SYNOPSIS
Objectives
Birth defects are the leading cause of infant mortality and are responsible for substantial child and adult morbidity. Documenting the variation in prevalence of birth defects among racial/ethnic subpopulations is critical for assessing possible variations in diagnosis, case ascertainment, or risk factors among such groups.
Methods
We used data from the Metropolitan Atlanta Congenital Defects Program, a population-based birth defects registry with active case ascertainment. We estimated the racial/ethnic variation in prevalence of 46 selected major birth defects among live births, stillbirths, and pregnancy terminations at >20 weeks gestation among mothers residing in the five central counties of metropolitan Atlanta between 1994 and 2005, adjusting for infant sex, maternal age, gravidity, and socioeconomic status (SES). We also explored SES as a potential effect measure modifier.
Results
Compared with births to non-Hispanic white women, births to non-Hispanic black women had a significantly higher prevalence of five birth defects and a significantly lower prevalence of 10 birth defects, while births to Hispanic women had a significantly higher prevalence of four birth defects and a significantly lower prevalence of six birth defects. The racial/ethnic disparities in the prevalence of some defects varied by SES, but no clear pattern emerged.
Conclusions
Racial/ethnic disparities were suggested in 57% of included birth defects. Disparities in the prevalence of birth defects may result from different underlying genetic susceptibilities; exposure to risk factors; or variability in case diagnosis, ascertainment, or reporting among the subpopulations examined. Policies that improve early diagnosis of birth defects could reduce associated morbidity and mortality.
Birth defects occur in approximately 3% of all live births and are a major contributing factor to infant mortality and childhood and adult disability.1,2 Evaluation of trends in the prevalence of birth defects and their distribution among subpopulations can help public health professionals and care providers better evaluate potential clusters, conduct etiologic and outcome research, determine health services needs, and target health care. Birth defects surveillance programs throughout the United States report state-specific estimates of prevalence, but estimates by maternal race/ethnicity are often unadjusted for important factors that may contribute to observed racial/ethnic variation.3,4 True racial/ethnic variation in the prevalence of birth defects may result from differential access to early and high-quality prenatal care, which may lead to differential patterns of prenatal diagnosis and pregnancy termination. Alternatively, some variation in prevalence by maternal race/ethnicity may represent different genetic or environmental risk factors. In some surveillance systems, variation by race/ethnicity may also reflect differential ascertainment and diagnosis of cases postnatally.
The Metropolitan Atlanta Congenital Defects Program (MACDP), a population-based, active birth defects surveillance system operating in the five central counties of metropolitan Atlanta, published 40 years of prevalence data for 67 major structural birth defects and chromosomal abnormalities, stratified by select infant and maternal characteristics, including race/ethnicity.3 We provide a more in-depth analysis of racial/ethnic variations in the prevalence of major birth defects using data from MACDP.
METHODS
MACDP is the oldest population-based birth defects surveillance program that uses active case ascertainment. Details of MACDP ascertainment methods have been published previously.3 Briefly, trained medical abstractors visit multiple sources—including hospitals with maternity services, pediatric tertiary care facilities, and perinatal offices—to actively ascertain cases of birth defects among live-born infants, stillborn infants, and elective pregnancy terminations at ≥20 weeks gestation. Birth defects are coded according to a modified British Paediatric Association six-digit coding scheme developed for MACDP that is similar to, but more specific than, the five-digit International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding system.5–7 Abstracted clinical information is reviewed for completeness and determination of diagnosis by the medical staff of MACDP, including pediatricians, clinical geneticists, and pediatric cardiologists.
Because we were interested in examining the variation in prevalence among non-Hispanic white (NHW), non-Hispanic black (NHB), and Hispanic infants, but standard information on Hispanic ethnicity first became available on vital records in Atlanta in 1994, we focused on the birth cohort of 1994–2005 among residents of the five central counties of Atlanta that were monitored by MACDP. For this evaluation, we included 46 types of birth defects (ICD-9-CM codes 740.000–759.999) for which overall prevalence data were available in MACDP. Descriptions of the defects and defect groups used in this article have been detailed previously.3 Birth defect cases identified by MACDP were included in the numerators of prevalence estimates. Using data from vital records provided by the Georgia Division of Public Health for the denominators, we calculated crude prevalence estimates for each birth defect per 10,000 live births, overall and by maternal race/ethnicity (NHW, NHB, and Hispanic). Cases whose maternal race/ethnicity was other non-Hispanic were excluded because the numbers were too small for detailed analyses. Those with no race/ethnicity recorded (<1%) were also excluded.
