Abstract
Steroids enantiomers are interesting compounds for detailed exploration of drug metabolizing enzymes, such as the UDP-glucuronosyltransferases (UGTs). We have now studied the glucuronidation of the enantiomers of estradiol, androsterone and etiocholanolone by the 19 human UGTs of subfamilies 1A, 2A and 2B. The results reveal that the pattern of human UGTs of subfamily 2B that glucuronidate ent-17β-estradiol, particularly 2B15 and 2B17, resembles the glucuronidation of epiestradiol (17α-estradiol) rather than 17β-estradiol, the main physiological estrogen. The UGTs of subfamilies 1A and 2A exhibit higher degree of regioselectivity than enantioselectivity in the conjugation of these estradiols, regardless of whether the activity is primarily toward the non-chiral site, 3-OH (UGT1A1, UGT1A3, UGT1A7, UGT1A8 and, above all, UGT1A10), or the 17-OH (UGT1A4). In the cases of etiocholanolone and androsterone, glucuronidation of the ent-androgens, like the conjugation of the natural androgens, is mainly catalyzed by UGTs of subfamilies 2A and 2B. Nevertheless, the glucuronidation of ent-etiocholanolone and ent-androsterone by both UGT2B7 and UGT2B17 differ considerably from their respective activity toward the corresponding endogenous androgens, whereas UGT2A1-catalyzed conjugation is much less affected by the stereochemistry differences. Kinetic analyses reveal that the Km value of UGT2A1 for ent-estradiol is much higher than the corresponding value in the other two high activity enzymes, UGT1A10 and UGT2B7. Taken together, the results highlight large enantioselectivity differences between individual UGTs, particularly those of subfamily 2B.
1. Introduction
UDP-glucuronosyltransferases (UGTs) are membrane enzymes of the endoplasmic reticulum that catalyze the transfer of the glucuronic acid moiety from UDP-glucuronic acid (UDPGA) to numerous different aglycone substrates, both xenobiotic and endogenous compounds [1–3]. The 19 functional human UGTs are divided into three subfamilies, 1A, 2A and 2B [4]. In all of them, exon 1 of the individual genes encodes about half of the respective proteins, the N-terminal domain with its substrate binding site. The rest of the exons, together, encode the C-terminal half of the enzymes. The latter part contains the C-terminal domain that carries the UDPGA binding site, the envelope helices, the single trans-membrane helix that anchors the proteins to the membrane, and the cytoplasmic tail [5]. The UGTs of subfamily 1A (UGT1As) share exons 2–5 and, due to this, the amino acid sequence of the entire C-terminal half of all the 9 UGT1As is identical. A corresponding exon sharing takes place between the two nasal UGTs of subfamily 2A, UGT2A1 and UGT2A2 [4,6], whereas UGT2A3 and the 7 isoforms of subfamily 2B do not share exons but, nonetheless, have a high degree of sequence identity among their C-terminal halves [4]. We have recently carried out a series of studies on the glucuronidation of different steroids by the human UGTs of subfamilies 1A, 2A, and 2B that were expressed as recombinant proteins in baculovirus-infected insect cells [7–9] and observed large and interesting differences among them in both regioselectivity and stereoselectivity of steroid glucuronidation by them.
The hormone 17β-estradiol (β-estradiol) is an important endogenous estrogen that is also used as a drug in hormone replacement therapy [10]. Androsterone and etiocholanolone are metabolites of testosterone, the most prevalent androgen in males. Enantiomers of these steroids have interesting and potentially useful clinical actions (vide infra). In addition, they are useful research tools when studying the substrate selectivity of the UGTs since they have the same physicochemical properties as the corresponding endogenous steroids, thereby enabling a unique distinction between effects due to lipophilicity and three-dimensional shape. We have previously demonstrated a clear preference of UGTs 1A1, 1A3, 1A7, 1A8, and 1A10 for the glucuronidation of β-estradiol, as well as 17α-estradiol (epiestradiol), at the 3-OH. UGT2B15 was selective for the 3-OH, but only exhibited considerable activity when epiestradiol was the substrate [7]. On the other hand, the 17-OH group of both diastereomers was favored by UGTs 2B4, 2B7 and 2B17, as well as UGT1A4, whereas UGTs 2A1 and 2A2 glucuronidated β-estradiol and epiestradiol at both hydroxyls [7]. Androsterone (3α-hydroxy-5α-androstan-17β-ol) and etiocholanolone (3α-hydroxy-5β-androstane-17β-ol) are diastereomers that differ only in the configuration of carbon 5. They have a single nucleophilic group, the 3-OH, but unlike the 3-OH group of β-estradiol and epiestradiol, the 3-OH in androsterone and etiocholanolone is bonded to a chiral centre and not to an aromatic ring (Fig. 1). From the glucuronidation point of view, a likely consequence of the chemical property and configuration differences between androsterone and etiocholanolone is that the pattern of human UGTs that glucuronidate them at the 3-OH (the only glucuronidation site in these steroids) resembles the UGTs that glucuronidate 17β-estradiol and/or 17α-estradiol at their respective 17-OH, rather than those which catalyze the 3-OH conjugation in these estrogens [7,9].
