Abstract
Phase I clinical trials exploring the use of autologous mesenchymal stem cell (MSC) therapy for the treatment of multiple sclerosis (MS) have begun in a number of centers across the world. MS is a complex and chronic immune-mediated and neurodegenerative disease influenced by genetic susceptibility and environmental risk factors. The ideal treatment for MS would involve both attenuation of detrimental inflammatory responses, and induction of a degree of tissue protection/regeneration within the CNS. Preclinical studies have demonstrated that both human-derived and murine-derived MSCs are able to improve outcomes in the animal model of MS, experimental autoimmune encephalomyelitis. How MSCs ameliorate experimental autoimmune encephalomyelitis is being intensely investigated. One of the major mechanisms of action of MSC therapy is to inhibit various components of the immune system that contribute to tissue destruction. Emerging evidence now supports the idea that MSCs can access the CNS where they can provide protection against tissue damage, and may facilitate tissue regeneration through the production of growth factors. The prospect of cell-based therapy using MSCs has several advantages, including the relative ease with which they can be extracted from autologous bone marrow or adipose tissue and expanded in vitro to reach the purity and numbers required for transplantation, and the fact that MSC therapy has already been used in other human disease settings, such as graft-versus-host and cardiac disease, with initial reports indicating a good safety profile. This article will focus on the theoretical and practical issues relevant to considerations of MSC therapy in the context of MS.
Keywords: adipose tissue, bone marrow, experimental autoimmune encephalitis, immunomodulation, mesenchymal stem cell, multiple sclerosis, multipotent stromal cell, neuroprotection, T lymphocyte, transdifferentiation
Clinical disability in multiple sclerosis (MS) is thought to be due to combined injury to oligodendrocytes and myelin (which normally facilitate rapid conduction along axons), and irreversible axonal loss. Following demyelination, some recovery of oligodendrocytes or differentiation of oligodendrocyte precursor cells (OPCs) can contribute to a degree of remyelination and restoration of neurological function. Repeated bouts of inflammatory demyelination followed by remyelination in part contribute to the clinical course of relapsing–remitting MS (RRMS). Axonal loss, which is already apparent early in disease, is particularly evident at sites of active inflammation, but also occurs more diffusely, and contributes to the development of permanent disability. With time, the underlying accumulation of injury results in progression of neurological disability, which has been referred to as secondary progressive MS (SPMS). Approximately 10–15% of patients with MS present with a progressive disease from onset (without experiencing remitting phases) and are referred to as having primary progressive MS (PPMS).
A large body of evidence supports the hypothesis that RRMS is an autoimmune disease mediated by autoreactive T cells capable of recognizing antigens normally compartmentalized in the brain. This self-recognition event may first occur in peripheral lymphatic tissue in response to brain antigens leaking from the CNS, or by molecular mimicry, a process whereby pathogens express antigens that, upon presentation by MHC molecules, bear a structural resemblance to human CNS antigens (for a review, see [1]). A growing array of immunomodulatory and immunosuppressive therapies are being used in the clinic for treating RRMS with increasing success, but have not been established as effective treatments for the progressive forms of MS. Thus, there continues to be a substantial unmet need for an MS therapy or combination therapy that can both abrogate the immune-mediated injury, as well as provide a degree of CNS tissue protection, and ideally, support functional regeneration of both myelinating and neural cells.
Mesenchymal stem cells: a versatile therapeutic tool
Mesenchymal stem cells (MSCs) have been referred to as mesenchymal stromal cells, marrow stromal cells and multipotent stromal cells, terms that reflect their tissue location and complex behaviors. The term ‘mesenchymal’ was initially used to indicate localization within the mesenchyme, a loose connective tissue present during development in the embryo or in adult dental pulp. While ‘mesenchymal’ remains a commonly used term, MSCs have since been found in almost all adult tissues, including bone marrow and adipose tissue. In their undifferentiated state, MSCs have a fibroblast-like appearance and are referred to as stromal cells. They provide substrate and growth factors to support neighboring cells, such as hematopoeitic stem cells in the bone marrow niche [2]. MSCs also have a multipotent stem cell capacity; with the appropriate cues, they readily differentiate into mesodermallineage products, such as adipose, cartilage and bone cells. In an effort to establish a consistent term, ‘multipotent mesenchymal stromal cells’ has been proposed by a panel of experts [3].
Their diverse functional capacities, and the ability to readily isolate, expand and manipulate them ex vivo, as well as their ability to traffic from blood to sites of tissue injury, have fueled much interest in MSCs as a potential therapeutic tool. Other advantages of MSCs are that they have been proven to be relatively safe in non-human primates and humans [4–6], and exhibit low immunogenicity compared with other types of stem cells [7–9], making them somewhat more amenable to allogeneic transplantation, although most proposals for use in autoimmune diseases have involved autologous cells. MSC-based therapies are now being explored in a host of neurological conditions [10], non-neurological autoimmune diseases [11] and nonautoimmune peripheral tissue injuries [5,12,13].