Using prevalence of birth defects among births to NHW women as a reference, we calculated adjusted prevalence ratios (PRs) and 95% confidence intervals (CIs) for birth defects among births to NHB and Hispanic women using Poisson regression models adjusted for maternal age (<20, 20–34, and ≥35 years), infant sex (male or female), gravidity (1 or >1), and socioeconomic status (SES) treated as a class variable. Adjusted PRs for chromosomal defects were stratified by maternal age and adjusted for all other covariates. We tested interaction on a multiplicative scale. Individual-level classification of SES was based on the percentage of people in a mother's census tract (CT) living below the federal poverty level (FPL).8 Four levels of SES were assigned based on CT poverty level: (1) ≥20.0% of the population below FPL; (2) 10.0%–19.9% below FPL; (3) 5.0%–9.9% below FPL; and (4) 0.0%–4.9% below FPL. A second set of adjusted Poisson regression models calculated PRs of birth defects of Hispanic and NHB people. To evaluate whether racial/ethnic disparities varied across CT poverty levels, a race-by-CT-poverty-level interaction term was introduced into the adjusted model. For models in which the interaction term was statistically significant (α=0.05), CT-poverty-level-specific adjusted PRs were calculated using the lowest CT-poverty-level quartile as the reference group. We conducted a subanalysis to determine whether maternal nativity affected estimates of birth prevalence. Mothers were coded as either U.S.-born (i.e., born in the 50 U.S. states, Washington, D.C., or U.S. territories) or foreign-born as recorded on the infant birth certificate.
RESULTS
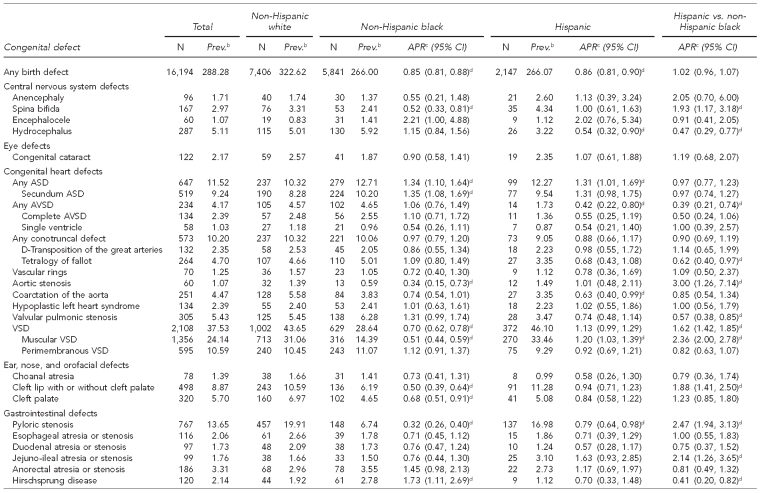
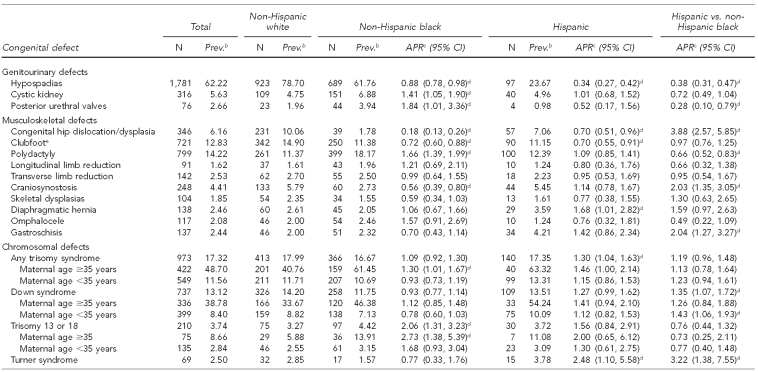
Of the 16,194 birth defects among 561,745 live births in metropolitan Atlanta from 1994 through 2005, NHW people had the highest prevalence of any birth defects (323 per 10,000 live births) followed by NHB people (266 per 10,000 live births) and Hispanic people (266 per 10,000 live births) (Table 1). After adjustment for maternal age, child sex, gravidity, and SES, births to both NHB and Hispanic women had a lower overall prevalence than births to NHW women (NHB women: adjusted PR=0.85, 95% CI 0.81, 0.88; Hispanic women: adjusted PR=0.86, 95% CI 0.81, 0.90). Of the 44 defect groups analyzed, the adjusted PR was <1 for 25 defects (57%) among both NHB and Hispanic infants. The overall prevalence for birth defects among Hispanic and NHB infants was similar (adjusted PR=1.02, 95% CI 0.96, 1.07), and 43% (n=18) of individual defect groups had a higher prevalence among Hispanic infants than among NHB infants.
