SYNOPSIS
Objectives.
We assessed chlamydia trends, individual-level risk factors, and population-level area-based socioeconomic measures (ABSMs) associated with chlamydia infection in women attending U.S. Public Health Service (PHS) Region X Infertility Prevention Project (IPP) family planning (FP) clinics from 1997–2006. We then explored these measures within racial/ethnic subpopulations.
Methods.
Using data from 667,223 chlamydia tests obtained from women aged 15–24 years screened in 201 FP clinics, we employed a generalized mixed model with logistic link, incorporating clinic and ZIP code as random effects to adjust for risk of chlamydia associated with individual- and population-level (areal) measures for the overall population and for each racial/ethnic subpopulation.
Results.
Significant racial/ethnic differences in chlamydia persisted after adjusting for individual and aggregate factors. Relatively steep chlamydia gradients were found across racial/ethnic ABSM levels. Compared with white women, infection risk was significantly higher for black (adjusted odds ratio [AOR] = 1.93), American Indian/Alaska Native (AOR=1.62), Asian/Pacific Islander (AOR=1.42), and Hispanic (AOR=1.28) women. The impact of population-level ABSMs on chlamydia varied across racial/ethnic groups and was generally modest. Among white women, there was a significant 4% relative annual increase in predicted chlamydia during the 10-year period 1997–2006. Chlamydia positivity over time did not change for racial/ethnic minority groups after adjusting for individual- and population-level factors.
Conclusions.
Racial/ethnic differences in chlamydia persisted over time and were not mitigated by adjustment for aggregate socioeconomic position or areal racial/ethnic measures. Changes in project strategies will be needed to address racial/ethnic disparities for chlamydial infection among young female FP clinic clients.
Surveillance and prevalence monitoring systems play an essential public health function in describing and evaluating the burden of chlamydial infection in the United States.1 Ideally, these data can help characterize geographic, gender, age, socioeconomic, and racial/ethnic inequities associated with this highly prevalent sexually transmitted infection (STI).2,3 Differences in STI estimates by racial/ethnic groupings are consistently evident in these case surveillance and regional, state, and local prevalence monitoring systems.4–8
Since 1988, the Region X Infertility Prevention Project (IPP), a prevalence monitoring system, has implemented screening and treatment for Chlamydia trachomatis within U.S. Public Health Service (PHS) Region X states (Alaska, Idaho, Oregon, and Washington).9 Consistent with U.S. Centers for Disease Control and Prevention (CDC) guidelines, the Region X IPP recommends chlamydia testing for all female clients aged <25 years seen at family planning (FP) clinics.10–12 From 1988 through the mid-1990s, chlamydia positivity in the IPP among female FP clients aged 15–24 years declined by more than 60%.13 However, the differential in disease estimates by racial/ethnic categories actually increased. In 1988, chlamydia in black FP clients was 62% higher than in white clients (16.4% vs. 10.1%), and this differential increased to 100% by 1996 (7.0% among black clients vs. 3.5% among white clients). From 1997–2004, chlamydia positivity among Region X IPP FP clinic clients increased, and differences between white and nonwhite clients remained.14
Recent frameworks to explain racial/ethnic disparities in STIs have focused on multilevel models with individual- and population-level factors.15–17 These models have varied in their nomenclature and structure, but generally include social determinants (e.g., measures of community poverty, crime, residential segregation, health-care systems, and health-care access), neighborhood indicators (e.g., racial composition and social capital), sexual partnership and network characteristics (e.g., concurrency and mixing patterns), and individual risk behaviors (e.g., condom use). Socioeconomic conditions have been linked to racial/ethnic STI differences.18–20 Research has also examined individual- and household-level socioeconomic position (SEP), population density, and racial/ethnic aggregate variables when assessing health disparities.21–24 Finally, ecological studies have linked social determinants and STI rates.25,26
Prevalence monitoring programs, including IPP, do not capture data on social determinants, sexual networks, or individuals' SEP.27 Efforts to measure population-level indicators and integrate them with individual-level STI test records are evolving, but pose complex issues.16 Measuring individual- and household-level SEP has been challenging, particularly when ascribing class status to adolescents or to women not employed outside the home.28 One approach to operationalizing population-level social determinants uses aggregate information about clients' residential areas.29,30 These area-based socioeconomic measures (ABSMs) have been calculated for various geographic units, including U.S. Census block groups, tracts, and ZIP codes. Census tracts may be the optimal aggregate level, but evidence suggests that estimates of STI case rates across selected ABSM gradients are relatively comparable for census and ZIP-code aggregation levels.31,32 Population-level ABSMs measured at the ZIP-code level can be linked to Region X IPP test records via the latter's client residential ZIP code.
Our goal was to assess whether race/ethnicity was a marker for other factors (e.g., SEP) related to STI acquisition among female clients attending Region X FP clinics. Study objectives were to (1) assess associations and temporal trends among individual-level risk factors, population-level social determinants, and chlamydia positivity in women aged 15–24 years tested at Region X IPP FP clinics from 1997–2006; and (2) explore these measures within racial/ethnic subgroups. We focused on 1997–2006 because the Region X IPP's second decade saw a marked shift in chlamydia trends relative to the first 10 years. This period also allowed use of newly developed U.S. Census 2000 methods for estimating social determinants with postal ZIP codes.
METHODS
Data sources
IPP individual-level chlamydia test records.
