trachelius and the order suffix -ida. The name Trachelius is probably derived from the Greek noun trachelos (neck).
Remarks: Here we confine the order Tracheliida to the family Tracheliidae, containing only one species, Trachelius ovum. For detailed description of this species, see Foissner et al. (1995).
Order Dileptida Jankowski, 1978
Improved diagnosis: Body broadly to rod-like dileptid, rarely rostrate. Proboscis agile, with ventral and dorsal side of similar length. Dorsal brush two- or multi-rowed and anisoarchistichad. Circumoral kinety hybrid, i.e., oral dikinetids in proboscis and monokinetids around oral bulge opening. Internal oral basket bulbous or obconical.
Type family: Dileptidae Jankowski, 1980.
Etymology: Composite of the stem of the generic name Dileptus and the order suffix -ida. The name Dileptus is derived from the Greek numeral di (two) and the Greek adjective leptos (thin, slender), referring to the two narrowed body ends.
Remarks: The order Dileptida comprises two families, Dimacrocaryonidae and Dileptidae, differing, especially, by the nuclear apparatus: one or two macronuclear nodules in the former while at least four nodules in the latter.
Dimacrocaryonidae fam. n.
Diagnosis: Dileptida with macronucleus in one or two nodules. Dorsal brush typically two-rowed, rarely multi-rowed. Oral apparatus dileptid.
Type genus: Dimacrocaryon Jankowski, 1967.
Etymology: Composite of the stem of the generic name Dimacrocaryon and the family suffix -idae. The name Dimacrocaryon is derived from the Greek numeral di (two), the adjective makros (large), and the noun karyon (nucleus), referring to the two macronuclear nodules.
Remarks: The family Dimacrocaryonidae comprises three genera: Dimacrocaryon Jankowski, 1967; Rimaleptus Foissner, 1984; and Monomacrocaryon gen. n.
Monomacrocaryon gen. n.
Diagnosis: Dimacrocaryonidae with oblong to cylindroidal macronucleus. Dorsal brush usually multi-rowed. Oral basket bulbous or obconical.
Type species: Monomacrocaryon terrenus (Foissner, 1981) comb. n., basionym: Dileptus terrenus Foissner, 1981.
Etymology: Composite of the Greek numeral mono (one), the adjective makros (large), and the noun karyon (nucleus), referring to the unsegmented macronucleus. Neuter gender.
Further species assignable: Monomacrocaryon tenue (Penard, 1922) comb. n., basionym: Dileptus tenuis Penard, 1922; Monomacrocaryon gigas (Claparède and Lachmann, 1859) comb. n., basionym: Amphileptus Gigas Claparède and Lachmann, 1859; Monomacrocaryon polyvacuolatum (Foissner, 1989) comb. n., basionym: Dileptus polyvacuolatus Foissner, 1989.
Remarks: Monomacrocaryon differs from all rhynchostomatians, except for Trachelius, by the unsegmented macronucleus. Monomacrocaryon is distinguished from Trachelius by the narrow body without lateral fossa (vs. broad with lateral fossa) and the oral ciliature (hybrid vs. dikinetidal circumoral kinety). Beginners may confuse Monomacrocaryon with Rimaleptus, in which the two macronuclear nodules are usually close together or sometimes abutting, thus appearing as a single, oblong structure.
Family Dileptidae Jankowski, 1980
Improved diagnosis: Dileptida with macronucleus in at least four moniliform or scattered nodules. Dorsal brush typically composed of more than three rows, rarely of one or two rows. Oral apparatus dileptid or paradileptid.
Type genus: Dileptus Dujardin, 1841.
Etymology: Composite of the stem of the generic name Dileptus and the family suffix -idae.
Remarks: Jankowski (1980) briefly diagnosed the family as follows: “with agile proboscis different from that of Trachelius”. We add the characters of the nuclear apparatus and ciliary pattern. The family Dileptidae comprises six genera: Dileptus Dujardin, 1841; Apodileptus gen. n.; Pseudomonilicaryon Foissner, 1997; Monilicaryon Jankowski, 1967; Paradileptus Wenrich, 1929; and Pelagodileptus Foissner et al., 1999.