Table 1.
Adjusteda prevalence ratios and 95% confidence intervals for selected congenital defects among non-Hispanic white, non-Hispanic black, and Hispanic infants: metropolitan Atlanta, 1994–2005
aAdjusted for maternal age, gravidity, child sex, and percent of population below the federal poverty level
bPer 10,000 live births
cReference group for APR is non-Hispanic white
dStatistically significant at α=0.05
eClubfoot not coexisting with a neural tube defect
Prev. = prevalence
APR = adjusted prevalence ratio
CI = confidence interval
ASD = atrial septal defect
AVSD = atrioventricular septal defect
VSD = ventricular septal defect
Five defects had a statistically significantly higher prevalence among NHB vs. NHW infants: Hirschsprung disease, polydactyly, trisomy 13 or 18, cystic kidney, and secundum atrial septal defect (ASD). Compared with NHW infants, NHB infants had a lower prevalence of 10 defects: congenital dislocation or dysplasia of the hip, pyloric stenosis, aortic stenosis, craniosynostosis, muscular ventricular septal defect (VSD), spina bifida, cleft lip with or without cleft palate, cleft palate, clubfoot without spina bifida, and hypospadias.
Four defects had a statistically significantly higher prevalence among Hispanic vs. NHW infants: ASD, muscular VSD, diaphragmatic hernia, and any trisomy syndrome. Compared with NHW infants, Hispanic infants had a lower prevalence of hypospadias, pyloric stenosis, coarctation of the aorta, avioventricular septal defect (AVSD), clubfoot, and congenital dislocation or dysplasia of the hip.
The comparison of Hispanic children with NHB children had the greatest number of defects (n=20) for which disparities existed. Of the 12 defects that had a statistically significant higher prevalence among Hispanic infants, six had an adjusted PR equal to or exceeding twice that for NHB infants.
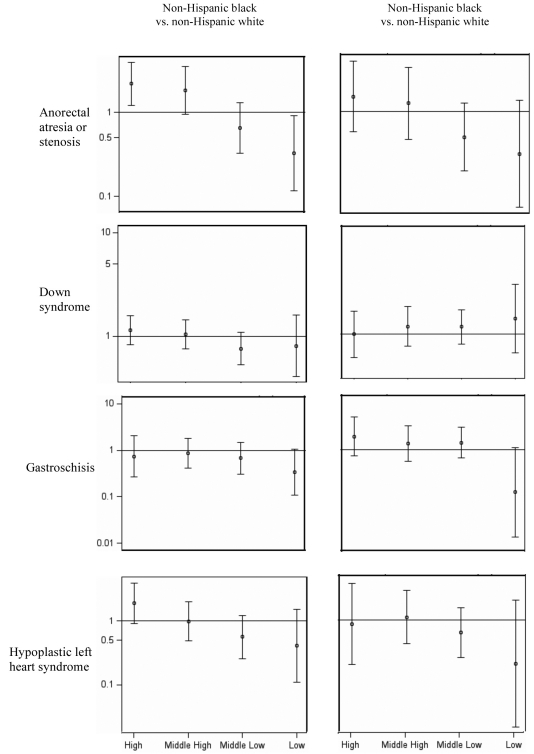
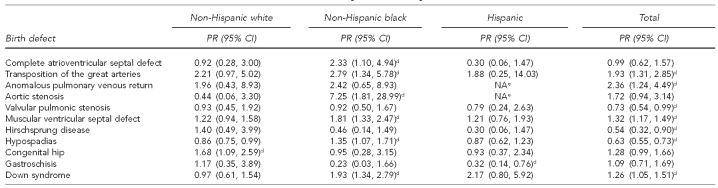
There was evidence of an interaction between race/ethnicity and CT poverty level for any defect and for a number of individual defects (43%); however, very small population sizes in many of the stratified analyses prohibited interpretable findings. For the few defects with sufficient data, there were no consistent patterns for the role of SES on racial/ethnic disparities in the prevalence of birth defects (Figure). The limited population size similarly limited the interpretability of the subanalysis examining maternal nativity. There was limited evidence of variations in prevalence by maternal nativity (Table 2). The strongest evidence was with gastroschisis, for which there was a lower prevalence among foreign-born Hispanic vs. U.S.-born Hispanic children (PR=0.32, 95% CI 0.14, 0.76). Foreign-born NHW children were more likely than their U.S.-born counterparts to have congenital dislocation of the hip (PR=1.68, 95% CI 1.09, 2.59). The prevalence of Down syndrome was twice as high among foreign-born compared with U.S.-born mothers for NHB (PR=1.93, 95% CI 1.34, 2.79) and Hispanic (PR=2.17, 95% CI 0.80, 5.92) people. Foreign-born NHB children were also more likely than their U.S.-born counterparts to have an increased prevalence of hypospadias (PR=1.35, 95% CI 1.07, 1.71) and several congenital heart defects including complete AVSD (PR=2.33, 95% CI 1.10, 4.94), transposition of the great arteries (PR=2.79, 95% CI 1.34, 5.78), aortic stenosis (PR=7.25, 95% CI 1.81, 28.99), and muscular VSD (PR=1.81, 95% CI 1.33, 2.47).