We identified 201 IPP FP clinics providing chlamydia testing during 1997–2006. The total population contained 737,691 test records for women aged 15–24 years, of which 667,223 records (90%) included valid Region X ZIP codes. All sites used a common clinic/laboratory form. Clinic staff collected patient data via interview during service provision. Measures included age; race; ethnicity; residential ZIP code; specimen collection date; having had a sex partner with chlamydia as FP clinic visit reason; self-reported behavioral risks in the last 60 days (i.e., new sex partner, multiple partners, or symptomatic partner); condom use at last sex; having had chlamydia in the past year; clinical findings consistent with an STI (e.g., cervicitis, ectopy, or pelvic inflammatory disease); and diagnostic test type and result. We developed a joint race/ethnicity measure.33 Records identified as Hispanic ethnicity—regardless of race—were assigned to race/ethnicity's Hispanic category.
Population-level measures.
Aggregate population-level measures relied on U.S. Census 2000 tables geocoded to ZIP-code tabulation areas (ZCTAs) and rural-urban commuting area (RUCA) codes (from 2006). ZCTAs are polygons generated by the Census reflecting U.S. Postal Service ZIP codes. We downloaded state-specific ZCTA records from U.S. Census 2000 Summary File (SF) 1 and 3 tables (n=1,485).34 Racial and Hispanic ethnicity counts came from SF1 tables P1, P7, and P11. Aggregate demographics were calculated as the percentage of the population identifying as part of a racial minority group and as Hispanic ethnicity. Given limited racial minority populations, we could not generate race-specific ABSMs. SF3 ABSMs included median household income (P53), percentage of the population below 100% of the federal poverty level (FPL) (P87), and percentage of the population aged ≥25 years without a high school diploma (P37). Aggregate interval-level social determinants were categorized consistent with prior research and item distributions.29 Median household incomes were recoded into quintiles within each state. We categorized urban-rural status via RUCA values for ZIP codes (n=1,832).35 RUCA codes are based on census tract population densities as well as primary and secondary work-commuting flow characteristics. Census tract RUCA values also were approximated at the ZIP-code level. Census and RUCA measures were merged with test records via client ZIP code.
Laboratory methods
Four state public health laboratories, a county health district laboratory, and the University of Washington Chlamydia Laboratory performed Region X IPP testing. Nucleic acid amplification technology (NAAT) tests included ligase chain reaction (LCx, Abbott Laboratories, Abbott Park, Illinois); target capture-transcription mediated amplification (TC-TMA) assays (Aptima Combo 2®, Gen-Probe, Inc., San Diego, California); and the latter's first-generation TMA. Non-NAATs included enzyme immunoassays (MicroTrak II®, Siemens Healthcare Diagnostics [formerly Dade Behring/Syva], Deerfield, Illinois); nucleic acid hybridization tests (Pace® 2, Gen-Probe, Inc.); nucleic acid hybridization assays (Digene Hybrid Capture® 2, Qiagen, Germantown, Maryland); and cell culture.
Statistical analyses
We calculated observed chlamydia positivity by dividing the number of positive tests by total positives and negatives, multiplied by 100. Positivity was stratified by demographic, behavioral, and aggregate characteristics (Table 1). We summarized relationships between chlamydia and individual- and population-level factors with odds ratios (ORs), 95% confidence intervals (CIs), and associated p-values, each generated by a generalized mixed model with logistic link, incorporating clinic as a random effect (to account for records clustering by clinic) and, for ABSM covariates, ZIP code as a random effect (to account for potential clustering and correlation of responses at the ABSM level).36 Adjusted ORs (AORs) and 95% CIs were also estimated with a generalized mixed model, first including individual-level factors and then adding population-level measures.
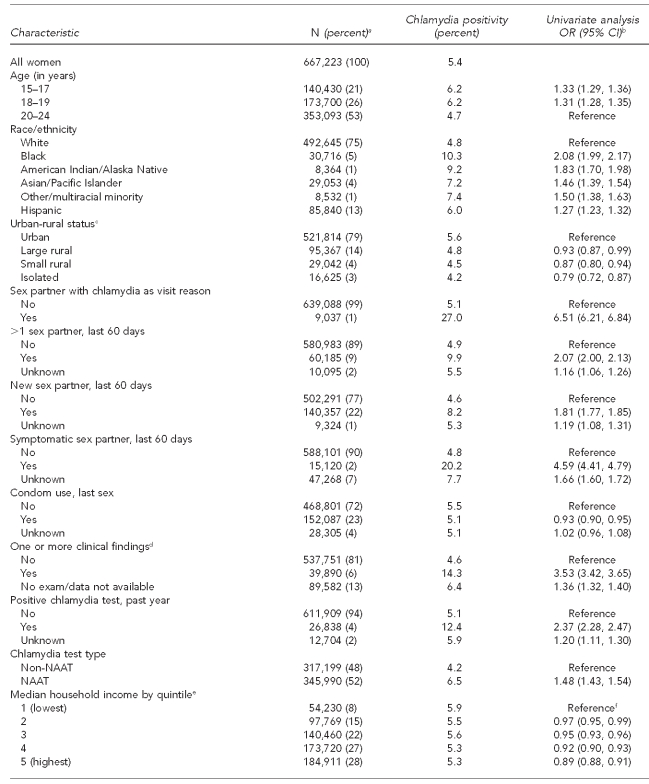
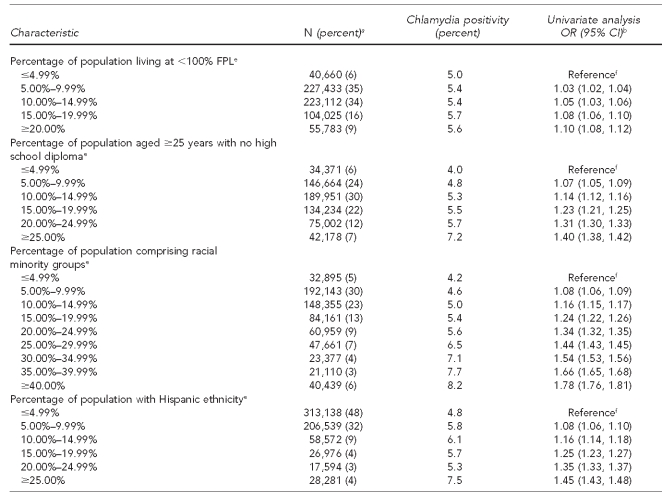
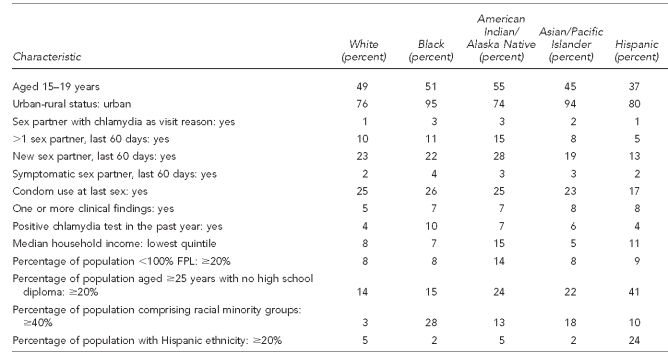
Table 1.