Apodileptus gen. n.
Diagnosis: Dileptidae with many scattered macronuclear nodules fusing in a single mass during ontogenesis.
Type species: Apodileptus visscheri (Dragesco, 1963) comb. n., basionym: Dileptus visscheri Dragesco, 1963.
Etymology: Composite of the Greek prefix apo (derived from) and the generic name Dileptus, referring to the Dileptus-like general organization. Masculine gender.
Remarks: Redescription of A. visscheri including its nuclear cycle will be published soon in our monograph on dileptids. Fusion of the macronuclear nodules is the ordinary state in dividing bimacronucleate and monilimacronucleate species (e.g., Paradileptus ovalis and Pseudomonilicaryon brachyproboscis; Vďačný and Foissner 2009; Wenrich 1929), while in multimacronucleate species (e.g., D. anatinus, D. jonesi, D. margaritifer) the nodules divide individually (Hayes 1938; Jones 1951; Golińska 1971). Thus, Apodileptus visscheri, in which the individual nodules fuse during cell division, is a conspicuous exception, justifying separation at genus level. An analogy exists in the hypotrichs, where the Pseudokeronopsinae are defined by the individually dividing macronuclear nodules (for a review, see Berger 2006).
Acknowledgements
Financial support was provided by the Austrian Science Fund (FWF projects P-19699-B17 and P-20360-B17), the Slovak Scientific Grant Agency (VEGA projects 1/0124/09 and 1/0600/11), and the Lake Biwa Museum (Comprehensive Research Project 06-02). The technical assistance of Robert Schörghofer, Andreas Zankl, and Mag. Barbara Harl is greatly appreciated. Special thanks to Dr. Yasushi Kusuoka for providing us the planktonic sample from Lake Biwa.
References
- Berger H. Monograph of the Urostyloidea (Ciliophora, Hypotricha) Monogr. Biol. 2006;85:1–1304. [Google Scholar]
- Claparède É., Lachmann J. Études sur les infusoires et les rhizopodes. Mém. Inst. natn. génev. 1859;6:261–482. [Google Scholar]
- Corliss J.O. 2nd ed. Pergamon Press; Oxford, New York, Toronto, Sydney, Paris, Frankfurt: 1979. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. [Google Scholar]
- Dragesco J. Capture et ingestion des proies chez les infusoires ciliés. Bull. Biol. Fr. Belg. 1962;96:123–167. [Google Scholar]
- Dragesco J. Révision du genre Dileptus, Dujardin 1871 (Ciliata Holotricha) (systématique, cytologie, biologie) Bull. Biol. Fr. Belg. 1963;97:103–145. [Google Scholar]
- Dragesco J., Métain C. La capture des proies chez Dileptus gigas (Cilié Holotriche) Bull. Soc. Zool. Fr. 1948;73:62–65. [Google Scholar]
- Dujardin F. Librairie Encyclopédique de Roret; Paris: 1841. Histoire naturelle des zoophytes. Infusoires, comprenant la physiologie et la classification de ces animaux et la maniére de les étudier a l’aide du microscope. [Google Scholar]
- Ehrenberg C.G. Verlag von Leopold Voss; Leipzig: 1838. Die Infusionsthierchen als vollkommene Organismen. Ein Blick in das tiefere organische Leben der Natur. [Google Scholar]
- Elwood H.J., Olsen G.J., Sogin M.L. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol. Biol. Evol. 1985;2:399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- Foissner W. Infraciliatur, Silberliniensystem und Biometrie einiger neuer und wenig bekannter terrestrischer, limnischer und mariner Ciliaten (Protozoa: Ciliophora) aus den Klassen Kinetofragminophora, Colpodea und Polyhymenophora. Stapfia (Linz) 1984;12:1–165. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Eur. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. Faunistic and taxonomic studies on ciliates (Protozoa, Ciliophora) from clean rivers in Bavaria (Germany), with descriptions of new species and ecological notes. Limnologica. 1997;27:179–238. [Google Scholar]
