Abstract
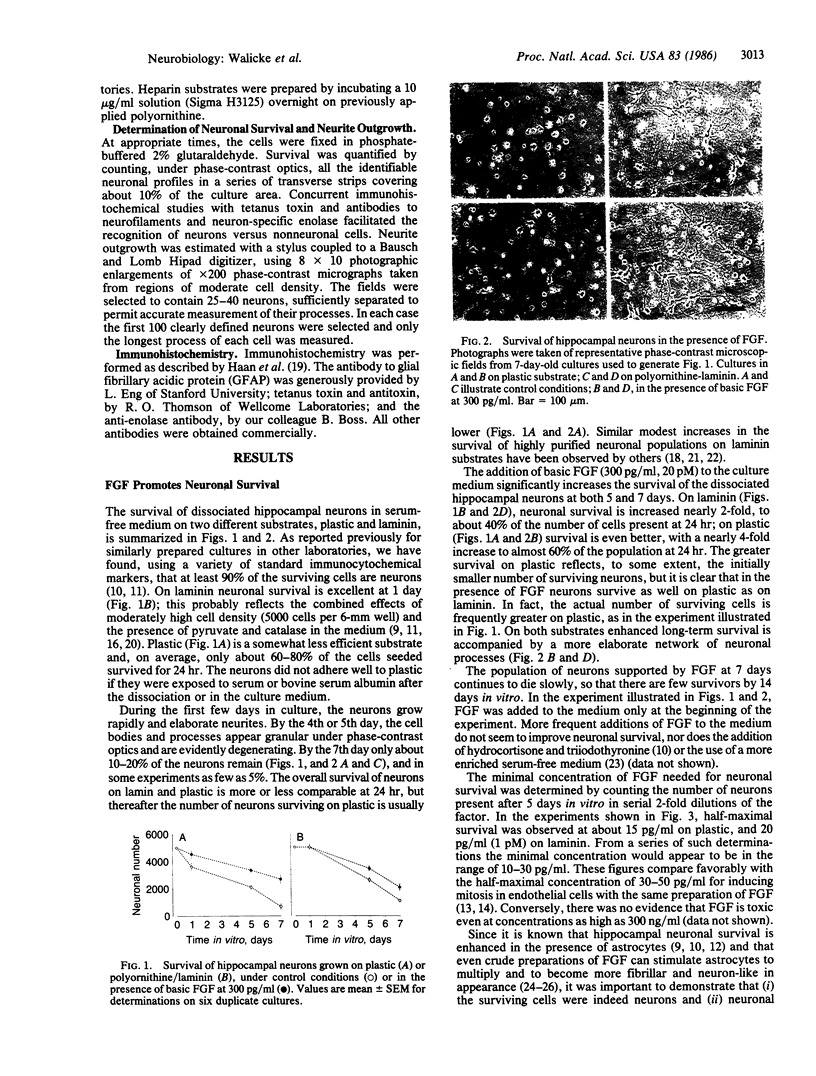
Basic fibroblast growth factor (FGF) has been found to increase neuronal survival and neurite extension in a highly purified population of fetal rat hippocampal neurons under well-defined serum-free cell culture conditions. In the presence of FGF, neuronal survival after 7 days in culture on a simple plastic substrate is increased 4-fold, to 54% of the initial population. Survival is increased 2-fold to 40% on polyornithine-laminin. When FGF was bound to plastic or heparin substrates, neurite outgrowth was significantly increased to lengths comparable to those seen with laminin; however, FGF produced no further increase in neurite outgrowth on laminin. Half-maximal survival was observed at FGF concentrations of about 15 pg/ml (1 pM); half-maximal process outgrowth occurred at about 375 pg/ml (20 pM). The responsive cells were identified as neurons by their labeling with tetanus toxin and by antibodies to neurofilaments and to the neuron-specific enolase. Astrocytes, identified by the presence of glial fibrillary acidic protein, constituted about 10% of cells present at 1 week both in the presence and in the absence of FGF. These results strongly suggest that, in addition to its known mitogenic effects on nonneuronal cells, FGF possesses neurotrophic activity for hippocampal neurons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Walker P. S., Fellows R. E. Properties of neurons from dissociated fetal rat brain in serum-free culture. J Neurosci. 1983 Dec;3(12):2448–2462. doi: 10.1523/JNEUROSCI.03-12-02448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979 Oct 1;187(3):469–493. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- Banker G. A. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980 Aug 15;209(4458):809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- Barbin G., Selak I., Manthorpe M., Varon S. Use of central neuronal cultures for the detection of neuronotrophic agents. Neuroscience. 1984 May;12(1):33–43. doi: 10.1016/0306-4522(84)90135-0. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K. New neuronal growth factors. Annu Rev Neurosci. 1984;7:149–170. doi: 10.1146/annurev.ne.07.030184.001053. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Baird A., Esch F., Ling N., Gospodarowicz D. Isolation and partial molecular characterization of pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5364–5368. doi: 10.1073/pnas.81.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calof A. L., Reichardt L. F. Motoneurons purified by cell sorting respond to two distinct activities in myotube-conditioned medium. Dev Biol. 1984 Nov;106(1):194–210. doi: 10.1016/0012-1606(84)90075-7. [DOI] [PubMed] [Google Scholar]
- Carbonetto S., Gruver M. M., Turner D. C. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J Neurosci. 1983 Nov;3(11):2324–2335. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan W. M., Fawcett J. W., O'Leary D. D., Stanfield B. B. Regressive events in neurogenesis. Science. 