Figure.
Adjusted prevalence ratiosa and 95% confidence intervals for selected birth defects by race/ethnicity and level of SES:b metropolitan Atlanta, 1994–2005
aPrevalence ratios plotted on logarithmic scale and adjusted for maternal age, infant sex, and gravidity
bFor SES levels, high = <5% of the population below the federal poverty level (FPL); middle high = 5%–9% below FPL; middle low = 10%–19% below FPL; and low = ≥20% below FPL.
SES = socioeconomic status
Table 2.
Prevalence ratiosa of non-native vs. U.S. native-bornb mothers by race/ethnicity for select birth defects,c 1997–2005
aMaternal nativity status was only available for live births.
bU.S. native includes mothers born in the 50 U.S. states, Washington, D.C., and U.S. territories.
cDefects for which at least one comparison reached statistical significance (α=0.05) were included.
dStatistically significant at α=0.05
eCase count was too small for estimation.
PR = prevalence ratio
CI = confidence interval
NA = not available
DISCUSSION
This article provides detailed estimates of birth prevalence for selected birth defects among NHW, NHB, and Hispanic infants using data from a population-based birth defects surveillance program with active case ascertainment. Prior studies have examined racial disparities in adjusted prevalence using population-based surveillance data; our results extend and corroborate several previously reported findings.9–11
Overall, disparities in the prevalence of specific birth defects were observed within nearly all organ systems. NHW infants had an overall higher prevalence of all defects as well as having a higher excess of cases for a majority of individual defects. Compared with NHW infants, NHB infants had a lower prevalence for several of the most common birth defects, such as muscular VSD, hypospadias, pyloric stenosis, clubfoot, and cleft lip with and without cleft palate.
Although recently published unadjusted national prevalence estimates for spina bifida show a significant disparity for Hispanic infants,10 MACDP data do not show a statistically significant increase in the prevalence of spina bifida among Hispanic women. This difference could be in part explained by MACDP's greater ability to capture fetal deaths compared with other -surveillance programs included in the national estimates.4 This lack of disparity corroborates recent data from California for U.S.-born Hispanic women; however, the California study did note a disparity between foreign-born Hispanic and NHW women.11 There were no cases of spina bifida among the 5,472 births to native Hispanic women compared with 26 cases among the 66,993 births to non-native Hispanic women in this study population, suggesting that nativity could be an important factor for identifying specific populations at greater risk for neural tube defects.
Unlike previous reports,11,12 NHB infants in this study had a lower prevalence of all congenital heart defects (CHDs) (73 per 10,000 live births) when compared with NHW and Hispanic infants (90 and 86 per 10,000 live births, respectively) (data not shown). This difference was largely driven by a lower prevalence of muscular VSD among NHB infants, which was 49% lower than among NHW infants and 41% lower than among Hispanic infants. In contrast, the prevalence of muscular VSD was slightly higher among Hispanic vs. NHW infants (adjusted PR=1.20, 95% CI 1.03, 1.39). The prevalence of perimembranous VSD did not differ significantly among the three racial/ethnic groups. Several previous studies found no variation by race for all VSDs in the aggregate,9,11,12 but this is the first study to report PRs for specific VSD subtypes. Muscular VSDs are generally milder defects that might be less likely to cause symptoms or come to medical attention, so the lower prevalence among NHB infants could reflect variations in access to diagnostic care or risk.13 When compared with NHW infants, NHB and Hispanic infants had a 34% and 31% higher prevalence, respectively, of ASD, which was primarily driven by a higher prevalence of secundum ASD.