Lessons from experimental autoimmune encephalomyelitis
Experimental autoimmune encephalomyelitis (EAE) is an animal model typically involving rodents and sometimes non-human primates that models some aspects of MS. It can be generated through the peripheral activation (or passive administration) of CNS-antigen reactive T cells, resulting in multifocal inflammatory CNS demyelination, axon loss and the emergence of neurological signs. While experiences with EAE have not always been predictive of the outcome in patients with MS, there are nonetheless several insightful lessons regarding MSCs that can be gleaned from the EAE studies. In two pioneering studies, bone marrow MSCs derived from either mice or humans injected intravenously (iv.) into mice were shown to ameliorate EAE disease signs and pathological features [14,15]. The central observation of those studies has since been reproduced by several groups using rodents with a variety of genetic backgrounds, and using a range of myelin antigens to induce EAE by active immunization or adoptive transfer of disease-causing T cells [16–25]. MSCs are effective in treating EAE models that follow a ‘chronic’ (nonremitting) uniphasic disease course, as well as models that follow a relapsing–remitting disease course [14,20]. While many of the EAE studies used bone marrow-derived MSCs, other tissue sources of MSCs ameliorate EAE, such as adipose-derived MSCs [20] and human exfoliated deciduous teeth-derived MSCs [24]. Allogeneic MSCs can also attenuate EAE; however, the potential for transplant rejection and graft-versus-host disease dampens the enthusiasm compared with autologous MSCs [26]. Another limitation in treating EAE is that bone marrow-derived MSCs are only effective when given before disease onset, yet have little effect if given after the disease has stabilized [14,16]. Much of the current research effort is aimed at further elucidating the mechanisms of action of MSCs, in order to understand how best to harness their therapeutic potential in the context of MS.
Mechanisms of therapeutic effect of MSCs
While there is little doubt that MSCs ameliorate EAE signs, the mechanisms by which they achieve this outcome continue to be debated. Three main hypotheses have been proposed. The first hypothesis is that MSCs modulate the immune system in such a way as to limit the potentially autoreactive responses that would cause tissue damage in the CNS. This hypothesis has garnered the most support from experimental results. The second hypothesis is that MSCs secrete neuroprotective factors, and stimulate endogenous repair mechanisms in the CNS that counteract ongoing tissue degeneration. The third hypothesis is that MSCs transdifferentiate into brain cells leading to cell replacement; however, most of the current evidence indicates that this is not a robust phenomenon.
MSCs modulate the immune system
It is widely reported that T-cell proliferation can be robustly inhibited by MSCs [7–9,17,24,27–33]. MSCs can inhibit both CD4 and CD8 T-cell proliferation, and have been shown to inhibit T lymphocytes derived from a variety of species, including human, non-human primates and rodents. The ability to inhibit T-cell proliferation appears to be quite general, as T cells activated with a number of paradigms, including CNS antigens presented by antigen presenting cells, polyclonal (antigen-nonspecific) stimulators, such as lectins or anti-CD3 antibodies, or allogeneic stimulation in mixed lymphocyte reactions, are all inhibited by MSCs. An exception to this generalized effect is one report showing that recall antigens B. pertussis or tetanus toxoid were not inhibited by MSCs, despite an effect on allogeneic stimulation in the same study [9].
Mesenchymal stem cells possess a wide array of molecules that can impact T-cell biology. T-cell proliferation can be inhibited by MSCs in a contact-dependent manner that involves ICAM-1 and VCAM-1, two adhesion molecules expressed by MSCs [8,33]. MSCs also secrete a variety of soluble products that have immuno-modulatory properties. In EAE, it was shown that a fragmented version of a chemokine receptor, CCL2, is critical for the ability of MSCs to inhibit disease [21]. Other molecules made by MSCs that have been demonstrated to inhibit T cells include indoleamine 2, 3-dioxygenase [28,34], nitric oxide [34], prostaglandin E2 (PGE2) [35], HLA-G [36], HGF [27], galectin 1 [37] and semaphorin 3A [37]. A role for TGF-β has been proposed, but remains controversial [27,28,34,38]. Proteomic studies have uncovered an extensive array of molecules secreted by MSCs; the list includes cytokines, chemokines and growth factors [39]. The relative importance of these mechanisms and molecules continues to be explored [32].
In addition to their effects on T-cell proliferation, MSCs can have a profound effect on T-cell differentiation. In EAE models, MSCs decrease proinflammatory Th1 and Th17 responses while promoting anti-inflammatory Th2 responses [14,19,21,24,35,38]. How well animal studies will predict the effect on inflammatory T-cell responses is less clear. Preclinical studies using human T cells demonstrated that both fetal- and adult-derived human MSCs or their secreted products can paradoxically increase Th17 responses [40,41], a feature that is exaggerated when MSCs are pre-conditioned with a proinflammatory molecule (IL-1β) [41]. The reason for this discrepancy may relate to competing mechanisms of action; for example, MSCs can influence Th17 responses in vitro through cell contact or soluble mechanisms that appear to depend on relative cell densities. At higher ratios of MSC:responder T cells, they inhibit IL-17 production through cell contact, whereas at lower MSC:T-cell ratios, MSCs can actually increase T-cell production of IL-17, an effect that appears to be mediated by IL-6 [31].
B-cell proliferation is also inhibited by MSCs; however, the proliferation block appears more selective than for T cells since proliferation of B cells in response to CpG (a mimic of pathogen-derived DNA) alone is refractory to MSC inhibition [38], while proliferation towards a mixture of CpG and factors that reflect an adaptive response (CD40L, anti-Ig antibodies, IL-2 and IL-4) can be readily inhibited [42–44]. Other B-cell responses inhibited by MSCs include secretion of immunoglobulins (IgM, IgG and IgA), chemotaxis towards chemokine gradients and differentiation of B cells from bone marrow precursors [42–44]. Tregs, which are potent inhibitors of adaptive T-cell and B-cell proliferation, can also be regulated by MSCs in some settings [31,35], but the role of Tregs in the context of the MSC effect on EAE remains undefined [14,38].