Characteristics of women aged 15–24 years and chlamydia positivity in U.S. Public Health Service Region X family planning clinics, 1997–2006
aTotal N per variable may not sum to 667,223 due to missing information on some test records. Percentages are based on number of responses in each category; percentages may not add to 100 due to rounding.
bCrude ORs and 95% CIs were produced via generalized mixed model with logistic link, incorporating clinic as a random effect (to account for records clustering by clinic) and, for ABSM covariates, ZIP code as a random effect (to account for potential clustering and correlation at the ABSM level).
cABSM based on rural-urban commuting area codes associated with client residential ZIP code. Urban is defined generally as U.S. Census Urbanized Areas (UA), large Urban Clusters (UC), or ZIP codes with high proportions of commuting into UAs or UCs. Large rural is defined as areas with a population of 10,000–49,999 and relatively lower commuting or secondary flow levels to UA and UC. Small rural is defined as areas with a population of 2,500–9,999 and secondary flow commuting statistics. Isolated areas are ZIP codes with primary flow to areas outside of UAs or UCs, as well as having little functional relationship to cities and towns.
dIncludes cervicitis, friable cervix, ectopy, and pelvic inflammatory disease
eABSM based on U.S. Census 2000 ZIP-code tabulation area associated with client residential ZIP code. Note: household median income quintiles calculated from state-specific ZIP-code tabulation area statistics due to variation in quintiles for Alaska, Idaho, Oregon, and Washington.
fBecause ABSMs generally appeared to have a linear relationship between increasing categories and chlamydia, we incorporated them assuming this linear model. This produces a single estimate of the increase in the odds per each incremental category (quintile or 5% increment depending on ABSM). The category-specific estimated ORs were then calculated using this single estimate.
OR = odds ratio
CI = confidence interval
NAAT = nucleic acid amplification technology
FPL = federal poverty level
ABSM = area-based socioeconomic measure
We evaluated interactions between race/ethnicity and each covariate to assess whether chlamydia associations were comparable across subpopulations. Because most interactions were significant, we generated racial/ethnic-specific models with associated AORs and 95% CIs. For the Figure (Panels 1 and 2), we evaluated the significance of temporal trends for selected characteristics with a generalized linear mixed model with logistic link, clinic as random effect, and years since 1997 as predictor. In Panel 3 of the Figure, we used laboratory test sensitivities and specificities to calculate racial/ethnic annual adjusted chlamydia positivities.13 A two-sided p-value <0.05 was considered statistically significant. We performed analyses using SAS® version 9.2.37
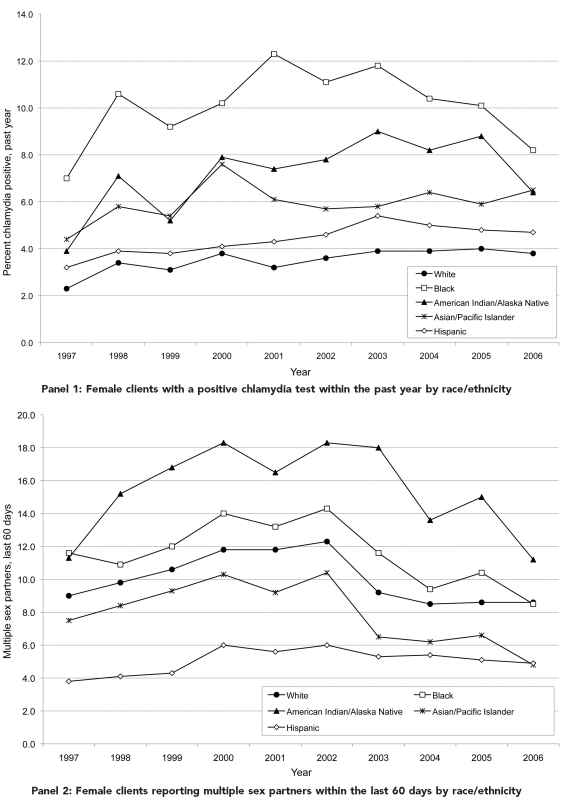
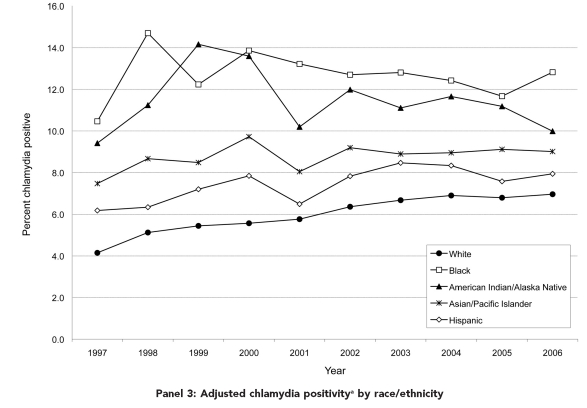
Figure.
