Abstract
Exposure to non-inherited maternal antigens (NIMA) during fetal and neonatal life can result in lifelong maternal microchimerism (MMc) and tolerance to NIMA+ allografts. We have previously shown that 40–50% of BDF1 female x B6 male offspring have multi-organ and multi-lineage MMc, while 70% have evidence of acquired maternal class I antigen in circulating PBMC and splenocytes. These features correlated with the presence of NIMAd-specific CD4+ Treg cells, while offspring lacking MMc also lacked NIMA-specific Tregs. Furthermore, after a DBA/2 heart transplant, NIMAd-specific CD4+ Treg cells rapidly mobilize to the allograft where they produce IL10 and TGFβ, suppressing early acute rejection, while mice deficient in MMc and NIMAd-specific Treg reject, allowing IFNγ-producing T effector cells to predominate in the grafts. We hypothesized that maternal cells occupy key sites of alloantigen presentation after transplant, sustaining pre-existing host Treg amidst a rising tide of donor alloantigen released from the graft. Using quantitative PCR to detect GFP transgeneic maternal cells, we found that transplant tolerance was associated with elevated MMc levels in blood, heart & lung, but surprisingly, not in liver. Rejection was associated with significantly lower levels of MMc in CD11b+ (p = 0.0001) and CD11c+ (p = 0.045) splenocytes, but not with differences in T cell MMc. Furthermore, compared with low pre-transplant baseline rate of maternal antigen acquisition, long-term graft survival was associated with an increased mean % of cells in blood [0.5% pre vs. 5.0% post] and spleen that were dimly positive for H-2Kd, indicative of de novo cell-surface alloantigen acquisition from the DBA/2 donor heart allograft. In contrast, NIMA-exposed mice that rejected their DBA/2 graft showed a transient increase in H-2Kd-dim cells in blood during rejection (day 9–12) but a complete absence of donor MHC acquisition 100 days after transplant. As was the case prior to transplant, antigen acquisition was largely confined to MHC class II+ professional APC. Conclusion: When a NIMA-expressing organ allograft is accepted, MMc persists, mainly distributed into the antigen-presenting cell compartment, where the bulk of graft-derived alloantigen for “semi-direct” presentation is also present.
Key words: transplantation tolerance, microchimerism, NIMA
Introduction
The clinical benefits of non-inherited maternal antigens (NIMA) in immune-tolerance were first noted by Owen et al.1 Since then, tolerogenic effects of NIMA have been documented at both T- and B-cell level in a variety of clinical settings.2–4
What is the basis of the NIMA benefit in graft survival? One possible answer is that many of us have already managed to accept semi-allogeneic cells from our mothers during ontogeny. Though fetal and maternal circulations are completely separated, fetal tissue is bathed with maternal blood in animals having a hemochorial placenta (e.g., mouse and human).5,6 Exchange of maternally derived progenitor and matured cells in feto-maternal interface during pregnancy7–10 and nursing in neonatal life11 results in maternal microchimerism (MMc).
Using an F1 backcross breeding model (BDF1 female × B6 male), we previously showed that about half of the H2b/b offspring were tolerant to heart allografts containing NIMA.12 The tolerance was associated with presence of NIMA-specific regulatory T cells (Tregs) in the allograft and allograft-draining lymph nodes.13 NIMA-specific Treg activity was measured by a delayed type of hypersensitivity assay and the numbers were strongly correlated with concentrations of MMc.11 In human studies, maternal alloantigens promote Treg proliferation in human fetal lymph nodes by a TGFβ-dependent mechanism, sparing the maternal cells and suppressing anti-NIMA alloreactivity during pregnancy.14 We have recently shown that NIMA-specific tolerance can be predicted by measuring Treg activity before transplantation.15 Because this observation, we hypothesized that offspring tolerant to a NIMA-expressing allograft would retain high levels of wide-spread MMc, and that this MMc would preferentially distribute toward cells involved in antigen presentation.
Results
NIMA-specific tolerance in NIMAd/GFP-exposed offspring.