Several observed disparities in this study have been documented previously. This study corroborates reports of a lower prevalence among NHB infants of craniosynostosis,14,15 hip dysplasia and dislocation,11 cleft palate,16 and cleft lip with or without cleft palate,10,11,16 as well as reports of lower prevalence among Hispanic infants for hypospadias.11,17
This study reports other disparities for the first time. For example, we found a lower adjusted prevalence of clubfoot among both NHB and Hispanic infants compared with NHW infants. Previous studies have found a higher prevalence among Asian and Pacific Islanders,18–20 but Moorthi et al. found no difference for isolated clubfoot among NHW, NHB, and Hispanic infants.21 We could only find one report that partially corroborated this finding, but the ICD-9-CM code used in that study was less specific and did not exclude clubfoot associated with a neural tube defect.11 It is possible that these mixed findings among studies reflected differences in populations, case definition, and methods of case ascertainment and classification.
Low SES has been shown to be associated with an increased prevalence of some birth defects; however, the findings in the literature have been inconsistent and at times contradictory, in part because of the use of varying measures of SES.22–25 Furthermore, the extent to which socioeconomic factors may explain or modify racial/ethnic disparities has not been well examined. Correa-Villaseñor et al. found that socioeconomic factors modified an observed white-black variation in risk for aortic stenosis,12 with the excess risk among white infants present only among infants in lower socioeconomic strata. It is important to determine whether lower rates of detection or incomplete ascertainment of birth defects among less affluent racial/ethnic minority groups is a possible or plausible explanation for the lower prevalence among non-Hispanic black and Hispanic infants. These data provided no clear evidence that racial/ethnic disparities in prevalence varied across CT poverty levels, but this study was insufficiently powered to detect interactions for many defects. This limitation underscores the need for larger studies that pool population-based surveillance data from multiple states.10
Limited evidence in the literature suggests that maternal nativity is an important factor contributing to variation among different racial/ethnic groups; however, variation and contradicting evidence exists as a result of different methodology used to group foreign-born mothers.26 A relatively modest population size limited this study's ability to fully examine the role of nativity on observed racial/ethnic disparities. Our finding of a nearly 70% lower prevalence of gastroschisis among U.S.-born Hispanic infants was similar to what has been reported elsewhere.27,28 No other studies could be found that evaluated disparities by nativity status separately for NHW and NHB race/ethnicity. As such, these findings, although limited by a small population size, illustrate the potential usefulness of examining the impact of maternal nativity by specific racial/ethnic groups, while also being mindful that the immigration patterns within the study population may limit generalizability. Larger studies of pooled data from multiple state birth defects surveillance programs will be useful to thoroughly investigate the impact of maternal country of birth on the prevalence of birth defects and increase the understanding of behavioral or nutritional risk factors associated with certain birth defects.
Strengths
This study had several strengths, the first of which was its use of the MACDP. Ascertainment relied on multiple data sources and extensive clinical review of case records. A second and unique strength of the study was the use of a standard nomenclature of CHD to code and classify cases in MACDP.29 All CHD cases were classified to improve the specificity of cardiac diagnoses and create groups of defects thought to be similar on embryological or morphogenetic bases. Third, this study adjusted for potential confounders of the apparent racial/ethnic disparities. Crude prevalence estimates stratified by race/ethnicity are typically reported annually in summary reports3,4 and in the peer-reviewed literature,9 leaving unanswered questions about the source of racial/ethnic variation. Finally, this was one of few studies to document racial/ethnic disparities in birth defects prevalence from surveillance data adjusting for CT-based measures of SES. Community measures may provide a better measure for SES than individual-level indicators in terms of environmental and behavioral risk factors and access to health care and may allow for better comparisons of populations across regions.30,31
Limitations
The findings were subject to several limitations. First, this study did not include identified fetuses with defects that were electively terminated before 20 weeks gestation. Although a proportion may have otherwise survived past 20 weeks, these fetuses did not meet the case definition. Better access to early prenatal care and early diagnosis of a birth defect may have resulted in a greater number of terminations at <20 weeks gestation for some racial/ethnic groups. Differences in rates of terminations across race/ethnicity and across age groups have been reported previously from MACDP.32,33
Second, temporal trends have been reported in the literature for some birth defects. We attempted to reduce the impact of temporal trends by restricting the study period to 10 years; however, the extent to which trends affected the reported prevalence is not known. Third, case counts were insufficient to include additional racial/ethnic groups, and we did not consider nationality. The results of comparisons between Hispanic and NHB infants highlight the misconception that racial/ethnic minority groups can be treated as an aggregate group. Furthermore, evidence suggests that disparities in birth defects exist among all racial/ethnic groups, yet there remains a dearth of quality studies producing stable estimates for many populations such as Native Americans or distinct Hispanic groups that may have unique genetic or cultural risk profiles. Because the likelihood of ascertainment and diagnosis of birth defects in a given infant may vary by whether the affected infant has isolated or multiple defects or a syndrome, it would be informative to examine the extent to which the observed racial/ethnic variations in prevalence of defects were evident by the phenotype of the baby. However, such analysis was not possible in our study, as all MACDP cases had not yet undergone such classification.