Temporal trends for selected population characteristics by race/ethnicity among female clients aged 15–24 years tested for chlamydia in U.S. Public Health Service Region X family planning clinics, 1997–2006
aEach annual data point is calculated as the weighted sum (based on proportion of each test type) of test-specific adjusted positivities. Test-specific adjusted positivity = (test-specific observed positivity + test specificity − 1) divided by (test sensitivity + test specificity − 1).
RESULTS
Univariate analyses—risk factors and chlamydia
Of the 667,223 uro-genital chlamydia tests completed during 1997–2006 at Region X IPP FP clinics, most were performed on women aged 20–24 years (53%) and white women (75%) (Table 1). Nine percent of women tested reported more than one sex partner, 22% reported a new sex partner, and 2% reported a symptomatic partner during the last 60 days. About 23% had used a condom at last sex. Only 1% of tests were performed on women reporting chlamydia exposure as a reason for their clinic visit. Four percent indicated a positive chlamydia test within the past year, while 6% had clinical findings consistent with an STI. Overall chlamydia positivity was 5.4%.
For individual-level factors, chlamydia positivity was higher among tests of adolescents, women reporting sexual risk behaviors, women who had clinical findings consistent with an STI, women indicating a chlamydial infection in the past year, and women with known contacts to someone with chlamydia; positivity was lower among tests of women reporting condom use at last sex. Slightly more than half (55%) of women tested reported no individual-level risks (i.e., history of chlamydia in the past year, recent sexual risk behaviors, chlamydia exposure as visit reason, or clinical findings consistent with an STI), ranging from 44% among black women to 62% among Hispanic women. For these tests among lower-risk women, chlamydia positivity by race/ethnicity varied: 3.1% of white, 4.2% of both Asian/Pacific Islander (A/PI) and Hispanic, 6.0% of American Indian/Alaska Native (AI/AN), and 8.0% of black women had positive tests (data not shown).
Regarding SEP ABSMs, 8% of tests came from areas representing each state's lowest quintile for median household income; 28% of tests came from ZIP codes ranked in the highest quintile. Nine percent were in ZIP codes identified with areas where ≥20% of the population was living below the FPL, and 19% came from areas where ≥20% of the adult population did not have a high school diploma. For racial and ethnic ABSMs, about 30% of tests came from areas where ≥20% of the population belonged to racial minority groups, and 7% came from ZIP codes with ≥20% Hispanic population. Urban areas accounted for 79% of tests (Table 1).
For SEP ABSMs, the gradient of chlamydia positivities ranged from 5.3% in the highest median income quintile to 5.9% in the lowest income quintile. Chlamydia positivity increased modestly as population poverty levels increased, ranging from 5.0% for tests from areas with <5% of the population living below the FPL to 5.6% for areas where ≥20% of the population was below the FPL. Chlamydia positivity also increased as level of education decreased: tests from areas with <5% of adults without high school diplomas had lower positivity (4.0%) than those from areas with ≥25% of adults with limited schooling (7.2%). For racial minority and Hispanic ethnicity ABSMs, the gradient of chlamydia positivities was steeper—ranging from 4.2% for tests in ZIP codes with <5% racial minority population to 8.2% in areas where ≥40% of residents were of nonwhite race. Areal Hispanic population and chlamydia varied from 4.8% positivity for areas with <5% Hispanic population to 7.5% positivity for areas with ≥25% Hispanic population. Finally, chlamydia positivity was highest in cities (5.6%), falling to 4.2% in isolated rural regions (Table 1).
Racial/ethnic differences and trends
Individual-level measures.
Table 2 provides racial/ethnic differences on individual- and population-level areal measures. Tests from Hispanic clients had the lowest proportion of adolescents aged 15–19 years (37%). AI/AN women were most likely to report recent multiple sex partners (15%), followed by black women (11%); tests from these two racial/ethnic minority groups also ranked relatively high on other risk measures—e.g., prior chlamydia infections in the past year, having a symptomatic sex partner in the last 60 days, and chlamydia exposure as a visit reason. Hispanic and white women ranked lowest for these three factors, although the range across all groups was often limited.
Table 2.
Racial/ethnic differences on individual- and population-level (areal) factors associated with chlamydial infection, U.S. Public Health Service Region X family planning clinics, 1997–2006
FPL = federal poverty level
For ABSMs, tests among AI/AN women were more likely than other groups to fall within the lowest quintile for household median income (15%), followed by Hispanic women (11%). Similarly, 14% of AI/AN tests came from areas where ≥20% of the population was below the FPL, while other racial/ethnic groups had fewer tests (8%–9%) from areas with high proportions of the population below the FPL. About 41% of Hispanic tests came from ZIP codes where ≥20% of the adult population did not have a high school degree. Tests among black clients were more likely to come from areas with ≥40% racial minority population (28%), compared with 3%–18% for other groups. Almost a quarter of Hispanic tests came from areas with ≥20% Hispanic population, compared with 2%–5% for other groups. Finally, black and A/PI clients who were tested were more likely to reside in urban areas (95% and 94%, respectively) compared with other groups.
Temporal trends.