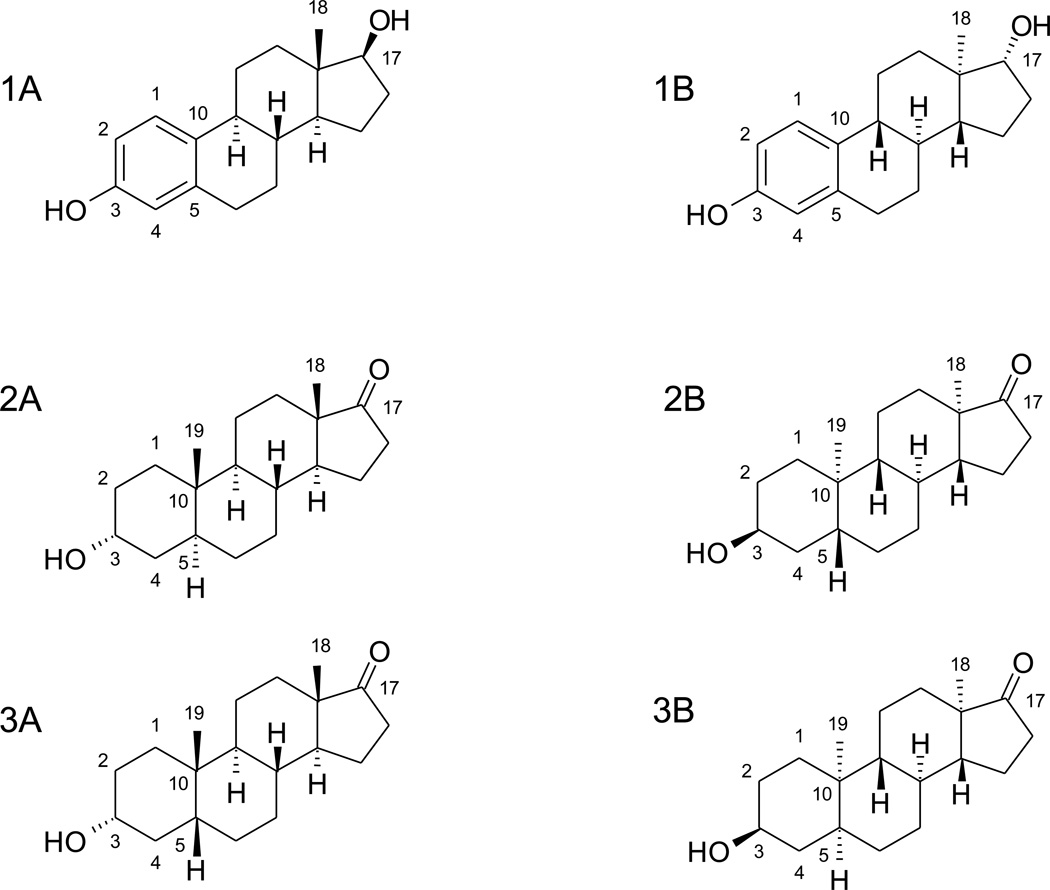
Figure 1.
Chemical structures of 17β-estradiol (1A), androsterone (2A), and etiocholanolone (3A), as well as their respective enantiomers, ent-17β-estradiol (1B), ent-androsterone (2B), and ent-etiocholanolone (3B).
In the present study we have examined the glucuronidation of the enantiomers of 17β-estradiol, androsterone and etiocholanolone (Figure 1). ent-Estradiol was previously reported to bind weakly to estrogen receptors and to lack estrogenic effects in rodent tissues [11], while another laboratory showed that it exhibits neuroprotective effects in human cell lines [12]. ent-Androsterone and ent-etiocholanolone were found to be substantially more active at GABAA receptors than their natural enantiomers [13]. However, the metabolic pathways these enantiomeric steroids (ent-steroids) undergo are not yet characterized. Like their natural enantiomers, they could go through both phase I and phase II metabolism and form both oxididized and conjugated metabolites. We have now examined how these molecules are conjugated by the human UGTs, comparing the glucuronidation patterns of them to the corresponding native steroids. The study presents first of its kind information on the glucuronidation of enantiomeric steroids and also provides new information regarding the substrate specificity of human UGTs for steroids.
2. Materials and Methods
2.1. Materials
Uridine 5′-diphosphoglucuronic acid tri-ammonium salt (UDPGA), 17β-estradiol, 17β-estradiol-3β-D-glucuronide sodium salt, and 17β-estradiol-17β-D-glucuronide sodium salt were purchased from Sigma-Aldrich (St. Louis, MO, USA). Testosterone-glucuronide was from National Measurement Institute (Pymble, Australia). ent-17β-Estradiol, ent-etiocholanolone and ent-androsterone were prepared as previously described [12,13]. Recombinant human UGTs 1A1, 1A3-1A10, 2B4, 2B7, 2B10, 2B11, 2B17, 2B28, and 2A1–2A3 were expressed as His-tagged proteins using baculovirus-infected insect cells [6,14]. The enzymes were used as enriched membranes samples. The relative expression level, per mg total protein, in each of the above-listed was evaluated using the monoclonal tetra-His antibodies (Qiagen, Hilden, Germany) as previously described [14]. The recombinant human UGT2B15 was purchased as “supersomes” from BD Biosciences (Franklin Lakes, NJ, USA). The latter UGT isoform lacked a C-terminal His-tag, preventing the comparison of its relative expression level to the other recombinant UGTs. The relative expression levels of the UGT batches that were used in this study were as follow: 1A1, 6.6; 1A3, 1.5; 1A4, 5.7; 1A5, 5.4; 1A6, 1.1; 1A7, 23.6; 1A8, 2.2; 1A9, 2.9; 1A10, 4.3; 2A1, 3.7; 2A2, 10.3; 2A3, 3.3; 2B4, 2.5; 2B7, 1; 2B10, 1; 2B11, 5.7; 2B17, 2.6; and 0.1 for 2B28.
