The c-Myc proto-oncogene encodes an enigmatic transcription factor that paradoxically has essential roles in the regulation of both proliferation and apoptosis. Deregulated c-myc expression combined with a loss of tumor suppressors, manifested in many types of human cancers, leads to tumorigenesis. Distinct threshold levels of c-Myc, which are regulated by various signaling pathways or deregulated in cancer, are correlated to different outcomes, such as cell cycle progression, transformation or apoptosis.1 But it is unclear whether elevated c-Myc leads to amplification of the same c-Myc target genes or whether an increase in c-Myc leads to regulation of additional targets. Considering that cell context also influences the biological outcomes initiated by elevated c-Myc, it is possible that additional cellular factors directly or indirectly modulate specific c-Myc target genes. Surprisingly, although c-Myc regulates thousands of genes, it is unclear which direct target genes mediate the different functions of c-Myc. In our recent report, we showed that a cofactor of c-Myc, the ARF tumor suppressor, switches the inherent function of c-Myc from a proliferative protein to an apoptotic protein by differentially controlling the regulation of specific direct target genes.2
Apoptosis induced by oncogenic c-Myc occurs with or without p53.3 In the ARF-MDM2-p53 pathway, overexpressed or deregulated c-Myc levels indirectly induce ARF, which then stabilizes p53 levels through direct binding and inhibition of MDM2, the E3 ubiquitin ligase of p53. The increased levels of p53 then induce apoptosis through transactivation of direct pro-apoptotic target genes, such as Bax and PUMA.3 c-Myc can also indirectly cause p53-dependent apoptosis without ARF by inducing a DNA damage response leading to activation of the ATM/ATR kinases and phosphorylation-mediated stabilization of p53.3 However, the mechanism of c-Myc-induced apoptosis independently of p53 has not been described. In our recent report, we demonstrated that ARF is necessary for the induction of a novel c-Myc target gene, Egr1, and that the Myc-ARF-Egr1 pathway is critical and specific for the ability of c-Myc to induce apoptosis independently of p53.2
In addition to ARF, there are many other cofactors that are necessary for, or influence, c-Myc transcriptional activity and biological functions. Max, TRRAP, Mediator and PTEFb are examples of cofactors that are essential for basic transcriptional activities, such as DNA binding, recruiting general machinery for chromatin modification and remodeling and the activation of RNA polymerase II.4 Other cofactors, such as the ubiquitin E3 ligases Skp2 and HectH9, stimulate c-Myc-induced transcription and cell cycle progression.4 The c-Myc cofactor, nucleophosmin (NPM), also enhances c-Myc-induced transcription and dramatically increases c-Myc-induced hyperproliferation and transformation.5 In contrast, other proteins, like ARF, interact with c-Myc to induce apoptosis. Interaction of c-Myc with Bin1 inhibits c-Myc transactivation and induces caspase-independent apoptosis through an unknown mechanism.3 Alternatively, c-Myc appears to be a cofactor of the transcription factor Miz1, since c-Myc is recruited to Miz1 target genes to inhibit transcription, leading to induction of apoptosis.3
Unlike other c-Myc cofactors, ARF has a dual role in regulating c-Myc transcriptional activity. ARF interacts directly with c-Myc and inhibits target genes, such as cdk4, tert and eIF4E, which possess canonical Myc E-box binding sites (CACGTG).6 Interestingly, ARF is recruited to these target genes with c-Myc to inhibit transcription, while also being recruited with c-Myc to the Egr1 promoter, which lacks canonical Myc binding sites, to activate transcription. While c-Myc can be recruited to the noncanonical Egr1 promoter without ARF, ARF significantly increases recruitment of c-Myc to the promoter and is necessary for transcriptional induction of Egr1 by c-Myc.2 Unlike Egr1, which mediates apoptosis, canonical c-Myc targets are linked to the ability of c-Myc to stimulate proliferation. Interestingly, we found that another Egr family member, Egr2, is a canonical target gene. Although we have not established a role for Egr2 in c-Myc mediated function, opposing roles of Egr1 and Egr2 have been found during adipocyte differentiation and T-cell function, supporting the idea that Egr1 and Egr2 have different roles in mediating c-Myc function.7,8