- Foissner W. An updated compilation of world soil ciliates (Protozoa, Ciliophora), with ecological notes, new records, and descriptions of new species. Eur. J. Protistol. 1998;34:195–235. [Google Scholar]
- Foissner W. Two new terricolous spathidiids (Protozoa, Ciliophora) from tropical Africa: Arcuospathidium vlassaki and Arcuospathidium bulli. Biol. Fertil. Soils. 2000;30:469–477. [Google Scholar]
- Foissner W. Two remarkable soil spathidiids (Ciliophora: Haptorida), Arcuospathidium pachyoplites sp. n. and Spathidium faurefremieti nom. n. Acta Protozool. 2003;42:145–159. [Google Scholar]
- Foissner W., Foissner I. Oral monokinetids in the free-living haptorid ciliate Enchelydium polynucleatum (Ciliophora, Enchelyidae): ultrastructural evidence and phylogenetic implications. J. Protozool. 1985;32:712–722. [Google Scholar]
- Foissner W., Foissner I. The fine structure of Fuscheria terricola Berger et al., 1983 and a proposed new classification of the subclass Haptoria Corliss, 1974 (Ciliophora, Litostomatea) Arch. Protistenk. 1988;135:213–235. [Google Scholar]
- Foissner W., Berger H., Blatterer H., Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems – Band IV: Gymnostomatea, Loxodes. Suctoria. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1995;1(95):1–540. [Google Scholar]
- Foissner W., Berger H., Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1999;3(99):1–793. [Google Scholar]
- Foissner W., Agatha S., Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa) with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Denisia. 2002;5:1–1459. [Google Scholar]
- Gabilondo R., Foissner W. Four new fuscheriid soil ciliates (Ciliophora: Haptorida) from four biogeographic regions. Acta Protozool. 2009;48:1–24. [Google Scholar]
- Gao S., Song W., Ma H., Clamp J.C., Yi Z., Al-Rasheid K.A.S., Chen Z., Lin X. Phylogeny of six genera of the subclass Haptoria (Ciliophora, Litostomatea) inferred from sequences of the gene coding for small subunit ribosomal RNA. J. Eukaryot. Microbiol. 2008;55:562–566. doi: 10.1111/j.1550-7408.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- Golińska K. Macronucleus in Dileptus cygnus and its changes in division. Acta Protozool. 1965;3:143–151. [Google Scholar]
- Golińska K. Comparative studies on the morphology of Dileptus anatinus sp. n. (Holotricha, Gymnostomata) Acta Protozool. 1971;8:367–378. [Google Scholar]
- Golińska K. Effect of puromycin on regeneration processes in Dileptus anatinus, Golinska 1971. Acta Protozool. 1974;12:289–306. [Google Scholar]
- Golińska K. The course of in situ remodelling of injured mouthparts in Dileptus (Ciliata, Gymnostomata) Acta Protozool. 1978;17:47–67. [Google Scholar]
- Golińska K. Regulation of ciliary pattern in Dileptus (Ciliata). I. Sensory cilia and their conversion into locomotor cilia. J. Embryol. Exp. Morph. 1982;68:99–114. [PubMed] [Google Scholar]
- Golińska K. Regulation of ciliary pattern in Dileptus (Ciliata). II. Formation of a cortical domain of sensory cilia from a domain of locomotor cilia. J. Cell Sci. 1983;62:459–475. doi: 10.1242/jcs.62.1.459. [DOI] [PubMed] [Google Scholar]
- Golińska K. Cortical organellar complexes, their structure, formation, and bearing upon cell shape in a ciliate, Dileptus. Protoplasma. 1991;162:160–174. [Google Scholar]