2.2 Glucuronidation assays
All the glucuronidation assays were done in triplicates, and negative control samples, including all the reaction assay components, with the exception of UDPGA, were carried out as well.
2.2.1 ent-Etiocholanolone and ent-androsterone
Samples for the ent-etiocholanolone and ent-androsterone screenings assays contained 50 µg (total protein) of the UGT source, 50 mM phosphate buffer pH 7.4, 10 mM MgCl2, 5 mM UDPGA, 5 % dimethylsulfoxide and 50 µM substrate, in a total volume of 90 µl. The reactions were carried out at 37°C for 60 min, initiated by the addition of UDPGA and terminated by the addition of 10 µl ice cold 4 M perchloric acid and transferred to ice for 10 min. The internal standard for the LC-MS analyses was 10 µl of 50 mM testosterone glucuronide and it was added at this stage. The proteins were sedimented by centrifugation at 16100 × g for 5 min and the supernatants were analyzed with UPLC/Q-TOF system (Waters/Micromass, Manchester, UK) as previously described [9]. Briefly: the glucuronides were separated using an Acquity BEH Shield RP18, 100 × 0.1 mm, 1.7 µm column (Waters, Milford, MA). The flow rate was 0.2 ml/min, the eluents were 0.1 % ammonium acetate (A) and acetonitrile (B), and the elution gradient we used was: 10–55 % B (0–3 min), 55–90 % B (3–3.5 min) 90–10 % B (3.5–3.6 min) and 10 % B (3.6–6 min). Ionization was done with negative-ion electrospray. The capillary, sample cone and extraction cone voltages were 3500, 45 and 2.0 V, respectively. The desolvation temperature was 250°C and the source temperature was 100°C. The desolvation and cone gas was nitrogen, at flow rates of 700 l/h and 200 l/h, respectively. The glucuronide peaks were identified by their mass and quantified using an external testosterone-glucuronide standard curve (since authentic glucuronides of androsterone, etiocholanolone or their enantiomers were not available).
2.2.2. ent-17β-estradiol
Incubation mixtures for ent-17β-estradiol glucuronidation assays contained either 5 (UGT1A10) or 50 µg (all other UGTs) UGT source (enriched membrane preparations, total protein), 50 mM phosphate buffer pH 7.4, 10 mM MgCl2, 5 mM UDPGA, 200 µM ent-17β-estradiol (in the screening assays) and 10% dimethylsulfoxide, in a total volume of 100 µl. Reaction initiation, incubation conditions and termination were as described above, except that no internal standard was added. The glucuronides in the supernatants were analyzed on a Shimadzu LC-10 model (Shimadzu Corporation, Kyoto, Japan). They were separated using a Chromolith SpeedROD rp-18e 50 × 4.6mm column (Merck, Darmstadt, Germany) at 30°C, employing a gradient of flow rates, starting at 1 ml/min (0–10 min), then 2 ml/min (10–23 min), and again 1 ml/min (23–25 min). The mobile phase consisted of 25 mmol/l phosphate buffer pH 3.0 and MeOH, 60/40 %. The retention time of ent-17β-estradiol 3-OH and 17-OH glucuronides were 5.7 and 10.7 min, respectively. The glucuronides were detected by fluorescence, excitation at 216 nm and emission at 316 nm, and were quantified using an external standard curves for either 17β-estradiol-17-glucuronide or 17β-estradiol-3-glucuronide. Kinetic analyses for the most active UGTs, UGT1A10, 2A1 and 2B7, were carried out in the same manner as the screening analyses, but using 8 different ent-17β-estradiol concentrations, 5, 10, 25, 50, 75, 100, 150, 200, 250 and 300 µM. Kinetic constants were derived from fitting the experimental data to either Michaelis-Menten or Michaelis-Menten with substrate inhibition equations, using GraphPad Prism 5 for Windows (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Glucuronidation of ent-β-estradiol
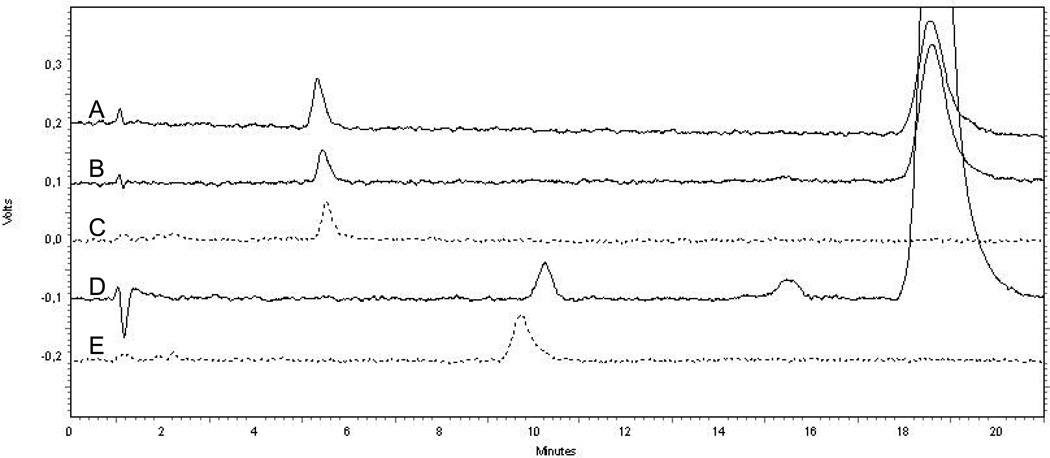
This compound can be glucuronidated at either the 3-OH or the 17-OH. In the absence of authentic glucuronides for either ent-17β-estradiol-3-glucuronide or ent-17β-estradiol-17-glucuronide, we had to make some assumptions. Examination of the chromatograms from the 17β-estradiol 3- and 17-glucuronide standards, the glucuronidation of ent-17β-estradiol with UGT1A10 and UGT2B7, and of 17β-estradiol by UGT1A10 (Fig. 2), strongly suggested that the relative elution order of the 3-glucuronide and 17-glucuronide of both enantiomers of 17β-estradiol is the same, even if the respective retention times are slightly different due to the different structure of each glucuronide (the glucuronides are diastereomers, not enantiomers, because glucuronides of both the native 17β-estradiol and ent-17β-estradiol contain the same, natural glucuronic acid). Based on this assumption, and the results with UGT1A10 and UGT2B7, we have concluded that under our experimental conditions, ent-17β-estradiol-3-glucuronide was eluted at 5.5 min, while the retention time of ent-17β-estradiol-17-glucuronide was 9.8 min (Fig. 2). Quantification of these two ent-17β-estradiol glucuronides was done using the respective glucuronides of the natural 17β-estradiol, as detailed elsewhere [7]. These values were used to calculate the activity of the different human UGTs, and the given values for each isoform were then corrected (normalized) for the relative expression level of each recombinant UGT, using the expression level of UGT2B7 per mg protein as 1.0 (see section 2.1.).