To explore MMc in the offspring undergoing a heterotopic heart transplant from a DBA/2 (H-2d) donor, we used a breeding model where the BDF1 mother is heterozygous for GFP as well as H-2d (Fig. 1) resulting in half of the offspring being homozygous for H-2b and one quarter of the offspring being H2b/b and GFP−/−. The latter mice that did not inherit H2d and GFP were nonetheless exposed to maternal GFP and H2d (NIMAd/GFP). The B6 female x BDF1-GFP+/− male control offspring that did not inherit H2d or GFP (termed NIPAd/GFP) had the same genotype as the NIMA-exposed offspring and thus served as excellent controls. This breeding model was used to distinguish maternal cells (H2d+ GFP+/−) from cells from DBA/2 heart allografts (H2d+ GFP−/−). DBA/2 hearts were transplanted in the abdominal cavities of NIMAd/GFP-exposed and NIPAd/GFP control offspring. As shown in Table 1, NIMA exposure resulted in tolerance in 47% of H-2b/b male offspring (N IMAd/GFP) to DBA/2 heart allografts, which is similar to the rate of tolerance in NIMAd-exposed offspring of GFPneg mothers.12,13,16 We found that 7/15 NIMAd-GFP-exposed offspring accepted the DBA/2 allografts. In contrast, none of the non-exposed control offspring (n = 4) accepted the allografts (p = 0.002). The tolerance was NIMA-specific since none of the NIMA-exposed offspring accepted a heart allograft from a third party donor (C3H) (Table 1).
Figure 1.
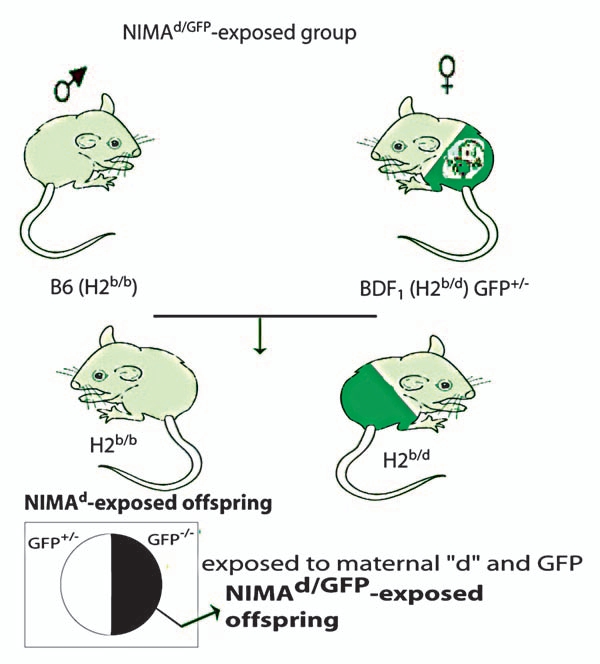
Breeding strategies to explore MMc in NIMA-exposed offspring after transplants: BDF1 GFP+/− female mice were crossed with B6 male mice to obtain NIMAd/GFP offspring, which were exposed to maternal H2d and GFP antigens. The breeding pair was switched to obtain non-exposed control (NIPAd/GFP) offspring. GFP+ maternal cells could be tracked and quantified after transplanting DBA/2 (H2d/d) hearts in the offspring.
Table 1.
NIMAd exposure results in tolerance in the offspring
| Recipient | Donor | n | Graft survival (days) | % of tolerant | Comparison | p value | |
| 1 | NIMAd | DBA/2 (H2d/d) | 16 | 9 × 4, 10, 11, 11, 12, 12, >80 × 7 | 44 | 1 vs. 2 | 0.026 |
| 2 | NIPAd | DBA/2 (H2d/d) | 8 | 9 × 3, 10, 10, 11, 11, 12 | 0 | 2 vs. 3 | ns |
| 3 | NIMAd | C3H (H2k/k) | 8 | 8, 9, 9, 10, 10, 11, 11, 12 | 0 | 1 vs. 3 | 0.022 |
| 4 | NIMAd/GFP | DBA/2 (H2d/d) | 15 | 11, 11, 12, 12, 13, 14, 15, 15, >90 × 7 | 47 | 4 vs. 5 | 0.002 |
| 5 | NIPAd/GFP | DBA/2 (H2d/d) | 4 | 8, 10, 10, 11 | 0 | ||
| 6 | B6 | DBA/2 (H2d/d) | 3 | 9, 10, 11 | 0 | ||
| 7 | B6 | B6 (H2b/b) | 3 | >100 × 3 | 100 |
Maternal microchimerism is elevated in tolerant vs. rejector mice.