- Golińska K. Formation and orientation of skeletal elements during development of oral territory in a ciliate, Dileptus. Acta Protozool. 1995;34:101–113. [Google Scholar]
- Golińska K., Afon’kin S.Yu. Preparatory changes and the development of the conjugation junction in a ciliate, Dileptus. Protoplasma. 1993;173:144–157. [Google Scholar]
- Grain J., Golińska K. Structure et ultrastructure de Dileptus cygnus Claparède et Lachmann, 1859, Cilié Holotriche Gymnostome. Protistologica. 1969;5:269–291. [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hayes M.L. Cytological studies on Dileptus anser. Trans. Am. Microsc. Soc. 1938;57:11–25. [Google Scholar]
- Hennig W. University of Illinois Press; Urbana, Chicago, London: 1966. Phylogenetic Systematics. [Google Scholar]
- Hörandl E. Paraphyletic versus monophyletic taxa––evolutionary versus cladistic classifications. Taxon. 2006;55:564–570. [Google Scholar]
- Hörandl E., Stuessy T.F. Paraphyletic groups as natural units of biological classification. Taxon. 2010;59:1641–1653. [Google Scholar]
- International Commission on Zoological Nomenclature (ICZN) 4th ed. The International Trust for Zoological Nomenclature; London: 1999. International Code of Zoological Nomenclature. [Google Scholar]
- Jankowski A.W. Novye rody klassov Gymnostomatea i Ciliostomea [New genera of the classes Gymnostomatea and Ciliostomea] Mater. IV. Konf. uč. Sekc. zool. (Kišinev) 1967:36. (in Russian) [Google Scholar]
- Jankowski A.W. Konspekt novoj sistemy tipa Ciliophora [Conspectus of a new system of the phylum Ciliophora] Trudy zool. Inst. (Leningrad) 1980;94:103–121. (in Russian) [Google Scholar]
- Jones E.E., Jr. Encystment, excystment, and the nuclear cycle in the ciliate Dileptus anser. J. Elisha Mitchell Scient. Soc. 1951;67:205–217. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil behandelten Prostomata. Tierwelt Dtl. 1931;21:181–398. [Google Scholar]
- Leipe D.D., Bernhard D., Schlegel M., Sogin M.L. Evolution of 16S-like ribosomal RNA genes in the ciliophoran taxa Litostomatea and Phyllopharyngea. Eur. J. Protistol. 1994;30:354–361. [Google Scholar]
- Lipscomb D.L. Parsimony, homology and the analysis of multistate characters. Cladistic. 1992;8:45–65. doi: 10.1111/j.1096-0031.1992.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Ludwig W., Strunk O., Westram R., Richter L., Meier H., Yadhukumar, Buchner A., Lai T., Steppi S., Jobb G., Förster W., Brettske I., Gerber S., Ginhart A.W., Gross O., Grumann S., Hermann S., Jost R., König A., Liss T., Lüßmann R., May M., Nonhoff B., Reichel B., Strehlow R., Stamatakis A., Stuckmann N., Vilbig A., Lenke M., Ludwig T., Bode A., Schleifer K.-H. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn D.H. 3rd ed. Springer; Dordrecht: 2008. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. [Google Scholar]
- Lynn D.H., Small E.B. Phylum Ciliophora Doflein, 1901. In: Lee J.J., Leedale G.F., Bradbury P., editors. 2nd ed. vol. 1. Allen Press; Lawrence, KS: 2002. pp. 371–656. (An Illustrated Guide to the Protozoa). [Google Scholar]
- Medlin L.K., Elwood H.J., Stickel S., Sogin M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Müller K. PRAP-computation of Bremer support for large data sets. Mol. Phylogenet. Evol. 2004;31:780–782. doi: 10.1016/j.ympev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Müller, O.F., 1773. Vermium terrestrium et fluviatilium, seu animalium infusoriorum, helminthicorum et testaceorum, non marinorum, succincta historia. Heineck & Faber, Havniae & Lipsiae.