The Figure provides temporal trends for selected project characteristics by race/ethnicity. Overall, records indicating a prior positive chlamydia test during the past year trended from 2.7% in 1997 to 4.7% in 2003, and then down slightly to 4.3% in 2006 (data not shown). In 1997, 2.3% of white clients tested and 7.0% of black clients tested indicated a prior positive chlamydia test during the past year. By 2006, 3.8% of white and 8.2% of black clients tested indicated prior infections. All racial/ethnic groups showed statistically significant increases in a prior positive chlamydia test during the past year (2%–5% annually) (Panel 1). For the total sample, tests among clients reporting multiple sex partners within the last 60 days trended from 8.5% in 1997 to 11.6% in 2002 before falling to 7.9% in 2006. In 1997, Hispanic women were least likely (3.8%) and black women most likely (11.6%) to report recent multiple sex partners. Over the 10-year period, this risk behavior fell significantly for all groups (2%–6% annually) except Hispanic women, for whom no change was found (Panel 2). Finally, chlamydia positivity adjusted for test type increased from 4.8% in 1997 to 7.5% in 2006. Racial/ethnic differences in adjusted positivity endured over time (Panel 3).
Multivariate analyses—risk factors and chlamydia
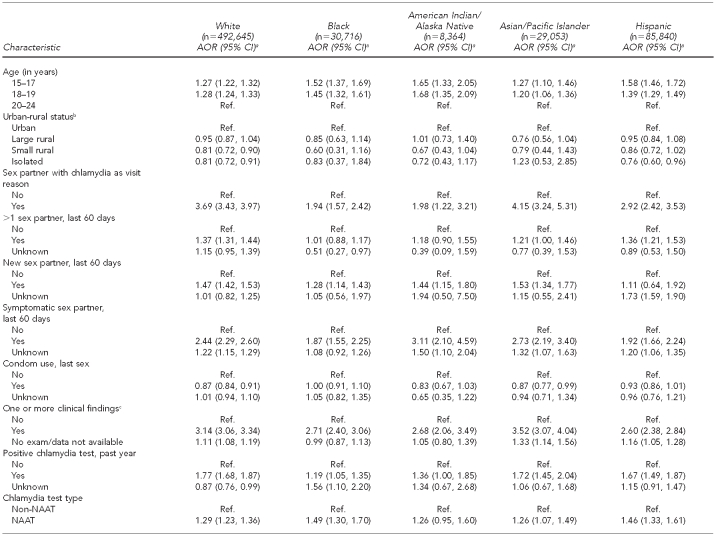
Racial/ethnic crude ORs were modestly reduced when individual-level covariates were added to the full-sample multivariate analysis. Relative to white women, chlamydia risk was still significantly higher for tests among black (AOR=1.93), AI/AN (AOR=1.62), A/PI (AOR=1.42), and Hispanic (AOR=1.28) women (data not shown). When population-level ABSMs were added to the individual-level model, racial/ethnic disparities did not change.
Table 3 summarizes multivariate analyses within racial/ethnic populations (excluding the “other/multiracial” category). The effects of most individual-level measures varied across racial/ethnic populations. The impacts of these risk measures on chlamydia positivity were often weakest for black clients and strongest among white clients—e.g., exposure to chlamydia as a visit reason, having had multiple partners and/or symptomatic partners in the last 60 days, and having had a positive chlamydia test in the past year. The pattern of results was different for those reporting having a new sex partner, with results ranging from an 11% increase in predicted chlamydia among Hispanic women to a 47% increase among white women. All groups showed a much higher risk of testing positive if clinical findings consistent with an STI had been identified. Condom use at last sex did not vary across groups and its impact was modest.
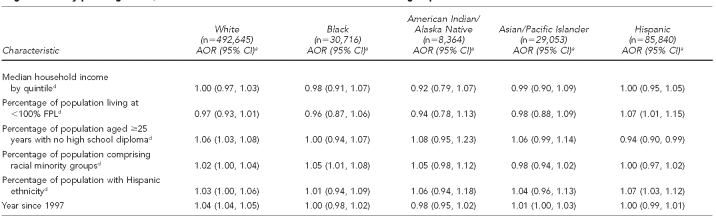
Table 3.
Risk of chlamydial infection in women aged 15–24 years seen in U.S. Public Health Service Region X family planning clinics, 1997–2006: multivariate results within racial/ethnic groups
aAORs and 95% CIs produced via generalized mixed model with logistic link, incorporating clinic as a random effect (to account for records clustering by clinic) and ZIP code as a random effect (to account for potential clustering and correlation of responses at the ABSM level)
bABSM based on rural-urban commuting area codes associated with client residential ZIP code
cIncludes cervicitis, friable cervix, ectopy, and pelvic inflammatory disease
dBased on U.S. Census 2000 ZIP-code tabulation areas associated with client residential ZIP code
AOR = adjusted odds ratio
CI = confidence interval
Ref. = reference category
NAAT = nucleic acid amplification technology
FPL = federal poverty level
ABSM = area-based socioeconomic measure
For SEP ABSMs, the percentage of the population living below the FPL was positively associated with chlamydia for Hispanic clients (AOR=1.07 per 5% increase). For most groups, increasing proportions of the adult population with low educational attainment yielded either stable or increasing chlamydia positivity. For Hispanic tests, however, as the proportion of people without a high school degree increased, chlamydia positivity fell (AOR=0.94 per 5% change). Household median income was unrelated to chlamydia positivity across groups. The racial minority ABSM was associated with chlamydia for black women (AOR=1.05 per 5% increase). The Hispanic ethnicity ABSM was associated with chlamydia for tests among Hispanic clients (AOR=1.07 per 5% increase). These population-level racial and ethnic minority measures were unrelated to chlamydia for all other racial/ethnic groups.
Among white women, we found a significant 4% relative annual increase in predicted chlamydial infection, while chlamydia positivity over time did not change for racial/ethnic minority groups after adjusting for individual- and population-level measures. The decreases in disparities shown in Panel 3 of the Figure are, thus, a result of increasing positivity in white women and stable positivities in other groups, and this finding was not mitigated by adjustment for other factors.