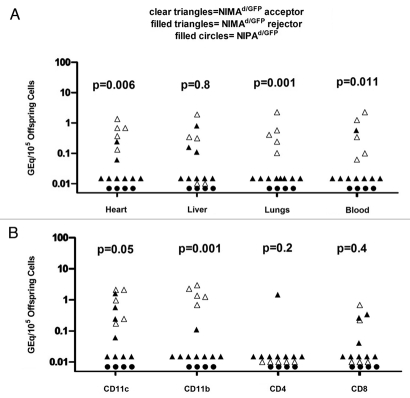
We collected the heart, lungs, liver and blood, previously shown to exhibit the highest levels of MMc,11 from offspring that had been transplanted with DBA/2 hearts. We found that NIMAd/GFP-exposed offspring that accepted DBA/2 heart allografts had significantly higher levels of GFP microchimerism in heart (p = 0.006), lungs (p = 0.001) and blood (p = 0.011) compared to mice that rejected the allograft; however, there was no significant difference in MMc level in the liver between tolerant and rejector mice (Fig. 2A). No GFP+ microchimerism was detected in any of the organs of NIPAd/GFP control offspring that all rejected their DBA/2 heart allografts acutely. When we sorted CD11b, CD11c, CD4 and CD8-positive cells from the spleen, we found that NIMAd/GFP-exposed graft acceptors had significantly higher levels of maternal CD11b (p = 0.001) and CD11c (p = 0.05)-positive cells in their spleen compared to rejector mice (Fig. 2B).
Figure 2.
Tolerant NIMAd/GFP offspring had higher levels of MMc than rejector offspring. (A) DNA was extracted after d 100 post-transplant from hearts, lungs, livers and blood of NIMAd/GFP offspring that either rejected or accepted DBA/2 hearts, and also from NIPAd/GFP offspring that uniformly rejected the allografts. Maternal GFP DNA was detected by a very sensitive qPCR. The p values shown in the figure are obtained after comparing the NIMAd/GFP-exposed acceptors and rejectors. (B) DNA was extracted from sorted CD11c, CD11b, CD4 and CD8-positive splenocytes. Maternal GFP DNA was detected by a qPCR. The p values shown in the figure are obtained after comparing the NIMAd/GFP-exposed acceptors and rejectors.
Tolerance in NIMA d-exposed regulator offspring is associated with alloantigen acquisition by host APC.
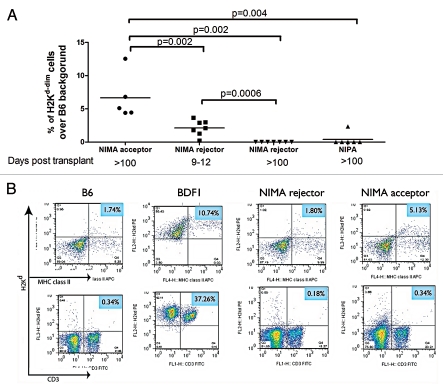
The estimated concentrations of maternal cells in adult F1 backcross mice is very low (1/105–1/104), a level that seems hardly sufficient to provide enough peripheral antigen for maintenance of tolerance to NIMA. One possibility is that signal amplification via antigen acquisition or trogocytosis, a process of surface membrane exchange between cells11,17 might achieve the levels needed to sustain antigen-specific Treg cell homeostasis. Indeed, NIMAd-exposed offspring with MMc contain much higher numbers of H-2Kd dimly + cells in their spleens and blood than offspring without detectable MMc, reaching levels approaching 0.5–1.0% of total spleen cells.11,16 To determine if heart allograft tolerance was also characterized by H2d antigen acquisition, peripheral blood was collected from the offspring at different time points after DBA/2 heart transplantation and stained with anti-mouse H-2Kd antibody. The median channel fluorescence of H-2Kd staining of the cells in NIMAd-exposed mice was intermediate between that of positive (BDF1) and negative (B6) parental strain controls (data not shown). Tolerant offspring had significantly higher levels of such H-2Kd-dim cells in their peripheral blood at day 100 post transplant than the rejector offspring, both at day 9–12 post transplant when the allografts were rejected (p = 0.002) and at day 100 (p = 0.002) (Fig. 3A). Interestingly, the rejector offspring had significantly higher levels of H2Kd-dim cells during rejection than on day 100 (p = 0.0006). Recent work suggests that the alloantigens acquired by host DC during rejection can immunize a naïve mouse.18 Dendritic cells internalize and re-present conformationally intact soluble MHC class I alloantigen for generation of alloantibody, suggesting that the H2Kd-dim APC in rejector mice may indeed play a functional role in modulating alloimmunity. To identify the cell subsets that dimly expressed H2Kd antigen in transplant recipients, dual staining with anti-mouse H2Kd antibody plus anti-mouse MHC class II and CD3 antibodies was performed on splenocytes. This revealed that primarily MHC class II+ cells, and not class IIneg T cells, dimly expressed H2Kd antigen (Fig. 3B). This suggests a mechanism whereby APC in tolerant offspring, already pre-loaded with NIMA by antigen acquisition from rare maternal cells, can maintain allospecific CD4+ Treg after transplantation.