Figure 2.
Elution profiles of the glucuronides generated by UGT1A10 from 17β-estradiol (A) and ent-17β-estradiol (B), as well as the glucuronide produced by UGT2B7 from ent-17β-estradiol (D). The chromatograms of the commercial glucuronide standards, 17β-estradiol-3-glucuronide (C) and 17β-estradiol-17-glucuronide (E), are shown in dash lines.
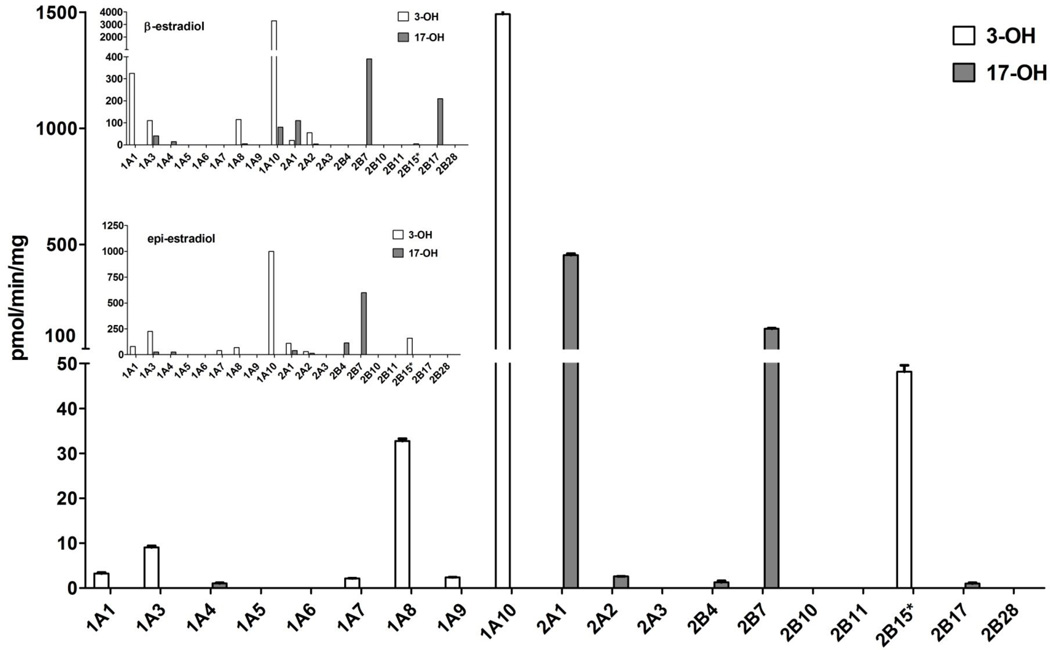
The 19 functional human UGTs (subfamilies 1A, 2A and 2B) were first screened for ent-17β-estradiol glucuronidation at a single substrate concentration, 200 µM, and the results of this screening are presented in Fig. 3. The general view emerging from these results is that (most) UGTs of subfamily 1A that catalyze ent-17β-estradiol glucuronidation, conjugate it at the 3-OH. On the other hand, most UGTs of subfamilies 2A and 2B that glucuronidate ent-17β-estradiol, only conjugate its 17-OH. There are two exceptions to this rule, however, the low but measurable activity of UGT1A4 is limited to the 17-OH, while UGT2B15 conjugates the 3-OH of ent-17β-estradiol (Fig. 3).
Figure 3.
Glucuronidation rates of ent-17β-estradiol by the human UGTs. The substrate concentration was 200 µM and the presented glucuronidation rates were corrected (normalized) for relative expression level of the recombinant UGTs. The inset shows a modified form of the corresponding activities toward 17β-estradiol (β-estradiol) and 17α-estradiol (epiestradiol) that were previously determined in our laboratory (Itäaho et al., 2008). UGT2B15* is marked by an asterisk since this recombinant UGT was for a commercial supplier and, therefore, its relative expression level could not be determined (see Methods for more details). Please note that since the expression level values are relative, and only some of the same enzyme batches that were used in the previous study were also employed in the current work, the normalized activity values between the insets and the main figure cannot be compared directly, even if the order of magnitude is the same. On the other hand, the ratio between the activities of different UGT can be validly compared.