DISCUSSION
From 1997–2006, chlamydia positivity at Region X IPP FP clinics ranged from 4.8% in white women to 10.3% among black women. Population-level areal socioeconomic measures were generally unrelated to chlamydia after accounting for individual characteristics, sexual risk behaviors, and clinical signs of infection. Racial and ethnic population ABSMs were associated with this STI for tests from black and Hispanic clients. Black women living in ZIP codes with ≥40% racial minority population had a 48% increased chlamydia risk compared with black women living in areas with <5% racial minority residents. Hispanic FP clients' risk increased 40% when residing in areas where the Hispanic population was ≥25% relative to areas with <5% Hispanic population. However, white women living in more racially and ethnically diverse ZIP codes did not show significant increases in chlamydia positivity. These results point toward further work needed in IPP on client sexual partner and network characteristics, as well as other social determinants.38–40 A few examples of potential measures include sex partner's race/ethnicity, concurrent partnerships, sexual network mixing patterns, and examination of community-level ABSMs not addressed in our analysis (e.g., percentage of single female heads of household and incarceration rates). The long-term challenges are threefold: (1) identifying a limited set of client sexual network indicators that can readily be collected during clinic visits and included in program databases, (2) facilitating the use of Census ABSMs with local clinic IPP information systems, and (3) developing effective interventions for clinic populations where network and population-level variables affect client STI acquisition.
We also found high chlamydia levels among tests of black and AI/AN women without any individual-level risk factors. This finding also suggests the need to characterize risks beyond individual client characteristics. But we acknowledge that our regional program captures a limited array of client risk indicators. The project could revisit whether other self-reported client behaviors or conditions (and their time frames) would be more relevant. Possibilities include substance use patterns, number of sex partners in the past year, number of steady/casual sex partners, and consistent condom use. It is important, though, to reiterate that FP clients' recent sexual risk behaviors were -independently associated with chlamydia. Client risk-reduction counseling is still a potentially effective intervention with all young female FP clients.
More broadly, our geocoded areal results provide an approach to enhancing FP chlamydia screening programs. In 2009, Region X Title X FP clinics screened only about 47% of female clients aged 15–24 years.41 Funding challenges limit screening rates. Identifying factors within this client population to maximize yield with available resources could improve program efficiency and efficacy. Region X FP clinics could prioritize ZIP codes with higher proportions of racial/ethnic minority populations as a practical way to inform decision-making about testing young FP clients, regardless of their race/ethnicity.
Beyond the Northwest, the other nine regional IPPs in the U.S. currently collect client demographics and use young age as a screening criterion at FP sites. Their screening coverage levels are also modest (approximately 50%). Most of these programs do not capture client risk behaviors or clinical findings. Yet, seven other IPPs do collect client ZIP codes. Population-level ABSMs could be generated from Census data and linked to client IPP test records, and chlamydia gradients could be assessed for clinics' client populations. If those STI test result gradients—on whichever social determinants were operationalized—were relatively steep among adolescent and young adult patients, for example, then clinics' age-based screening criteria could be augmented to prioritize specific residential areas. This type of data analysis could provide an empirical basis to increase case finding when universal screening is not practical.
Finally, we must consider the increase in chlamydia over time among white FP clients. Possible explanations have already been mentioned—i.e., other unmeasured individual risk behaviors, partner characteristics, and sexual network indicators. Two broader factors may also play roles. First, there may have been changes in the community composition or the types of young female residents who sought FP clinic services during these 10 years. Secondly, clinics have multiple funding streams that support reproductive health services such as STI testing. Some FP agencies may shift service mix based on the availability of other resources, such as clients' insurance coverage or eligibility for other federal programs where direct care and STI services could be reimbursed.
Acknowledging that young female FP client chlamydia trends have stabilized or even increased, Region X IPP has modified some program practices. FP clinics have expanded their outreach to men and their male client STI services. They have also enhanced services to women—e.g., re-screening of chlamydia-positive clients three to 12 months following initial infection and use of expedited partner therapy. Finally, in 2009, Region X IPP began collecting one sexual network measure, asking clients if their sex partners had concurrent partners.
Limitations
Our study's limitations include the following: (1) potential misclassification of client race/ethnicity, affecting group designations;42 (2) technical constraints involving ABSMs—i.e., we could not operationalize population-level measures at smaller aggregate units, nor could we generate more detailed race-specific ABSMs or assess temporal changes in areal measures; and (3) exogenous factors (e.g., clinic population changes, testing frequency, and variation in screening coverage) that could have affected STI trends.43 Methodological issues have also been raised about modeling population- and individual-level measures, and interpreting multilevel results.44–51 Finally, our results cannot be applied to FP female clients from other settings or the general population.
CONCLUSIONS
Racial/ethnic disparities in chlamydial infection have been found throughout the Region X IPP FP program for more than 20 years. Differences in positivity between white and nonwhite young female clients have fallen somewhat, but this improvement is a function of increasing chlamydia diagnoses among white clients. Based on incorporating population-level ABSMs using Census data at the ZCTA level with individual-level test records, our results are not consistent with positing a role for client SEP to account for chlamydia positivity within racial/ethnic minority groups or racial/ethnic disparities in general among young female clients screened in Region X IPP FP clinics. Race/ethnicity was not a marker for socioeconomic factors potentially related to this STI. However, racial/ethnic population-level measures were associated with chlamydial infection among black and Hispanic clients, suggesting that other social determinants and sexual network indicators may play a role in racial/ethnic disparities for chlamydia. Regardless, individual-level measures such as prior STI history, sexual risk behaviors, and clinical findings were more useful for explaining chlamydia positivity. Where these individual-level measures are not available, as in most other regional IPPs, then our general approach applied to ABSMs from U.S. Census 2010 tables could be useful in exploring STI gradients for these population-level measures. To the extent that chlamydia gradients exist for one or more population-level indicators, then programs could implement more systematic testing and effective case finding in this priority client population.