Figure 3.
H2Kd-dim cells in NIMAd-exposed regulator offspring: (A) Peripheral blood was collected at different time points after transplantations with DBA/2 hearts and stained with anti-mouse H2Kd antibody. (B) Splenocytes from NIMAd-exposed offspring were stained with anti-mouse H2Kd, CD3 and MHC class II antibodies and analyzed by a flow cytometer.
Discussion
The proposal put forward by Starzl et al. that Mc is required for transplantation tolerance was, and still is, very controversial.20 An early study of tolerance to a maternal kidney transplant from our lab,21 and a more recent mouse study,22 tend to support a Mc-tolerance linkage due to functional inactivation (anergy) or clonal deletion of cytotoxic T effector (TE) cells. However, these experiments did not separate effects due to circulating microchimeric cells themselves, from effects of autologous APC that may have acquired antigens from these cells or from the graft.17 We found that MMc was tightly correlated with levels of NIMA-specific Tregs.11 Microchimerism itself can be either broadly distributed or narrowly restricted to certain cell lineages. Recently, studies by Chan et al.23,24 suggest that while “full” allograft tolerance is associated with multi-lineage chimerism, a “split tolerance” may result when certain lineages such as T cells are accepted, while B cells and APC are eliminated by the host, resulting in rejection of subsequent skin and islet allografts. The finding that MMc is present in different organs and cell lineages in the NIMA-exposed tolerant offspring in current study suggests that in this breeding model a portion of the offspring have developed a fully tolerant state toward the BDF1 mother that is maintained during acceptance of a DBA/2 heart allograft. However, the other half of offspring lacking pre-transplant regulation and multi-lineage MMc15 appear to develop at best a split tolerance, allowing a somewhat prolonged graft survival but eventual rejection (see Table 1).
In our previous analysis of antigen acquisition in healthy non-transplanted NIMAd offspring, we reported that the % of cells dimly expressing maternal H2Kd (0.1–4.0%) were 10–100-fold higher than % of maternal cells determined by direct maternal DNA detection in the same tissue sample.11 We interpreted this to mean that maternal BDF1 antigen had been acquired by host cells, as described previously for DC transfers between mouse strains.17 In contrast, peripheral blood cells expressing surface H2Kd in tolerant mice at 100 days post transplant was between 4 and 12% (Fig. 3), or approximately ten-fold higher than what we had observed pre-transplant. This suggests that a large proportion of the trogocytosis after transplant is derived from the DBA/2 heart donor. We hypothesize that in addition to membrane transfer that occurs during rare encounters with maternal cells in tissues, encounters with DBA/2 donor passenger leukocytes and endothelium will occur more frequently after transplant.
The post-transplant detection of acquired H2Kd antigen expression in class II+ antigen-presenting cells, the very cells in which MMc is most prevalent, continues a pattern of widespread MMc and Tregs before transplantation,11 and suggests a role of the maternal BDF1 cells and antigens in generation and maintenance of NIMA-specific Tregs during heart transplant tolerance induction. Such Tregs, which develop naturally in response to the BDF1 mother are likely equipped to prevent both acute and chronic rejection of a DBA/2 heart transplant, since they were primed on both direct and indirect pathways.25 We have evidence from mRNA analysis of day eight transplants that these Tregs rapidly mobilize to the graft.