Most UGTs that catalyze ent-17β-estradiol glucuronidation exhibit rather low rates, at least under the conditions employed in these screening assays (Fig. 3). These conditions were not optimized with respect to the substrate concentration for each individual isoform. Nonetheless, the results clearly demonstrate that 3 individual human UGTs, one from each subfamily, catalyze ent-17β-estradiol glucuronidation at much higher rates than most others. UGT1A10 is by far the most active enzyme in ent-17β-estradiol glucuronidation (Fig. 3). UGT2A1 exhibited about 30% of the rate of UGT1A10, and it is the most active human UGT in the glucuronidation of ent-17β-estradiol at the 17-OH. Like UGT2A1, UGT2B7 also conjugates only the 17-OH of ent-17β-estradiol, and its normalized turnover rate under the screening conditions is barely above 10% of the rate exhibited by UGT1A10 towards the 3-OH. Nevertheless, in case ent-17β-estradiol would mainly be glucuronidated in the liver, the relatively high activity of UGT2B7 is an important observation since it is far more active in ent-17β-estradiol glucuronidation than any other hepatic UGT, because neither UGT1A10 nor UGT2A1 are expressed in the liver at measurable levels [6,15,16].
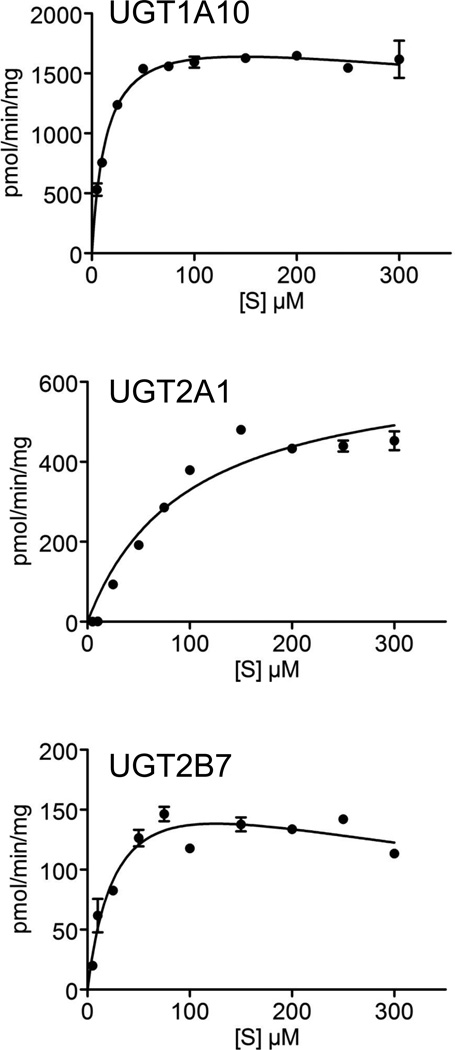
Subsequently, we have examined the kinetics of ent-17β-estradiol glucuronidation by the 3 most active human UGTs, each of them toward its respective target hydroxyl (Fig. 4). The kinetic parameters that were derived from the presented curves are listed in Table 1. These results not only reinforce the particularly high activity of UGT1A10 in ent-17β-estradiol glucuronidation; they also reveal that it has the lowest Km value toward this substrate. The Km value of UGT2B7 was higher, about double the respective UGT1A10 value, but the largest Km value was exhibited by UGT2A1, nearly 7 fold higher than in UGT1A10 (Table 1). It may also be noted here that the kinetic curves for UGTs 1A10 and 2B7 revealed mild substrate inhibition that might also be affected by limited solubility of ent-17β-estradiol at concentrations above 100 µM, even in the presence of 10 % dimethylsulfoxide.
Figure 4.
Kinetics of ent-17β-estradiol glucuronidation by UGT1A10 (A), UGT2A1 (B) and UGT2B7 (C). The formation of ent-17β-estradiol-3-glucuronide was examined in A, whereas the formation of ent-17β-estradiol-17-glucuronide is shown in curves B and C. The results with UGT2A1 (B) were best fitted to the Michaelis-Menten model, whereas the kinetics of UGT1A10 (A) and UGT2B7 (C) were fitted to the Michaelis-Menten with substrate inhibition model. All the assays were done in triplicates. The derived kinetic constants are listed in Table 1.
Table 1.
Kinetic constants for ent-17β-estradiol glucuronidation by recombinant UGTs. In the case of UGT1A10 the formed glucuronide was ent-17β-estradiol-3-glucuronide, whereas with UGTs 2A1 and 2B7 the 17β-glucuronide was formed. Normalized Vmax values are shown on the top, and the actual rates for the 3 UGTs are presented in brackets. The Ki values for UGT1A10 and 2B7 are extrapolated from the enzyme kinetic equations.
| Vmax (pmol/min/mg) |
Km (µM) |
Ki (µM) |
|
|---|---|---|---|
| UGT1A10 | 1954 ± 92 (8401 ± 396) |
14.3 ± 1.9 | 1547 ± 587 |
| UGT2A1 | 651 ± 50 (2904 ± 187) |
97.5 ± 19 | − |
| UGT2B7 | 204 ± 27 (204 ± 27) |
29.7 ± 8.6 | 533 ± 235 |
3.2. Glucuronidation of ent-androsterone and ent-etiocholanolone
ent-Androsterone and ent-etiocholanolone each have a single glucuronidation site, the respective 3-OH, but like in the case of ent-17β-estradiol, we lacked authentic glucuronide standards to quantify glucuronide production. The challenge in their cases is higher since the analytical method was LC-MS that requires an internal standard, and to overcome this and obtain a reasonable estimate of the glucuronidation rates of both ent-androsterone and ent-etiocholanolone we have adopted the solution we have previously used [9], namely employing the available testosterone-glucuronide as an internal standard that has similar chemical properties.