Dendritic cell (DC) biology can be modified in a number of ways. MSCs suppress the ability of DCs to trigger T-cell proliferation through a mechanism that involves perturbations to the DC cytoskeleton, and reduced expression of antigen-presenting and costimulatory molecules [28,45–49]. The production of potentially proinflammatory cytokines by DCs is inhibited by MSCs, while anti-inflammatory IL-10 is reportedly increased [35,48,49]. Differentiation and maturation of DC from monocytic precursors can also be blocked [46,47]. The in vivo relevance of these well-documented in vitro phenomena remains to be established; in EAE, in vivo MSC therapy reportedly did not influence DC phenotype [14]. Macrophages appear similarly affected by MSCs, as are DCs, with skewing towards an anti-inflammatory cytokine profile and reduced antigen presenting function being reported [50]. Inhibition of natural killer cells, invariant NK T cells and γδ T cells by MSCs and MSC-secreted products, such as PGE2, has been reported [35,38,51]. The relevance that suppressing these types of cells might have in MS is unclear, as they have both protective and tissue-damaging roles. In summary, MSCs display a wide array of effects on the immune system (both innate and adaptive). These would appear to be, on balance, mostly anti-inflammatory, and thus would be predicted to limit new disease activity and improve the outcome of patients with MS.
Impact of the local microenvironment on MSCs’ immunomodulatory behavior
An important consideration for MSC therapy in humans relates to the broad range of exposures that can modulate MSC biology and may be encountered in vivo in humans, which may be considerably more complex than the range of stimuli modeled in animal housing facilities. Preclinical work comparing MSCs derived from MS patients to healthy donor MSCs revealed that most biological parameters of MSCs were comparable between the groups. These parameters included the MSC proliferative capacity, a range of phenotypic markers, cytokine production, Toll-like receptor (TLR) responsiveness and importantly, their ability to inhibit T-cell and DC responses. One reported exception was that MS patient-derived MSCs produced significantly larger amounts of the chemokine CXCL10 (IP-10), which is capable of chemoattracting immune cells [52].
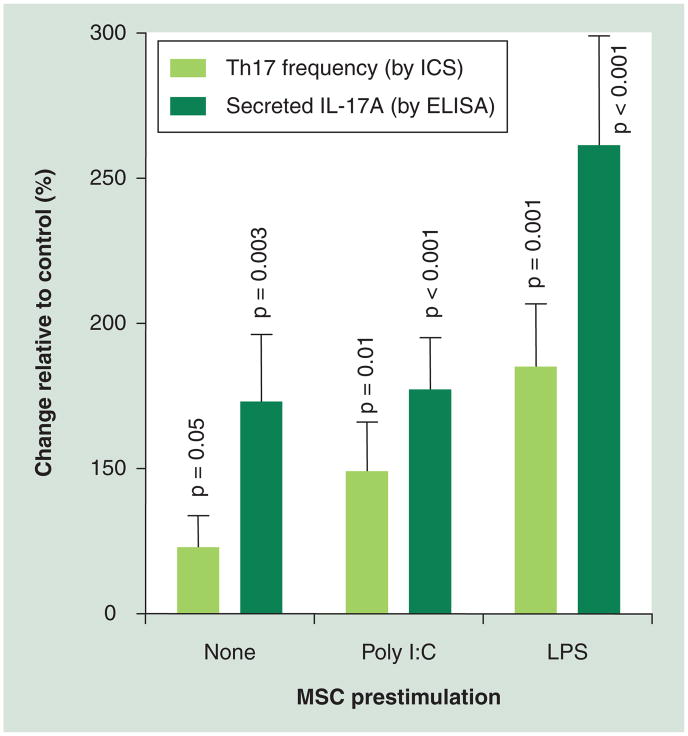
There are likely to be other parameters that may distinguish response profiles of MSC documented in animals from those of human MSCs. The relevance of such differences to our ability to predict the impact of injecting MS patient-derived autologous MSCs into MS patients remains to be elucidated. Some of the factors that MSCs may encounter in vivo in humans could theoretically interfere with the ability of MSCs to inhibit the immune system; for example, MSCs express TLRs, such as TLR4, which responds to lipopolysaccharide (LPS) from Gram-negative bacteria, and TLR3, which recognizes double-stranded RNA, which is found in some viruses and can mimicked in the lab by the chemical poly I:C. The exposure of MSCs to LPS or poly I:C has been shown to reduce certain immunosuppressive properties of MSC, and induce the secretion of proinflammatory cytokines [53,54], including IL-6, which is known to contribute to the induction of Th17 responses that may be detrimental in MS [32]. In support of this are data from our laboratory (Figure 1), indicating that pre-exposure of human MSCs to either LPS or poly I:C can significantly enhance the capacity of MSCs to induce Th17 responses. The magnitude of the LPS effect was even greater than that previously described for IL-1β [41]. Another factor that may influence MSCs responses in vivo is ATP, a molecule released during tissue damage, which impairs the ability of MSCs to inhibit T-cell proliferation and enhances their secretion of proinflammatory cytokines [53]. On the other hand, other factors in the local microenvironment may augment the immunosuppressive effects of MSC, such as the stress-inducible molecule NAD [55] and IFN-γ [33,38]. Other proinflammatory factors that modify MSC immune properties include IFN-α/-β, TNF-α and SDF-1 (for a review, see [56]).