The findings in this study support the concept of a three way model of NIMA-specific transplant tolerance induction, in which host cells, BDF1 maternal cells, and DBA/2 fully allogeneic cells participate in construction of layers of tolerance able to resist both acute and chronic rejection. These findings may be relevant to human renal transplant tolerance, in which both host and donor (passenger) cells, as well as NIMAs, appear to play a role.26
Materials and Methods
Source of mice.
C57 BL/6 (B6, H-2b/b), DBA/2 (H-2d/d), B6D2F1 (BDF1, a cross of B6 and DBA/2; H-2b/d), and B6C3F1 (a cross of B6 and C3H/He; H2b/k) were purchased from Harlan Sprague Dawley (Indianapolis, IN). B6-GFP mice (C57BL/6-Tg(ACTB-EGFP)1Osb/J; H-2b/b GFP+/−), which have a hemizygous GFP transgene under chicken beta actin promoter, were purchased from The Jackson Laboratory (Bar Harbor, ME). The care and breeding of animals were performed under institutional guidelines.
Heterotopic heart transplantation.
Heterotopic vascularized heart transplantation was conducted using an intraabdominal microsurgical technique described previously in reference 27.
Flow cytometry.
Live cells dimly expressing maternal class I MHC antigens were quantified in the NIIMAd-exposed offspring using PE-labeled anti-H2Kd antibody (BD Biosciences) in the spleen and peripheral blood. B6 and BDF1 cells were used as negative and positive controls, respectively. In some experiments, cells were also stained for anti-mouse MHC class II, CD3 and CD19 antibodies (BD Biosciences). The data were analyzed using FlowJo software (Treestar).
Cell sorting.
Splenocytes were incubated with magnetic bead conjugated antibodies against CD11c, CD11b, CD4 and CD8 (Miltenyi Biotech, Auburn, CA) for 15 minutes. CD11c+ and CD11b+, CD4+ and CD8+ cells were sorted using the autoMACS sorter (Miltenyi Biotech) according to the manufacturer's protocols. CD11c subsets were sorted prior to CD11b and CD4 subsets to avoid myeloid dendritic cells (CD11b+) in sorted macrophage (CD11b+) population and CD4+ dendritic cells in CD4+ T cells respectively. The purity of sorted cells was >97% for CD4, CD8 and CD11b subsets, and 85% for CD11c subset.
DNA extraction and quantitative polymerase chain reaction (qPCR).
DNA was extracted using QIAamp DNA extraction kit (Qiagen) according to the manufacturer's protocol. The extracted DNA was quantified using a nanodrop (NanoDrop products). The detailed technique of qPCR and the sequences of primers and probe specific for maternal GFP and H2Dd sequences were described in details previously in reference 11. Briefly, a standard curve was obtained after serially diluting maternal BDF1 GFP+/− DNA into B6 DNA. The standard curve was used to quantify DNA. A PCR reaction was reaction was performed with 1 µg of genomic DNA (equivalent to about 106 cells), 20 picomolar of each primer, 7.5 picomolar of probe, and 25 µl of Taqman Universal PCR Mastermix (Applied Biosystem) in 50 µl of total reaction volume. The qPCR program used was 50°C for 2 minutes, 95°C for 10 minutes, followed by fifty cycles of 95°C for 15 seconds and 60°C for one minute.
Statistics.
Data was analyzed using GraphPad Prism 5 software (GraphPad Software). To analyze graft survivals in different groups, log rank test was used. For rest of the data, the non-parametric Mann-Whitney test was used.
Acknowledgments
We would like to thank Steve Schumacher for technical assistance in typing mice, DNA extraction and qPCR. P.D. designed experiments, and performed all experiments and heterotopic heart transplantation, analyzed data and prepared manuscript. W.J.B. helped in overall experiment designing and manuscript editing. This work was supported by 5R01AI066219-04 from the NIH (to W.J.B.).