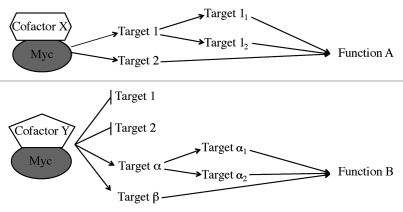
Few direct c-Myc target genes like Egr1 have been identified that mediate a specific c-Myc function, giving rise to the idea that it takes multiple target genes to mediate any c-Myc function. The Egr transcription factors regulate the expression of a large number of target genes, some of which have been shown to be indirect targets of c-Myc.9 Perhaps c-Myc directly induces a variety of transcription factors, such as Egr1 and Egr2, in different cell contexts depending on the interaction of specific cofactors, which in turn can sequentially or cooperatively regulate a large number of genes. In this “Cofactor Switch” model, a cofactor like ARF could control a large number of genes for specific c-Myc functions (Fig. 1).
Figure 1.
The cofactor switch model. In the first scenario (upper part) Myc and cofactor X regulate the expression of numerous target genes, some of which may be transcription factors themselves (Target 1) that regulate their own target genes. The various direct or indirect targets then mediate a specific function (Function A) of c-Myc. In the second scenario (lower part) cofactor Y allows Myc to induce different target genes (α and β) and may block the ability of Myc to induce targets 1 and 2. As in the first scenario, some of the targets may be transcription factors that regulate their own targets that mediate Myc Function B indirectly. The different cofactors may be differentially expressed depending on conditions or cell type and may be competitive for Myc binding. The model also assumes that basal cofactors will be associated with Myc in both scenarios.
The Myc-ARF-Egr1 pathway may play an important role in restraining c-Myc-induced tumorigenesis. Our finding that Egr1 is essential for p53-independent, c-Myc-induced apoptosis, and the observations that Egr1 expression is low or lost in many human tumors10,11 suggest that the Myc-ARF-Egr1 pathway plays a key role in c-Myc-induced apoptosis and indicates that this pathway is disabled in tumors even with an intact ARF gene. Successful chemotherapy strategies often depend on induction of apoptosis in the targeted tumor. Further understanding the mechanism controlling this pathway could be critical for future cancer therapeutic approaches by unleashing the inherent apoptotic function of c-Myc.
Comment on: Boone DN, et al. Proc Natl Acad Sci USA. 2010;108:632–637. doi: 10.1073/pnas.1008848108.
References
- 1.Murphy DJ, et al. Cancer Cell. 2008;14:447–457. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boone DN, et al. Proc Natl Acad Sci USA. 108:632–637. doi: 10.1073/pnas.1008848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer N, et al. Semin Cancer Biol. 2006;16:275–287. doi: 10.1016/j.semcancer.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Cowling VH, et al. Semin Cancer Biol. 2006;16:242–252. doi: 10.1016/j.semcancer.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, et al. Proc Natl Acad Sci USA. 2008;105:18794–18799. doi: 10.1073/pnas.0806879105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Y, et al. Nature. 2004;431:712–717. doi: 10.1038/nature02958. [DOI] [PubMed] [Google Scholar]
- 7.Boyle KB, et al. Cell Death Differ. 2009;16:782–789. doi: 10.1038/cdd.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins S, et al. Eur J Immunol. 2008;38:528–536. doi: 10.1002/eji.200737157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubosaki A, et al. Genome Biol. 2009;10:41. doi: 10.1186/gb-2009-10-11-r121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joslin JM, et al. Blood. 2007;110:719–726. doi: 10.1182/blood-2007-01-068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, et al. Cancer Genom Proteom. 2007;4:377–385. [PubMed] [Google Scholar]