Figure 1. Impact of environmental exposures on mesenchymal stem cell-mediated increases in human Th17 responses.
Conditioned media collected from MSCs that received no prestimulation (none), or from MSCs that were prestimulated with poly I:C or LPS, were added to a Th17 differentiation assay. The Th17 differentiation assay used primary human peripheral blood mononuclear cells, activated with anti-CD3 and a differentiation cocktail, including IL-23, anti-IFN-γ and anti-IL-4. The percentage of Th17+ CD4 T cells was determined by ICS for IL-17A, and surface staining for CD4. The amount of secreted IL-17A was determined by ELISA. Data pooled from six independent experiments are expressed as the change relative to the Th17 responses of the differentiation assay, when no MSC media was added (control). MSC conditioned media significantly increased Th17 responses as compared to control (p-values shown). The statistical analysis was performed using student t-test.
ICS: Intracellular cytokine staining; LPS: Lipopolysaccharide; MSC: Mesenchymal stem cells; Poly I:C: Polyinosinic:polycytidylic acid.
Together, these observations further emphasize the point that predicting in vivo MSC behavior in humans who live in a highly variable ‘dirty’ environment is likely to be considerably more complex than in genetically inbred animals that are housed in specific pathogen-free environments. Careful monitoring of immune responses in patients will need to be conducted in clinical trials to measure disease-relevant immune responses, including T-cell proliferation and differentiation, as well as to assess factors that influence these responses.
MSC effects in the CNS: protection & repair
While most of the clinical effects in EAE are thought to be due to immune modulation in peripheral lymphoid tissue, emerging evidence also supports a therapeutic role for MSCs within the CNS [18,57–61]. Upon iv. injection of MSCs, they will migrate predominantly to the lung, but a small fraction of cells make their way to other tissues, including secondary lymphoid tissue, bone marrow, as well as into the CNS [4,62–64]. Migration of MSCs into the CNS may be more efficient in disease states, as shown in EAE where iv. injected MSCs are recruited to the site of injury, reportedly localizing to sites of demyelination even weeks after EAE induction; the actual numbers that reach the CNS even in this context appear to be quite small [19,23]. Migration to the site of injury has also been observed in the context of other neurological insults, including ischemia and traumatic injuries [65]. A recent report using in vivo cell tracking techniques in patients with MS and amyotrophic lateral sclerosis was consistent with the notion that MSCs can migrate to the CNS [66]. The mechanisms by which MSCs migrate to the CNS and other other tissue injury sited continue to be studied; chemokines, such as fractalkine and SDF-1, have been implicated [67]. The ability of MSCs to traffic from blood into damaged/inflamed tissues is a potential advantage as a therapeutic agent such as this would obviate the need for CNS injections.
Since the number of MSCs that enter the CNS upon iv. injection is rather small, other routes of injection have been explored that would maximize delivery into the brain tissue [59,68]. In one study, intracerebroventricular or intracerebroparenchymal injection attenuated mild EAE, although MSCs were ineffective in treating more severe EAE models when delivered in this manner [25].
One rationale for introducing MSCs into the CNS relates to their apparent capacity to induce endogenous repair mechanisms and contribute to the release of protective factors. Evidence for this includes the observation that MSCs induce neuronal precursor cells to differentiate into oligodendrocytes [69] and will stimulate axon regeneration in a lysolecithin injury model [70]. These events appear to occur independently of the immune system, indicating that MSCs can act directly on endogenous repair systems. Upon iv. injection in EAE, MSCs migrated to sites of demyelination in the spinal cord, where their presence was associated with increased oligodendrogenesis, and also appeared to trigger endogenous neuronal precursor cell activity [19]. How MSCs might stimulate such repair is not fully elucidated, although it has been demonstrated that MSCs are capable of secreting neurotrophic molecules, such as BDNF, that may account for some of the regenerative effects [15,19,20]. To maximize the potential for tissue regeneration, transplantation strategies using a combination of both MSCs and neural precursor cells have been explored. Transplanting MSCs with oligodendrocyte precursor cells improved myelination in myelin-deficient shiverer mice [71]. In addition to stimulating endogenous repair, MSCs may provide a degree of protection against ongoing tissue injury in the CNS through mechanisms including switching microglia to a protective state, protecting neurons from apoptosis and attenuating oxidative tissue injury (for a review, see [60]).
Despite such evidence for MSCs contributing to repair/protection within the CNS, the major clinical effect of MSCs in EAE is still likely to be due to immune modulation. Even in situations where MSCs are injected into the CNS, they appear to migrate to peripheral lymphoid tissues and inhibit T-cell activation [18,19]. In one study where MSCs were manipulated to become neurotrophic factor secreting cells, they could prevent neuron death and attenuate EAE clinical signs, but were also found to potently inhibit T-cell activation [22], making it difficult to ascertain the relative contribution of these distinct mechanisms. On the other hand, when MSCs were differentiated towards a neuronal lineage, they lost their ability to suppress T-cell proliferation, and no longer attenuated EAE [72]. Current effort is directed at optimizing both the immunomodulatory and neuroprotective potential of MSCs.