1984 Sep 21;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Eccleston P. A., Silberberg D. H. Fibroblast growth factor is a mitogen for oligodendrocytes in vitro. Brain Res. 1985 Aug;353(2):315–318. doi: 10.1016/0165-3806(85)90221-4. [DOI] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Fibroblast and epidermal growth factors: their uses in vivo and in vitro in studies on cell functions and cell transplantation. Mol Cell Biochem. 1979 May 21;25(2):79–110. doi: 10.1007/BF00228991. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Gurney M. E. Suppression of sprouting at the neuromuscular junction by immune sera. Nature. 1984 Feb 9;307(5951):546–548. doi: 10.1038/307546a0. [DOI] [PubMed] [Google Scholar]
- Haan E. A., Boss B. D., Cowan W. M. Production and characterization of monoclonal antibodies against the "brain-specific" proteins 14-3-2 and S-100. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7585–7589. doi: 10.1073/pnas.79.23.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Reichardt L. F. Laminin is associated with the "neurite outgrowth-promoting factors" found in conditioned media. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2183–2187. doi: 10.1073/pnas.82.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Skaper S. D., Barbin G., Varon S. Cholinergic neuronotrophic factors. Concurrent activities on certain nerve growth factor-responsive neurons. J Neurochem. 1982 Feb;38(2):415–421. doi: 10.1111/j.1471-4159.1982.tb08645.x. [DOI] [PubMed] [Google Scholar]
- Matthew W. D., Patterson P. H. The production of a monoclonal antibody that blocks the action of a neurite outgrowth-promoting factor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):625–631. doi: 10.1101/sqb.1983.048.01.066. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Seifert W. A neurotrophic factor (NTF) released from primary glial cultures supports survival and fiber outgrowth of cultured hippocampal neurons. J Neurosci Res. 1982;8(2-3):195–204. doi: 10.1002/jnr.490080209. [DOI] [PubMed] [Google Scholar]
- Pettmann B., Weibel M., Sensenbrenner M., Labourdette G. Purification of two astroglial growth factors from bovine brain. FEBS Lett. 1985 Sep 9;189(1):102–108. doi: 10.1016/0014-5793(85)80851-6. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Bartlett P. F., Gavrilovic J., Lisak R. P., Rattray S. Mitogens for glial cells: a comparison of the response of cultured astrocytes, oligodendrocytes and Schwann cells. Brain Res. 1981 Aug;254(1):19–35. doi: 10.1016/0165-3806(81)90056-0. [DOI] [PubMed] [Google Scholar]
- Saneto R. P., de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free, chemically defined medium. Proc Natl Acad Sci U S A. 1985 May;82(10):3509–3513. doi: 10.1073/pnas.82.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak I., Skaper S. D., Varon S. Pyruvate participation in the low molecular weight trophic activity for central nervous system neurons in glia-conditioned media. J Neurosci. 1985 Jan;5(1):23–28. doi: 10.1523/JNEUROSCI.05-01-00023.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Edgar D. Neurotrophic factors. Science. 1985 Jul 19;229(4710):238–242. doi: 10.1126/science.2409599. [DOI] [PubMed] [Google Scholar]
- Togari A., Dickens G., Kuzuya H., Guroff G. The effect of fibroblast growth factor on PC12 cells. J Neurosci. 1985 Feb;5(2):307–316. doi: 10.1523/JNEUROSCI.05-02-00307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]