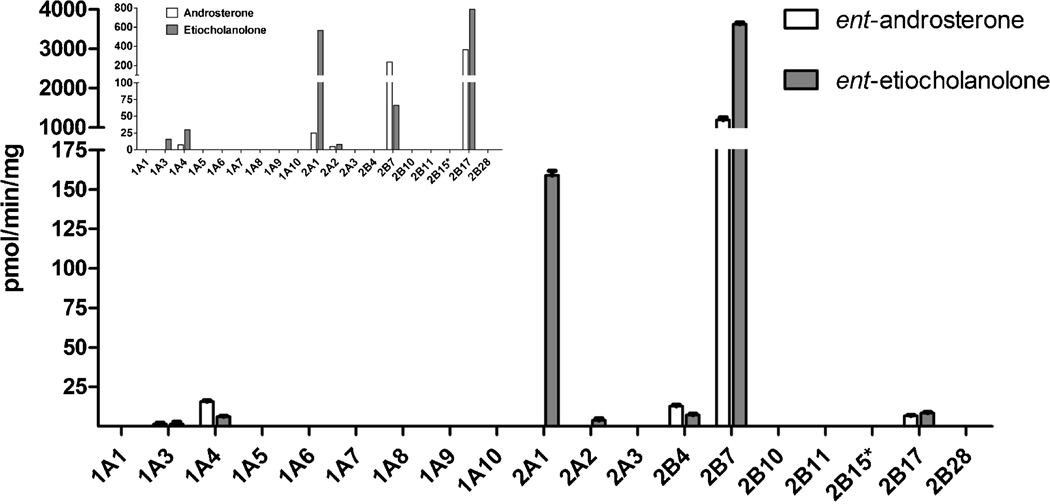
The human UGTs of subfamily 1A, with the exception of UGT1A4 and to a limited extent UGT1A3, too, do not conjugate ent-androsterone or ent-etiocholanolone (Fig. 5). UGTs 2A1 and 2A2 catalyze the glucuronidation of ent-etiocholanolone, but not ent-androsterone. UGTs of subfamily 2B that glucuronidate ent-etiocholanolone, however, also catalyze ent-androsterone glucuronidation (Fig. 5). The most remarkable finding that emerges from these results is the exceptionally high glucuronidation rate of UGT2B7 toward the two ent-androgens, particularly ent-etiocholanolone. It is also worth noting that UGT2B17 exhibits a low glucuronidation rate toward both ent-etiocholanolone and ent-androsterone, a somewhat surprising observation considering the relatively high activity of this isoform toward the natural androsterone and, particularly, etiocholanolone [9]. The latter is particularly interesting when comparing UGT2B17 to UGT2A1 since the large difference in glucuronidation rate of UGT2A1 between ent-etiocholanolone to ent-androsterone is similar to the large preference for etiocholanolone over androsterone that this enzyme exhibited when conjugation of the respective natural androgens was studied [9].
Figure 5.
Glucuronidation rates of ent-androsterone and ent-etiocholanolone by the human UGTs. The substrate concentrations were 50 µM and the presented glucuronidation rates were corrected (normalized) for relative expression level of the recombinant UGTs. The inset shows a modified form of the corresponding activities toward androsterone and etiocholanolone that were previously determined in our laboratory (Sten et al., 2009b). See legend to Fig. 3 for further details about UGT2B15* and correction (normalization) of the activities according to the relative expression level of individual recombinant UGTs.
4. Discussion
We have carried out a series of assays in order to determine the glucuronidation of the enantiomers of 3 physiological steroids by the human UGTs of subfamilies 1A, 2A and 2B. These ent-steroids might serve as interesting lead molecules for drug development and, independently, they provide unique tools to define further the substrate specificity of individual human UGTs.
The results clearly demonstrate that several different human UGTs can glucuronidate each of the 3 tested ent-steroids (Figs. 3 and 5). Nevertheless, the number of UGTs that may be considered as main contributors to these reactions is small and their expression level in different tissues must be taken into account when predicting in vivo glucuronidation. The results strongly suggest that UGT2B7 will be the most important enzyme in hepatic glucuronidation of both ent-androsterone and ent-etiocholanolone, and that these reactions would not be significantly affected by the lack of UGT2B17 due to the del/del genetic polymorphism in the gene encoding UGT2B17 [17]. It is less clear, however, which UGTs will be the most important in the in vivo metabolism of ent-17β-estradiol in humans. Nevertheless, if we assume that the glucuronidation of ent-17β-estradiol will mainly take place in the liver, it is worth pointing out that the two enzymes that catalyze the highest rates of its glucuronidation, UGT1A10 and UGT2A1 (Figs. 3 and 4, Table 1), are extra-hepatic proteins [6,15,16]. Hence, it is likely that the UGT that will mainly contribute to the formation of ent-17β-estradiol glucuronide in the human body will be UGT2B7. In such a case, the major glucuronide of ent-17β-estradiol in the human urine will be ent-17β-estradiol-17-glucuronide, not ent-17β-estradiol-3-glucuronide. It is also predicted that ent-17β-estradiol-3-glucuronide will be generated, mainly by UGT1A10 in the intestine, a reaction that may take place during first-pass metabolism, if ent-17β-estradiol would be given orally, or formed during enterohepatic circulation.