Little evidence for neural transdifferentiation of MSCs in CNS inflammatory injury
An initial reason for using MSCs was that they might transdifferentiate into brain cells. Early results supporting transdifferentiation in vivo were subsequently shown to reflect an artifact of cell fusion between MSC and endogenous brain cells (for a review, see [73]). Since then, several investigators have found that MSCs do have the capacity to express oligodendrocyte, astrocyte and neuron phenotypic markers in vitro, as well as following injection into animals induced with EAE [18,23,72]. However, a recent study indicated that MSC fusion artifacts can be increased when EAE is induced [74]. Whether or not actual transdifferentiation can occur, it has still not been clearly demonstrated that phenotypic similarities of MSC to neural cells within the CNS can translate into functional replacement of lost brain cells within damaged tissue. Other arguments against transdifferentiation playing a major role in the observed benefits of MSC in EAE include the rapid onset of therapeutic effect of MSCs, which would appear to be inconsistent with the time needed for transdifferentiation to occur; the observation by a number of groups that relatively low numbers of MSCs reach the CNS even in the inflamed state; and the ongoing challenge that long-term engraftment of MSCs in the CNS has remained difficult to achieve (for a review, see [60,61]).
Expert commentary
Will MSC injections have a sustainable effect?
Key unanswered questions relate to the durability and reversibility of the immune-modulating effects of MSC (e.g., inhibition of T-cell proliferation). The literature is not unanimous on this point; some studies indicate that the MSC-induced T-cell inhibition is reversible [8,27], while other studies suggest it is irreversible, reminiscent of classical anergy (a state of functional unresponsiveness) that can be released upon addition of exogenous IL-2 [7,14]. The classical anergy model has also been challenged by a report showing that IL-2 could not release T cells from their unresponsive state [29]. There has been further evidence presented for ‘split-anergy’ induction by MSCs, as defined by a block in proliferation, but no effect on the production of effector cytokines (as shown for IFN-γ) or other early activation markers (CD25 and CD69) [29]. While the goal of treating auto-immunity remains inhibition of pathogenic immune responses, including those of T cells, such effects have to be tempered to avoid a more global immunosuppression that could manifest in failed immune competence. This point is particularly relevant since MSCs inhibit proliferation of both T and B cells in a fairly nonspecific manner that need not discriminate between autoreactive or antipathogen responses. It is encouraging that human recall responses to tetanus toxoid and B. pertussis may be less readily inhibited by MSCs, suggesting that immunological memory to prior pathogen exposure will not be affected [9].
Delineating the precise effects of MSC and the durability of such effects on T-cell activation will be crucial in refining possible therapies that need to deal with ongoing aberrant T-cell responses, as well as de novo T-cell responses possibly against other brain antigens that may be released as the disease evolves from relapse to relapse. Would repeated MSC injection be required and if so, when? At the time of relapse? During remission, before T-cell activation against new brain antigens occurs? There is also emerging evidence that unexpected crosstalk exists between the immune system and MSCs. T lymphocytes and innate γδ T cells can directly kill MSCs through apoptotic or cytotoxic mechanisms [24,51]. This observation is worth exploring further as it may have implications for the dosing of MS patients that already have a high level of baseline T-lymphocyte activation, which could rapidly kill MSCs.
The current consensus in the literature indicates that the main effects of MSCs relevant to treatment of RRMS relate to the peripheral immune modulatory capacity of MSCs (for which iv. injection may suffice). The potential capacity of MSCs to contribute to a protective and/or growth-promoting environment within the CNS is also worthy of defining in the context of MS, including whether such effects can be achieved with iv. administration or whether an intrathecal approach is much more efficient/necessary. Published protocols refer to both iv. and intrathecal injection of MSCs [66,75,76]. One notes that recent animal studies have shown that MSCs have the potential to form fibrous scar tissue in the brain, a process that is exacerbated by the proinflammatory environment that occurs during EAE [25], underscoring the importance of carefully weighing the risks and benefits of the different approaches to MSC therapy as new data emerges.
Response to stem cell clinics: ‘medical tourism’
An issue that has been much discussed in the lay press relates to the phenomenon of ‘stem cell clinics’, where patients may travel considerable distances to receive stem cells for a variety of conditions, including MS. This growing trend of so-called ‘medical tourism’ presents many difficulties to patients, caregivers, scientific communities and the national health systems that support them. Consensus statements have been released by leaders in the field that point out that any future use of stem cells, including MSCs, should be conducted in the context of organized clinical trials where robust design and sufficient follow-up allow for effective monitoring of both safety and efficacy outcomes, and where scientific studies can be incorporated to dissect the actual mechanisms underlying in vivo effects of intervention [59,68,77]. Other key elements that need to be established for the appropriate future use of MSCs in clinical trials include well-defined inclusion and exclusion criteria, carefully validated protocols for isolation, purification, storage and administration of MSC, and ethical reviews that take into consideration new data that influence the balance of risk and benefit for different types of MSCs therapy.
Five-year view
Mesenchymal stem cells have gone from the bench to the bedside in a relatively short period of time. Phase I clinical trial results have been reported for MSC therapy in graft-versus-host disease (for a review, see [6]) and cardiac disease (for a review, see [5]), with favorable safety profiles, but mixed clinical results. Some early experiences with MSCs in MS have also been reported in small studies that indicate a good safety profile [75,76,78–80]. A Phase II/III clinical trial using intrathecal and iv. injection of bone marrow-derived autologous MSCs reported headache and fever in the majority of patients, with no major side effects except the possible toxicity from dimethyl sulfoxide (the preservative used during MSC cryopreservation), and the authors demonstrated that T-cell proliferation to lectins was reduced in data pooled from amyotrophic lateral sclerosis and MS, but no significant clinical effect was found, albeit the study size and follow-up time were insufficient to draw any firm conclusions [66]. A Phase I clinical trial has been initiated at the Cleveland Clinic (OH, USA) using autologous iv. administered MSCs in patients with MS [81], and extensive ancillary biological studies, including evaluation of in vivo effects of MSCs on MS-relevant immune responses, will be conducted in collaboration with the experimental therapeutics program at the Montreal Neurological Institute (Canada). A recent Phase IIA study plan was published for iv. transplantation of autologous MSCs in MS; the study will follow well-defined clinical and imaging parameters and is using a novel analysis of optic nerves to track CNS repair [82].