Acknowledgments
The authors thank Dr. James Hughes of the University of Washington Department of Biostatistics in Seattle, Washington, for providing statistical consultations and Alexandra Fine for providing database support on this article.
Footnotes
This work was supported by the U.S. Public Health Service Region X Infertility Prevention Project; the U.S. Department of Health and Human Services, Office of Population Affairs (OPA); and the Centers for Disease Control and Prevention (CDC), Division of STD Prevention (DSTDP). The data are part of the CDC/DSTDP National Chlamydia Prevalence Monitoring Surveillance System. This work did not require Institutional Review Board determination.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of OPA or CDC.
REFERENCES
- 1.Eng TR, Butler WT, editors. Institute of Medicine. The hidden epidemic: confronting sexually transmitted diseases. Washington: National Academies Press; 1997. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (US), National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of STD Prevention. STD health disparities. [cited 2009 Jun 13]. Available from: URL: http://www.cdc.gov/STD/health-disparities/default.htm.
- 3.Department of Health and Human Services (US), Office of Disease Prevention and Promotion. Healthy People 2010. [cited 2010 Jun 9]. Available from: URL: http://www.healthypeople.gov/2010/redirect.aspx?url=/2010. [PubMed]
- 4.Centers for Disease Control and Prevention (US), National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of STD Prevention. Atlanta: CDC; 2008. Sexually transmitted disease surveillance, 2007. [Google Scholar]
- 5.Einwater LA, Ritchie JM, Ault KA, Smith EM. Gonorrhea and chlamydia infection among women visiting family planning clinics: racial variation in prevalence and predictors. Perspect Sex Reprod Health. 2005;37:135–40. doi: 10.1363/psrh.37.135.05. [DOI] [PubMed] [Google Scholar]
- 6.Klausner JD, McFarland W, Bolan G, Hernandez MT, Molitor F, Lemp GF, et al. Knock-knock: a population-based survey of risk behavior, health care access, and Chlamydia trachomatis infection among low-income women in the San Francisco Bay area. J Infect Dis. 2001;183:1087–92. doi: 10.1086/319276. [DOI] [PubMed] [Google Scholar]
- 7.Gorgos L, Fine D, Marrazzo J. Chlamydia positivity in American Indian/Alaska Native women screened in family planning clinics, 1997–2004. Sex Transm Dis. 2008;35:753–7. doi: 10.1097/OLQ.0b013e31816d1f7d. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (US), National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of STD Prevention. Atlanta: CDC; 2009. Sexually transmitted disease surveillance 2007 supplement, Chlamydia Prevalence Monitoring Project annual report 2007. [Google Scholar]
- 9.Britton TF, DeLisle S, Fine D. STDs and family planning clinics: a regional program for chlamydia control that works. Am J Gynecol Health. 1992;6:80–7. [PubMed] [Google Scholar]
- 10.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006 [published erratum appears in MMWR Recomm Rep 2006;55(36):997] MMWR Recomm Rep. 2006;55(RR-11):1–94. [PubMed] [Google Scholar]
- 11.U.S. Preventive Services Task Force. Screening for chlamydial infection: recommendations and rationale. Am J Prev Med. 2001;20(3 Suppl):90–4. [PubMed] [Google Scholar]
- 12.Office of Management and Budget (US) The Program Assessment and Rating Tool (PART) for the Office of Family Planning. Washington: OMB; 2005. [Google Scholar]
- 13.Fine D, Dicker L, Mosure D, Berman S Region X Infertility Prevention Project. Increasing chlamydia positivity in women screened in family planning clinics: do we know why? Sex Transm Dis. 2008;35:47–52. doi: 10.1097/OLQ.0b013e31813e0c26. [DOI] [PubMed] [Google Scholar]
- 14.Fine D, Marrazzo J. Beyond race/ethnicity: positivity of Chlamydia trachomatis among women attending Region 10 family planning clinics in the U.S., 1997-2005, by individual risks and area-based socioeconomic measures. Presented at: International Society for Sexually Transmitted Disease Research; 2007 Jul 30-Aug 1; Seattle. [cited 2010 Jun 13]. Also available from: URL: http://www.eventure-online.com/-eventure/publicSearch.do?action=save&congressId=642. [Google Scholar]
- 15.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2010. [cited 2011 Aug 30]. Establishing a holistic framework to reduce inequities in HIV, viral hepatitis, STDs, and tuberculosis in the United States. Also available from: URL: www.cdc.gov/socialdeterminants. [Google Scholar]
- 16.Aral SO, Padian NS, Holmes KK. Advances in multilevel approaches to understanding the epidemiology and prevention of sexually transmitted infections and HIV: an overview. J Infect Dis. 2005;191(Suppl 1):S1–6. doi: 10.1086/425290. [DOI] [PubMed] [Google Scholar]
- 17.Hogben M, Leichliter JS. Social determinants and sexually transmitted disease disparities. Sex Transm Dis. 2008;35(12 Suppl):S13–8. doi: 10.1097/OLQ.0b013e31818d3cad. [DOI] [PubMed] [Google Scholar]
- 18.Ellen JM, Kohn RP, Bolan GA, Shiboski S, Krieger N. Socioeconomic differences in sexually transmitted disease rates among black and white adolescents, San Francisco, 1990 to 1992. Am J Public Health. 1995;85:1546–8. doi: 10.2105/ajph.85.11.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen DA, Farley TA, Mason K. Why is poverty unhealthy? Social and physical mediators. Soc Sci Med. 2003;57:1631–41. doi: 10.1016/s0277-9536(03)00015-7. [DOI] [PubMed] [Google Scholar]