Abbreviations
- Mc
microchimerism
- MMc
maternal microchimerism
- NIMA
non-inherited maternal antigen
- NIPA
non-inherited paternal antigen
- Tregs
T regulatory cells
- TE
T effector cells
- APC
antigen presenting cells
- MHC
major histocompatibility complex
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for Actively Acquired Tolerance to Rh Antigens. Proc Natl Acad Sci U S A. 1954;40:420–424. doi: 10.1073/pnas.40.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–1664. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 3.Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 4.van Rood JJ, Loberiza FR, Jr, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, et al. Effect of tolerance to non-inherited maternal antigens on the occurrence of graftversus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 5.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 6.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 7.Dutta P, Burlingham WJ. Tolerance to noninherited maternal antigens in mice and humans. Current opinion in organ transplantation. 2009;14:439–447. doi: 10.1097/MOT.0b013e32832d6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta P, Burlingham WJ. Stem cell microchimerism and tolerance to non-inherited maternal antigens. Chimerism. 2010;1:2–10. doi: 10.4161/chim.1.1.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta P, Dart ML, Schumacher SM, Burlingham WJ. Fetal microchimerism persists at high levels in c-kit stem cells in sensitized mothers. Chimerism. 2010;1:51–55. doi: 10.4161/chim.1.2.14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta P, Burlingham WJ. Microchimerism: tolerance vs. sensitization. Curr Opin Organ Transplant. 2011;16:359–365. doi: 10.1097/MOT.0b013e3283484b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. 2009;114:3578–3587. doi: 10.1182/blood-2009-03-213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrassy J, Kusaka S, Jankowska-Gan E, Torrealba JR, Haynes LD, Marthaler BR, et al. Tolerance to noninherited maternal MHC antigens in mice. J Immunol. 2003;171:5554–5561. doi: 10.4049/jimmunol.171.10.5554. [DOI] [PubMed] [Google Scholar]
- 13.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–6761. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 14.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta P, Dart M, Roenneburg DA, Torrealba JR, Burlingham WJ. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant. 2011;11:1296–1301. doi: 10.1111/j.1600-6143.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molitor-Dart ML, Andrassy J, Haynes LD, Burlingham WJ. Tolerance induction or sensitization in mice exposed to noninherited maternal antigens (NIMA) Am J Transplant. 2008;8:2307–2315. doi: 10.1111/j.1600-6143.2008.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 18.Curry AJ, Pettigrew GJ, Negus MC, Easterfield AJ, Young JL, Bolton EM, et al. Dendritic cells internalise and re-present conformationally intact soluble MHC class I alloantigen for generation of alloantibody. Eur J Immunol. 2007;37:696–705. doi: 10.1002/eji.200636543. [DOI] [PubMed] [Google Scholar]
- 19.Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–877. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood K, Sachs DH. Chimerism and transplantation tolerance: cause and effect. Immunol Today. 1996;17:584–587. doi: 10.1016/s0167-5699(96)10069-4. [DOI] [PubMed] [Google Scholar]
- 21.Burlingham WJ, Grailer AP, Fechner JH, Jr, Kusaka S, Trucco M, Kocova M, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995;59:1147–1155. [PubMed] [Google Scholar]
- 22.Bonilla WV, Geuking MB, Aichele P, Ludewig B, Hengartner H, Zinkernagel RM. Microchimerism maintains deletion of the donor cell-specific CD8+ T cell repertoire. J Clin Invest. 2006;116:156–162. doi: 10.1172/JCI26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan WF, Razavy H, Anderson CC. Differential susceptibility of allogeneic targets to indirect CD4 immunity generates split tolerance. J Immunol. 2008;181:4603–4612. doi: 10.4049/jimmunol.181.7.4603. [DOI] [PubMed] [Google Scholar]
- 24.Chan WF, Razavy H, Luo B, Shapiro AM, Anderson CC. Development of either split tolerance or robust tolerance along with humoral tolerance to donor and third-party alloantigens in nonmyeloablative mixed chimeras. J Immunol. 2008;180:5177–5186. doi: 10.4049/jimmunol.180.8.5177. [DOI] [PubMed] [Google Scholar]
- 25.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nature Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowska-Gan ESA, Sollinger HW, Pirsch JD, Hofmann MR, Haynes LD, Armbrust MJ, et al. Pre-transplant immune regulation predicts allograft outcome: bidirectional regulation correlates with excellent renal transplant function in living-related donor-recipient pairs. Transplantation. doi: 10.1097/TP.0b013e31823e46a0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutta P, Hullett DA, Roenneburg DA, Torrealba JR, Sollinger HW, Harn DA, et al. Lacto-N-fucopentaose III, a Pentasaccharide, Prolongs Heart Transplant Survival. Transplantation. 2011;90:1071–1078. doi: 10.1097/TP.0b013e3181f8f296. [DOI] [PubMed] [Google Scholar]