As clinical trials proceed, desired milestones to be reached within the next 5 years will include a careful monitoring of immunological and neurobiological parameters in MS patients before and after MSC transplantation, focusing on responses that are the most relevant to MS including immune effects on Th1, Th2 and Th17 T-cell responses, as well as the impact of therapy on CD8 T cells, NK cells, B-cell responses and myeloid cell profiles. Biological measures in CSF may provide insights into the impact of MSC therapy on the balance of neuroinflammation and neurodegeneration and repair, for which correlation with selected clinical outcomes and particularly emerging brain imaging approaches, will be most informative. Relating the impact of therapy on clinical and imaging parameters to the profile and magnitude of MSC effects on immunological responses, and careful consideration of individual patients, as well as monitoring adverse reactions that could include infections or immune responses that may indicate a proinflammatory environment (i.e., Th17 increases) will be paramount. Ancillary paraclinical studies of interest would be ones to examine MS patient blood samples and MS patient-derived MSCs, to determine whether the desired immunomodulation occurs when MS patient T cells are used as the responders or whether MS patient-derived MSCs attenuate potentially damaging proinflammatory T-cell responses as compared with healthy control MSCs. These studies would further elucidate the relative immune modulatory capacity of MS-derived MSC and the relative responsiveness of MS patient-derived immune cells to MSC effects, helping to dissect relevant therapeutic mechanisms of MSCs and most effectively adapt this promising approach to the MS spectrum. If results indicate that MSCs are safe and well tolerated in MS patients, then future clinical trials will be planned to address, in a more definitive fashion, the efficacy of treatment.
Key issues.
Multiple sclerosis (MS) is a chronic immune-mediated and neurodegenerative disease.
Mesenchymal stem cells (MSCs) are a versatile therapeutic tool with the potential to treat tissue injuries, including CNS inflammatory and degenerative injuries.
Both murine- and human-derived MSCs have shown robust efficacy in reducing disease activity and CNS injury in experimental autoimmune encephalomyelitis, an immune model of MS.
Several mechanisms of action are proposed for the therapeutic effect of MSCs in experimental autoimmune encephalomyelitis.
MSCs modulate several aspects of the immune system, generally downregulating immune responses such as T-lymphocyte proliferation and inflammatory cytokine production, which are thought to contribute to MS disease activity.
The local microenvironement can influence MSCs’ immunomodulatory behavior; for example, through engagement of Toll-like receptors during pathogen-associated exposure.
In addition to effects on the peripheral immune system, MSCs can have effects within the CNS, where they have the potential to mediate protection and repair. CNS-compartmentalized mechanisms of action are relevant since MSCs can migrate from the blood to the CNS at the site of injury, or can be injected into the CNS.
There is little evidence that transdifferentiation into brain cells represents a relevant therapeutic mechanism of MSCs in CNS inflammatory injury.
Future questions include whether single MSC injections will have a sustainable effect in vivo or whether repeat dosing will be required, and whether long-term MSC treatment regimens will prove to have acceptable safety profiles. In response to stem cell clinics and ‘medical tourism’, several organized clinical trials are now underway to test MSCs for treatment of MS.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Amit Bar-Or is supported by NIH funding in collaboration with Jeffrey Cohen at the Cleveland Clinic Foundation to carry out mechanistic studies on the effects of mesenchymal stem cells in a Phase I clinical trial of mesenchymal stem cells in multiple sclerosis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1•.Bar-Or A, Darlington PJ. The immunology of MS. In: Cohen JA, Rudick RA, editors. MS Therapeutics. 4. Cambridge University Press; Cambridge, UK: 2011. Comprehensive description of the immunological aspects of multiple sclerosis (MS) [Google Scholar]
- 2.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 4.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29(2):244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 5.Flynn A, O’Brien T. Stem cell therapy for cardiac disease. Expert Opin Biol Ther. 2011;11(2):177–187. doi: 10.1517/14712598.2011.543894. [DOI] [PubMed] [Google Scholar]
- 6.Kebriaei P, Robinson S. Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy. 2011;13(3):262–268. doi: 10.3109/14653249.2010.549688. [DOI] [PubMed] [Google Scholar]
- 7•.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. Linked the ability of mesenchymal stem cells (MSCs) to inhibit T-cell proliferation to a clinical benefit in allograft survival. [DOI] [PubMed] [Google Scholar]
- 8.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 9.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171(7):3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 10.Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10(7):649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- 11.Dazzi F, Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract Res Clin Haematol. 2011;24(1):49–57. doi: 10.1016/j.beha.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Abreu SC, Antunes MA, Pelosi P, Morales MM, Rocco PR. Mechanisms of cellular therapy in respiratory diseases. Intensive Care Med. 2011 doi: 10.1007/s00134-011-2268-3. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Hu G, Su J, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 14••.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. Established MSCs as a possible therapy for MS, and demonstrated in vivo T-cell suppression by MSCs. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Li Y, Chen J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195(1):16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61(3):219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 17.Pedemonte E, Benvenuto F, Casazza S, et al. The molecular signature of therapeutic mesenchymal stem cells exposes the architecture of the hematopoietic stem cell niche synapse. BMC Genomics. 2007;8:65. doi: 10.1186/1471-2164-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Kassis I, Grigoriadis N, Gowda-Kurkalli B, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008;65(6):753–761. doi: 10.1001/archneur.65.6.753. Used in vivo tracking technology to characterize the localization of MSCs in the CNS, and provided evidence for regeneration of brain cells. [DOI] [PubMed] [Google Scholar]