- 20.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 21.Hallfors DD, Iritani BJ, Miller WC, Bauer DJ. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health. 2007;97:125–32. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halpern CT, Hallfors D, Bauer DJ, Iritani B, Waller MW, Cho H. Implications of racial and gender differences in patterns of adolescent risk behavior for HIV and other sexually transmitted diseases. Perspect Sex Reprod Health. 2004;36:239–47. doi: 10.1363/psrh.36.239.04. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. In: Smedley BD, Stith AY, Nelson AR, editors. Washington: National Academies Press; 2003. [PubMed] [Google Scholar]
- 24.Manhart LE, Marrazzo JM, Fine D, Kerani RP, Golden MR. Selective testing criteria for gonorrhea among young women screened for chlamydial infection: contribution of race and geographic prevalence. J Infect Dis. 2007;196:731–7. doi: 10.1086/520517. [DOI] [PubMed] [Google Scholar]
- 25.Pouget ER, Kershaw TS, Niccolai LM, Ickovics JR, Blankenship KM. Associations of sex ratios and male incarceration rates with multiple opposite-sex partners: potential social determinants of HIV/STI transmission. Public Health Rep. 2010;125(Suppl 4):70–80. doi: 10.1177/00333549101250S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen D, Spear S, Scribner R, Kissinger P, Mason K, Wildgen J. “Broken windows” and the risk of gonorrhea. Am J Public Health. 2000;90:230–6. doi: 10.2105/ajph.90.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger N. Putting health inequities on the map: social epidemiology meets medical/health geography—an ecosocial perspective. GeoJournal. 2009;74:87–97. [Google Scholar]
- 28.Braveman P, Cubbin C, Marchi K, Egerter S, Chavez G. Measuring socioeconomic status/position in studies of racial/ethnic disparities: maternal and infant health. Public Health Rep. 2001;116:449–63. doi: 10.1093/phr/116.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krieger N, Chen JT, Waterman PD, Rehkopf GH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the Public Health Disparities Geocoding Project. Am J Public Health. 2003;93:1655–71. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–9. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger N, Chen JT, Waterman PD, Rehkopf D, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312–23. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger N, Waterman PD, Chen JT, Soobader MJ, Subramanian SV. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures—the Public Health Disparities Geocoding Project (US) Public Health Rep. 2003;118:240–60. doi: 10.1093/phr/118.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Office of Management and Budget (US), Tabulation Working Group, Interagency Committee for the Review of Standards for Data on Race and Ethnicity. Washington: OMB; 1999. Feb 17, Draft provisional guidance on the implementation of the 1997 standards for federal data on race and ethnicity. [Google Scholar]
- 34.Census Bureau (US) Census 2000 summary file 1 and summary file 3. [cited 2009 Jun 8]. Available from: URL: http://factfinder.census.gov/servlet/DatasetMainPageServlet.
- 35.WWAMI Rural Health Research Center. Rural-urban commuting area codes (RUCAs) [cited 2009 Jun 14]. Available from: URL: http://depts.washington.edu/uwruca.
- 36.McCullagh P, Nelder JA. Generalized linear models. 2nd ed. New York: Chapman and Hall; 1989. [Google Scholar]
- 37.SAS Institute, Inc. SAS®: Version 9.2. Cary (NC): SAS Institute, Inc.; 2008. [Google Scholar]
- 38.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(Suppl 1):S115–22. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 39.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26:250–61. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Aral SO, Adimora AA, Fenton KA. Understanding and responding to disparities in HIV and other sexually transmitted infections in African Americans. Lancet. 2008;372:337–40. doi: 10.1016/S0140-6736(08)61118-6. [DOI] [PubMed] [Google Scholar]
- 41.Department of Health and Human Services (US), Office of Population Affairs. Tools and documents. [cited 2010 Oct 11]. Available from: URL: http://www.hhs.gov/opa/familyplanning/toolsdocs/index.html.
- 42.Winker MA. Measuring race and ethnicity: why and how? JAMA. 2004;292:1612–4. doi: 10.1001/jama.292.13.1612. [DOI] [PubMed] [Google Scholar]
- 43.Miller WC. Epidemiology of chlamydial infection: are we losing ground? Sex Transm Infect. 2008;84:82–6. doi: 10.1136/sti.2007.028662. [DOI] [PubMed] [Google Scholar]
- 44.Geronimus AT. Invited commentary: using area-based socioeconomic measures—think conceptually, act cautiously. Am J Epidemiol. 2006;164:835–40. doi: 10.1093/aje/kwj314. [DOI] [PubMed] [Google Scholar]
- 45.Geronimus AT, Bound J, Neidert LJ. On the validity of using census geocode characteristics to proxy individual socioeconomic characteristics. J Am Stat Assoc. 1996;91:529–37. [Google Scholar]
- 46.Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol. 1998;148:475–86. doi: 10.1093/oxfordjournals.aje.a009673. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: a multilevel analysis of Massachusetts births, 1989–1991. Am J Epidemiol. 2006;164:823–34. doi: 10.1093/aje/kwj313. [DOI] [PubMed] [Google Scholar]
- 48.Subramanian SV. The relevance of multilevel statistical methods for identifying causal neighborhood effects. Soc Sci Med. 2004;58:1961–7. doi: 10.1016/S0277-9536(03)00415-5. [DOI] [PubMed] [Google Scholar]
- 49.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Subramanian et al respond to “Think conceptually, act cautiously”. Am J Epidemiol. 2006;164:841–4. doi: 10.1093/aje/kwj313. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8:621–8. [PubMed] [Google Scholar]
- 51.Soobader M, LeClere FB, Hadden W, Maury B. Using aggregate geographic data to proxy individual socioeconomic status: does size matter? Am J Public Health. 2001;91:632–6. doi: 10.2105/ajph.91.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]