Most UGTs of subfamily 1A that exhibit considerable activity toward the 3-OH ent-17β-estradiol, including UGT1A10 (Fig. 3), do not glucuronidate ent-androsterone and ent-etiocholanolone at the 3-OH (Figs. 1 and 5). UGT1A4 clearly differs from most other UGT1As since it catalyzes low rates of ent-17β-estradiol glucuronidation at the 17-OH (Fig. 3), as well as low glucuronidation rates of ent-androsterone and ent-etiocholanolone (Fig.5). Among the UGTs of subfamily 2B, particularly UGT2B17 and UGT2B15, there are noteworthy similarities between their respective activities toward ent-17β-estradiol and the previous results with 17β-estradiol and epiestradiol (Fig. 3). UGT2B17 is highly active toward the natural estradiol but nearly inactive in epiestradiol and ent-17β-estradiol glucuronidation. UGT2B15, on the other hand, does not conjugate 17β-estradiol at any meaningful rates, but catalyzes the glucuronidation of both epiestradiol and ent-17β-estradiol at significantly higher rates. The latter conjugation, however, is directed toward the 3-OH, not the 17-OH as most other UGT2Bs (Fig. 3). In the case of androsterone and etiocholanolone, UGT2B17, the most active human UGTs in the glucuronidation of these natural androgens, is nearly inactive toward their unnatural enantiomers (Fig. 5). In contrast to UGT2B17 and most UGT2Bs, UGT2B7 is highly active in the conjugation of epiestradiol and ent-17β-estradiol (Fig. 3) and, on the other hand, it is also highly active in the glucuronidation of the ent-androgens, particularly ent-etiocholanolone (Fig. 5).
The combined results described above suggest to us that for many human UGTs, regardless of whether their preferential glucuronidation site is at the 3-OH, the 17α-OH, or the 17β-OH, the configuration of carbon 17 is a much more important structural element than the configuration of the other chiral centers in the tested steroids. In addition, it emerges from the results with many UGTs that when androsterone, etiocholanolone, ent-androsterone and ent-etiocholanolone are bound to the different UGTs, the 3-OH of these androgens occupy the position that, in the cases of bound estradiols, is occupied by the 17-OH. This possibility is in agreement with the findings that while UGT1A10 does not conjugate any of the androgens that were tested by us in this study or the previous one [9], UGT1A4 glucuronidates all these androgens, even if at very low rates (Fig. 5). On the other hand, UGT2B7 and UGT2B17 that exhibit high activity toward the 17-OH of one or more of the estradiols - 17β-estradiol, epiestradiol, or ent-17β-estradiol, but not their 3-OH (Fig. 3), catalyze high glucuronidation rates of some or all of the natural and unnatural enantiomers of androsterone and etiocholanolone. Moreover, in the case of UGT2B17, the high 17β-estradiol glucuronidation activity was almost fully abolished by changing the configuration of C17 of the estrogen backbone, namely in epiestradiol and in ent-17β-estradiol (Fig. 3). Similarly, the high activity of UGT2B17 toward the 3-OH of androsterone and etiocholanolone was nearly abolished by changing the configuration of the 3-OH of these androgens, resulting in very low activity toward the enantiomers of both steroids (Fig. 5). At the same time, the activity of UGT2B7 toward the same hydroxyl groups was stimulated, at least in comparison to most other UGTs, resembling the high activity of UGT2B7 toward epiestradiol [7].
The above suggestion that estradiol binds to different UGTs in an opposite orientation, considering the 3-OH, the 17-OH and the bound UDPGA, was formulated based on the activity results. Currently we know too little about the detailed structure of the substrate binding site of the human UGTs to permit us to analyze the results based on differences in the three-dimensional shapes of the different estradiol substrates. For example, at present there is no explanation for the apparent opposite binding of 17β-estradiol by different UGTs, at least if we accept the assumption that UGTs that mainly catalyze its glucuronidation at the 3-OH, like UGT1A1 and UGT1A10, bind it so that the 3-OH is close to the catalytic His (His39 in UGT1A1 numbering, see model in [5]), whereas UGTs that only catalyze glucuronidation of the 17-OH of 17β-estradiol, like UGT2B7 and UGT2B17, bind this substrate so that its 17-OH is facing their “catalytic His” (His35 in UGT2B7 [18]).
In conclusion, the current results suggest that the tested ent-steroids would be glucuronidated in the human body, and that the contribution of UGT2B7 to such reactions will be major. The contribution of UGT2B17 is expected to be very small, a large difference from the contribution of this enzyme to the glucuronidation of natural 17β-estradiol, androsterone and etiocholanolone. The results of this study also advance our efforts to understand, and subsequently predict, the interactions of individual human UGTs with different compounds, both xenobiotics and endogenous molecules. The results highlight the importance of the configuration of C17 in estradiols and raise the possibility that the 3-OH of androgens occupies, in some UGTs, the same space in the substrate binding site as the 17-OH of the different estradiol stereoisomers. Future studies are needed, however, to accept or reject this possibility, particularly since solving the 3-D structure of several UGTs with bound steroids may not be achieved very soon.