- Pan H., Gao F., Li J., Lin X., Al-Farraj S.A., Al-Rasheid K.A.S. Morphology and phylogeny of two new pleurostomatid ciliates, Epiphyllum shenzhenense n. sp. and Loxophyllum spirellum n. sp. (Protozoa, Ciliophora) from a mangrove wetland, South China. J. Eukaryot. Microbiol. 2010;57:421–428. doi: 10.1111/j.1550-7408.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- Penard E. Georg & Cie; Genève: 1922. Études sur les infusoires d’eau douce. [Google Scholar]
- Posada D., Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Raikov I.B. Nuclei of ciliates. In: Hausmann K., Bradbury B.C., editors. Ciliates: Cells as Organisms. Fischer Verlag; Stuttgart, Jena, New York: 1996. pp. 221–242. [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schewiakoff W. Organizacia i sistematika infusoria Aspirotricha (Holotricha auctorum) [The organization and systematics of the infusoria Aspirotricha (Holotricha auctorum)] Zap. imp. Akad. Nauk (8e Série) 1896;4:1–395. (in Russian) [Google Scholar]
- Schmidt S.L., Foissner W., Schlegel M., Bernhard D. Molecular phylogeny of the Heterotrichea (Ciliophora, Postciliodesmatophora) based on small subunit rRNA gene sequences. J. Eukaryot. Microbiol. 2007;54:358–363. doi: 10.1111/j.1550-7408.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- Schönborn W. A. Ziemsen; Wittenberg Lutherstadt: 1966. Beschalte Amöben (Testacea) [Google Scholar]
- Schrank F. von P. Bd. 3. Stein; Landshut bei Krüll: 1803. (Fauna Boica. Durchgedachte Geschichte der in Baiern einheimischen und zahmen Thiere). [Google Scholar]
- Shimano S., Sanbe M., Kasahara Y. Linkage between light microscopic observations and molecular analysis by single-cell PCR for ciliates. Microbes Environ. 2008;23:356–359. doi: 10.1264/jsme2.me08532. [DOI] [PubMed] [Google Scholar]
- Song W., Wilbert N. Taxonomische Untersuchungen an Aufwuchsciliaten (Protozoa, Ciliophora) im Poppelsdorfer Weiher, Bonn. Lauterbornia. 1989;3:2–221. [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J. A rapid bootstrap algorithm for the RAxML web-servers. Syst. Biol. 2008;75:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Strüder-Kypke M.C., Lynn D.H. Sequence analyses of the small subunit rRNA gene confirm the paraphyly of oligotrich ciliates sensu lato and support the monophyly of the subclasses Oligotrichia and Choreotrichia (Ciliophora, Spirotrichea) J. Zool. (London) 2003;260:87–97. [Google Scholar]
- Strüder-Kypke M.C., Wright A.-D.G., Foissner W., Chatzinotas A., Lynn D.H. Molecular phylogeny of litostome ciliates (Ciliophora, Litostomatea) with emphasis on free-living haptorian genera. Protist. 2006;157:261–278. doi: 10.1016/j.protis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Strüder-Kypke M.C., Kornilova O.A., Lynn D.H. Phylogeny of trichostome ciliates (Ciliophora, Litostomatea) endosymbiotic in the Yakut horse (Equus caballus) Eur. J. Protistol. 2007;43:319–328. doi: 10.1016/j.ejop.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates, Inc.; Sunderland, MA: 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. [Google Scholar]
- Thamm M., Schmidt S.L., Bernhard D. Insights into the phylogeny of the genus Stentor (Heterotrichea, Ciliophora) with special emphasis on the evolution of the macronucleus based on SSU rDNA data. Acta Protozool. 2010;49:149–157. [Google Scholar]
- Vďačný P., Foissner W. Morphology, conjugation, and postconjugational reorganization of Dileptus tirjakovae n. sp. (Ciliophora, Haptoria) J. Eukaryot. Microbiol. 2008;55:436–447. doi: 10.1111/j.1550-7408.2008.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vďačný P., Foissner W. Description of four new soil dileptids (Ciliophora, Haptoria), with notes on adaptations to the soil environment. Acta Protozool. 2008;47:211–230. [PMC free article] [PubMed] [Google Scholar]