- 19.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57(11):1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantin G, Marconi S, Rossi B, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27(10):2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 21••.Rafei M, Campeau PM, Aguilar-Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182(10):5994–6002. doi: 10.4049/jimmunol.0803962. Demonstrated that soluble CCL2 mediates the MSCs’ ability to attenuate experimental autoimmune encephalitis, and provided detailed mechanistic data showing that soluble CCL2 suppresses Th17 responses. [DOI] [PubMed] [Google Scholar]
- 22.Barhum Y, Gai-Castro S, Bahat-Stromza M, Barzilay R, Melamed E, Offen D. Intracerebroventricular transplantation of human mesenchymal stem cells induced to secrete neurotrophic factors attenuates clinical symptoms in a mouse model of multiple sclerosis. J Mol Neurosci. 2010;41(1):129–137. doi: 10.1007/s12031-009-9302-8. [DOI] [PubMed] [Google Scholar]
- 23.Gordon D, Pavlovska G, Uney JB, Wraith DC, Scolding NJ. Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2010;69(11):1087–1095. doi: 10.1097/NEN.0b013e3181f97392. [DOI] [PubMed] [Google Scholar]
- 24.Yamaza T, Kentaro A, Chen C, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1(1):5. doi: 10.1186/scrt5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Grigoriadis N, Lourbopoulos A, Lagoudaki R, et al. Variable behavior and complications of autologous bone marrow mesenchymal stem cells transplanted in experimental autoimmune encephalomyelitis. Exp Neurol. 2011;230(1):78–89. doi: 10.1016/j.expneurol.2011.02.021. Demonstrated in animal models that MSCs can form potentially pathogenic scar tissues in CNS. [DOI] [PubMed] [Google Scholar]
- 26.Rafei M, Birman E, Forner K, Galipeau J. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009;17(10):1799–1803. doi: 10.1038/mt.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 28.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 29.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 30.Kang HS, Habib M, Chan J, et al. A paradoxical role for IFN-γ in the immune properties of mesenchymal stem cells during viral challenge. Exp Hematol. 2005;33(7):796–803. doi: 10.1016/j.exphem.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Liu XJ, Zhang JF, Sun B, et al. Reciprocal effect of mesenchymal stem cell on experimental autoimmune encephalomyelitis is mediated by transforming growth factor-β and interleukin-6. Clin Exp Immunol. 2009;158(1):37–44. doi: 10.1111/j.1365-2249.2009.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanz TV, Opitz CA, Ho PP, et al. Mouse mesenchymal stem cells suppress antigen-specific TH cell immunity independent of indoleamine 2,3-dioxygenase 1 (IDO1) Stem Cells Dev. 2010;19(5):657–668. doi: 10.1089/scd.2009.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184(5):2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 36.Nasef A, Mathieu N, Chapel A, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84(2):231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 37.Lepelletier Y, Lecourt S, Renand A, et al. Galectin-1 and semaphorin-3A are two soluble factors conferring T-cell immunosuppression to bone marrow mesenchymal stem cell. Stem Cells Dev. 2010;19(7):1075–1079. doi: 10.1089/scd.2009.0212. [DOI] [PubMed] [Google Scholar]
- 38.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 39.Skalnikova H, Motlik J, Gadher SJ, Kovarova H. Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics. 2011;11(4):691–708. doi: 10.1002/pmic.201000402. [DOI] [PubMed] [Google Scholar]
- 40.Guo Z, Zheng C, Chen Z, et al. Fetal BM-derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur J Immunol. 2009;39(10):2840–2849. doi: 10.1002/eji.200839070. [DOI] [PubMed] [Google Scholar]
- 41•.Darlington PJ, Boivin MN, Renoux C, et al. Reciprocal Th1 and Th17 regulation by mesenchymal stem cells: implication for multiple sclerosis. Ann Neurol. 2010;68(4):540–545. doi: 10.1002/ana.22065. Preclinical study using adult human MSCs showing that MSC products have the potential to paradoxically increase Th17 responses while decreasing Th1. [DOI] [PubMed] [Google Scholar]
- 42.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 43.Tabera S, Perez-Simon JA, Diez-Campelo M, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93(9):1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- 44.Asari S, Itakura S, Ferreri K, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37(5):604–615. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aldinucci A, Rizzetto L, Pieri L, et al. Inhibition of immune synapse by altered dendritic cell actin distribution: a new pathway of mesenchymal stem cell immune regulation. J Immunol. 2010;185(9):5102–5110. doi: 10.4049/jimmunol.1001332. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 47.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Sun B, Wang D, et al. Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T-cell tolerance. Scand J Immunol. 2008;68(6):607–615. doi: 10.1111/j.1365-3083.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 49.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 50.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5(2):e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. Reciprocal interactions between human mesenchymal stem cells and γδ T cells or invariant natural killer T cells. Stem Cells. 2009;27(3):693–702. doi: 10.1634/stemcells.2008-0687. [DOI] [PubMed] [Google Scholar]