Highlights.
Conjugation of enantio-steroids by human UDP-glucuronosyltransferases
Enantio-steroids as a unique tool to explore stereoselectivity in glucuronidation
Many UGTs are more regio-selective than stereo-selective in steroid metabolism
Acknowledgments
We thank Johanna Mosorin for superb technical assistance. This study was supported, in parts, by the Sigrid Juselius Foundation (MF), the Magnus Ehrnrooth foundation (NS and MF), and by the NIH Grant GM47969 (DFC).
Abbreviations
- Epiestradiol
17α-estradiol
- UDPGA
UDP-glucuronic acid
- UGT
UDP-glucuronosyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab. 2000;1:143–161. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- 2.Radominska-Pandya A, Czernik PJ, Little JM, Battaglia E, Mackenzie PI. Structural and functional studies of UDP-glucuronosyltransferases. Drug Metab Rev. 1999;31:817–899. doi: 10.1081/dmr-100101944. [DOI] [PubMed] [Google Scholar]
- 3.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, et al. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677–685. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- 5.Laakkonen L, Finel M. A molecular model of the human UDP-glucuronosyltransferase 1A1, its membrane orientation, and the interactions between different parts of the enzyme. Mol Pharmacol. 2010;77:931–939. doi: 10.1124/mol.109.063289. [DOI] [PubMed] [Google Scholar]
- 6.Sneitz N, Court MH, Zhang X, Laajanen K, Yee KK, Dalton P, et al. Human UDP-glucuronosyltransferase UGT2A2: cDNA construction, expression, and functional characterization in comparison with UGT2A1 and UGT2A3. Pharmacogenet Genomics. 2009 doi: 10.1097/FPC.0b013e3283330767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itaaho K, Mackenzie PI, Ikushiro S, Miners JO, Finel M. The configuration of the 17-hydroxy group variably influences the glucuronidation of beta-estradiol and epiestradiol by human UDP-glucuronosyltransferases. Drug Metab Dispos. 2008;36:2307–2315. doi: 10.1124/dmd.108.022731. [DOI] [PubMed] [Google Scholar]
- 8.Sten T, Bichlmaier I, Kuuranne T, Leinonen A, Yli-Kauhaluoma J, Finel M. UDP-glucuronosyltransferases (UGTs) 2B7 and UGT2B17 display converse specificity in testosterone and epitestosterone glucuronidation, whereas UGT2A1 conjugates both androgens similarly. Drug Metab Dispos. 2009;37:417–423. doi: 10.1124/dmd.108.024844. [DOI] [PubMed] [Google Scholar]
- 9.Sten T, Kurkela M, Kuuranne T, Leinonen A, Finel M. UDP-glucuronosyltransferases in conjugation of 5alpha- and 5beta-androstane steroids. Drug Metab Dispos. 2009;37:2221–2227. doi: 10.1124/dmd.109.029231. [DOI] [PubMed] [Google Scholar]
- 10.Gambrell RD., Jr The menopause: benefits and risks of estrogen-progestogen replacement therapy. Fertil Steril. 1982;37:457–474. doi: 10.1016/s0015-0282(16)46149-2. [DOI] [PubMed] [Google Scholar]
- 11.Payne DW, Katzenellenbogen JA. Binding specificity of rat alpha-fetoprotein for a series of estrogen derivatives: studies using equilibrium and nonequilibrium binding techniques. Endocrinology. 1979;105:745–753. doi: 10.1210/endo-105-3-743. [DOI] [PubMed] [Google Scholar]
- 12.Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17beta-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- 13.Katona BW, Krishnan K, Cai ZY, Manion BD, Benz A, Taylor A, et al. Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABAA receptors by ent-androgens. Eur J Med Chem. 2008;43:107–113. doi: 10.1016/j.ejmech.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Kurkela M, Patana AS, Mackenzie PI, Court MH, Tate CG, Hirvonen J, et al. Interactions with other human UDP-glucuronosyltransferases attenuate the consequences of the Y485D mutation on the activity and substrate affinity of UGT1A6. Pharmacogenet Genomics. 2007;17:115–126. doi: 10.1097/FPC.0b013e328011b598. [DOI] [PubMed] [Google Scholar]
- 15.Ohno S, Nakajin S. Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos. 2009;37:32–40. doi: 10.1124/dmd.108.023598. [DOI] [PubMed] [Google Scholar]
- 16.Itäaho K, Court M, Uutela P, Kostiainen R, Radominska-Pandya A, Finel M. Dopamine is a low affinity and high specificity substrate for the human UDP-glucuronosyltransferase 1A10. Drug Metab Dispos. 2009;37:768–775. doi: 10.1124/dmd.108.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobsson J, Ekstrom L, Inotsume N, Garle M, Lorentzon M, Ohlsson C, et al. Large differences in testosterone excretion in Korean and Swedish men are strongly associated with a UDP-glucuronosyl transferase 2B17 polymorphism. J Clin Endocrinol Metab. 2006;91:687–693. doi: 10.1210/jc.2005-1643. [DOI] [PubMed] [Google Scholar]
- 18.Miley MJ, Zielinska AK, Keenan JE, Bratton SM, Radominska-Pandya A, Redinbo MR. Crystal structure of the cofactor-binding domain of the human phase II drug-metabolism enzyme UDP-glucuronosyltransferase 2B7. J Mol Biol. 2007;369:498–511. doi: 10.1016/j.jmb.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]