- Vďačný P., Foissner W. Ontogenesis of Dileptus terrenus and Pseudomonilicaryon brachyproboscis (Ciliophora, Haptoria) J. Eukaryot. Microbiol. 2009;56:232–243. doi: 10.1111/j.1550-7408.2009.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vďačný P., Orsi W., Foissner W. Molecular and morphological evidence for a sister group relationship of the classes Armophorea and Litostomatea (Ciliophora, Intramacronucleata, Lamellicorticata infraphyl. nov.), with an account on basal haptorid litostomateans. Eur. J. Protistol. 2010;46:298–309. doi: 10.1016/j.ejop.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Vďačný P., Bourland W.A., Orsi W., Epstein S.S., Foissner W. Phylogeny and classification of the Litostomatea (Protista, Ciliophora), with emphasis on free-living taxa and the 18S rRNA gene. Mol. Phylogenet. Evol. 2011;59:510–522. doi: 10.1016/j.ympev.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Vinnikova N.V. Conjugation in Dileptus anser (O. F. M.) (Gymnostomatida, Tracheliidae) Acta Protozool. 1974;12:275–287. (in Russian with English title translation and English summary) [Google Scholar]
- Vinnikova N.V. Fine structural changes of the macronuclei of Dileptus anser O. F. M. during conjugation. Acta Protozool. 1974;13:97–106. (in Russian with English title translation and English summary) [Google Scholar]
- Vinnikova N.V. Conjugation in the ciliate Dileptus anser. I. Ultrastructure of micronuclei during mitosis and meiosis. Protistologica. 1976;12:7–24. [Google Scholar]
- Visscher J.P. Feeding reactions in the ciliate, Dileptus gigas, with special reference to the function of trichocysts. Biol. Bull. Mar. Biol. Lab. (Woods Hole) 1923;45:113–143. [Google Scholar]
- Visscher J.P. Conjugation in the ciliated protozoon, Dileptus gigas, with special reference to the nuclear phenomena. J. Morphol. 1927;44:383–415. [Google Scholar]
- Wenrich D.H. Observations on some freshwater ciliates (Protozoa) II. Paradileptus, n. gen. Trans. Am. Microsc. Soc. 1929;48:352–365. [Google Scholar]
- Wiens J.J. Coding morphological variation within species and higher taxa for phylogenetic analysis. In: Wiens J.J., editor. Phylogenetic Analysis of Morphological Data. Smithsonian Institution Press; Washington, London: 2000. pp. 115–145. [Google Scholar]
- Wright A.-D.G., Lynn D.H. Monophyly of the trichostome ciliates (Phylum Ciliophora: Class Litostomatea) tested using new 18S rRNA sequences from the vestibuliferids, Isotricha intestinalis and Dasytricha ruminantium, and the haptorian, Didinium nasutum. Eur. J. Protistol. 1997;33:305–315. [Google Scholar]
- Wright A.-D.G., Lynn D.H. Phylogenetic analysis of the rumen ciliate family Ophryoscolecidae based on 18S ribosomal RNA sequences, with new sequences from Diplodinium, Eudiplodinium, and Ophryoscolex. Can. J. Zool. 1997;75:963–970. [Google Scholar]
- Wright A.-D.G., Dehority B.A., Lynn D.H. Phylogeny of the rumen ciliates Entodinium, Epidinium and Polyplastron (Litostomatea: Entodiniomorphida) inferred from small subunit ribosomal RNA sequences. J. Eukaryot. Microbiol. 1997;44:61–67. doi: 10.1111/j.1550-7408.1997.tb05693.x. [DOI] [PubMed] [Google Scholar]
- Wrześniowski A. Beobachtungen über Infusorien aus der Umgebung von Warschau. Z. Wiss. Zool. 1870;20:467–511. [Google Scholar]
- Xu K., Foissner W. Morphology, ontogenesis and encystment of a soil ciliate (Ciliophora, Haptorida), Arcuospathidium cultriforme (Penard, 1922), with models for the formation of the oral bulge, the ciliary patterns, and the evolution of the spathidiids. Protistology. 2005;4:5–55. [Google Scholar]