- 52•.Mazzanti B, Aldinucci A, Biagioli T, et al. Differences in mesenchymal stem cell cytokine profiles between MS patients and healthy donors: implication for assessment of disease activity and treatment. J Neuroimmunol. 2008;199(1–2):142–150. doi: 10.1016/j.jneuroim.2008.05.006. Demonstration that MSCs derived from MS patients are overall similar to healthy controls, including their ability to suppress T cells. [DOI] [PubMed] [Google Scholar]
- 53.Ferrari D, Gulinelli S, Salvestrini V, et al. Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12 and increases the homing capacity and production of proinflammatory cytokines. Exp Hematol. 2011;39(3):360–374. e1–5. doi: 10.1016/j.exphem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Liotta F, Angeli R, Cosmi L, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26(1):279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 55.Fruscione F, Scarfi S, Ferraris C, et al. Regulation of human mesenchymal stem cell functions by an autocrine loop involving NAD+ release and P2Y11- mediated signaling. Stem Cells Dev. 2010;20(7):1183–1198. doi: 10.1089/scd.2010.0295. [DOI] [PubMed] [Google Scholar]
- 56.Greco SJ, Rameshwar P. Microenvironmental considerations in the application of human mesenchymal stem cells in regenerative therapies. Biologics. 2008;2(4):699–705. doi: 10.2147/btt.s2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karussis D, Kassis I. The potential use of stem cells in multiple sclerosis: an overview of the preclinical experience. Clin Neurol Neurosurg. 2008;110(9):889–896. doi: 10.1016/j.clineuro.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265(1–2):131–135. doi: 10.1016/j.jns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Freedman MS, Bar-Or A, Atkins HL, et al. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 2010;16(4):503–510. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- 60.Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24(1):59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Uccelli A, Morando S, Bonanno S, Bonanni I, Leonardi A, Mancardi G. Mesenchymal stem cells for multiple sclerosis: does neural differentiation really matter? Curr Stem Cell Res Ther. 2011;6(1):69–72. doi: 10.2174/157488811794480744. [DOI] [PubMed] [Google Scholar]
- 62.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 63.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 64.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 65.Sykova E, Jendelova P. Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ. 2007;14(7):1336–1342. doi: 10.1038/sj.cdd.4402140. [DOI] [PubMed] [Google Scholar]
- 66•.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. Provides evidence for in vivo trafficking of MSCs using magnetic resonance imaging and reports on their immunomodulatory effect in humans with neurological disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22(3):415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 68.Kassis I, Vaknin-Dembinsky A, Karussis D. Bone marrow mesenchymal stem cells: agents of immunomodulation and neuroprotection. Curr Stem Cell Res Ther. 2011;6(1):63–68. doi: 10.2174/157488811794480762. [DOI] [PubMed] [Google Scholar]
- 69.Rivera FJ, Siebzehnrubl FA, Kandasamy M, et al. Mesenchymal stem cells promote oligodendroglial differentiation in hippocampal slice cultures. Cell Physiol Biochem. 2009;24(3–4):317–324. doi: 10.1159/000233256. [DOI] [PubMed] [Google Scholar]
- 70.Cho JS, Park HW, Park SK, et al. Transplantation of mesenchymal stem cells enhances axonal outgrowth and cell survival in an organotypic spinal cord slice culture. Neurosci Lett. 2009;454(1):43–48. doi: 10.1016/j.neulet.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 71.Cristofanilli M, Harris VK, Zigelbaum A, et al. Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells Dev. 2011 doi: 10.1089/scd.2010.0547. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 72.Matysiak M, Orlowski W, Fortak-Michalska M, Jurewicz A, Selmaj K. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J Neuroimmunol. 2011;233(1–2):106–111. doi: 10.1016/j.jneuroim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Wright KT, El Masri W, Osman A, Chowdhury J, Johnson WE. Bone marrow for the treatment of spinal cord injury: mechanisms and clinical application. Stem Cells. 2011;29(2):169–178. doi: 10.1002/stem.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kemp K, Gordon D, Wraith DC, et al. Fusion between human mesenchymal stem cells and rodent cerebellar Purkinje cells. Neuropathol Appl Neurobiol. 2011;37(2):166–178. doi: 10.1111/j.1365-2990.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang J, Zhang H, Hua B, et al. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler. 2009;15(5):644–646. doi: 10.1177/1352458509104590. [DOI] [PubMed] [Google Scholar]
- 76.Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4(1):50–57. [PubMed] [Google Scholar]
- 77.Siatskas C, Payne NL, Short MA, Bernard CC. A consensus statement addressing mesenchymal stem cell transplantation for multiple sclerosis: it’s time! Stem Cell Rev. 2010;6(4):500–506. doi: 10.1007/s12015-010-9173-y. [DOI] [PubMed] [Google Scholar]
- 78.Scolding N. Adult stem cells and multiple sclerosis. Cell Prolif. 2011;44(Suppl 1):35–38. doi: 10.1111/j.1365-2184.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riordan NH, Ichim TE, Min WP, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227(1–2):185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 81.Mahad DP, Cohen JA. Mesenchymal stem cell transplantation to treat multiple sclerosis. In: Cohen JA, Rudick RA, editors. Multiple Sclerosis Therapeutics. 4. Cambridge University Press; Cambridge, UK: 2011. [Google Scholar]
- 82.Connick P, Kolappan M, Patani R, et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12:62. doi: 10.1186/1745-6215-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]