Finally, an observed disparity in the prevalence of a birth defect could be explained by differences in diagnosis, ascertainment, or reporting by race/ethnicity. Some birth defects may be more susceptible to artifactual prevalence variability based on defect severity and the consequential ability to detect and confirm a diagnosis,34 although there was no pattern to suggest that diagnostic variability accounted for all the disparities in prevalence.
CONCLUSIONS
Racial/ethnic variation in the birth prevalence of most birth defects exists, but the magnitude of the variation is modest. The reasons for racial/ethnic variations in the prevalence of birth defects are not well understood. These data provide evidence to suggest that socioeconomic factors explain some of the variation in birth defect prevalence, with a hypothesis that inequity in access to quality medical and diagnostic services may explain a lower observed prevalence among poor racial/ethnic minority groups. Further examination of this interaction using both individual- and community-level measures of SES could shed more light on the impact of the availability and access to health-care services on the confirmed diagnosis of a birth defect. Disparities might be further explained by the differential use of elective pregnancy terminations, varying exposure to environmental teratogens, and differing genotypic profiles.
Studies that are sufficiently powered to include smaller racial/ethnic minority groups and report on prevalence among foreign-born mothers by country of birth would be helpful to understand the role of cultural orientation on the risk of birth defects. Studies that have adequate data to examine recurrence risk and how this risk might vary by race/ethnicity and other factors would also be helpful in understanding the possible role of genetics in the observed disparities in prevalence. Identifying and corroborating these disparities could help guide studies to elucidate the underlying reasons for them and, thereby, facilitate the development of effective intervention and prevention strategies that target more vulnerable populations. Additionally, more population-based studies are needed to further explain possible racial/ethnic variations in the survival of children with birth defects and to evaluate the potential impact of delayed diagnosis or undiagnosed and untreated birth defects on the reduced survival of minorities.35–37
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). This study was approved by the CDC Institutional Review Board.
REFERENCES
- 1.Petrini J, Damus K, Russell R, Poschman K, Davidoff MJ, Mattison D. Contribution of birth defects to infant mortality in the United States. Teratology. 2002;66(Suppl 1):S3–6. doi: 10.1002/tera.90002. [DOI] [PubMed] [Google Scholar]
- 2.Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57(1):1–5. [PubMed] [Google Scholar]
- 3.Correa A, Cragan JD, Kucik JE, Alverson CJ, Gilboa SM, Balakrishnan R, et al. Reporting birth defects surveillance data 1968–2003 [published erratum appears in Birth Defects Res A Clin Mol Teratol 2008;82:41-62] Birth Defects Res A Clin Mol Teratol. 2007;79:65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities (US) Birth defects surveillance data from selected states, 1999–2003. Birth Defects Res A Clin Mol Teratol. 2006;76:894–960. doi: 10.1002/bdra.20336. [DOI] [PubMed] [Google Scholar]
- 5.British Paediatric Association. British Paediatric Association Classification of Diseases. London: British Paediatric Association; 1979. [Google Scholar]
- 6.World Health Organization. International classification of diseases, ninth revision, clinical modification. [cited 2009 Oct 15]. Available from: URL: http://www.who.int/classifications/icd/en.
- 7.Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities (US) MACDP defect code list. [cited 2011 Aug 25]. Available from: URL: http://www.cdc.gov/ncbddd/birthdefects/documents/MACDPcode0807.pdf.
- 8.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57:186–99. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:E32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 10.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael SL, Shaw GM, Kaidarova Z. Congenital malformations in offspring of Hispanic and African-American women in California, 1989–1997. Birth Defects Res A Clin Mol Teratol. 2004;70:382–8. doi: 10.1002/bdra.20012. [DOI] [PubMed] [Google Scholar]
- 12.Correa-Villaseñor A, McCarter R, Downing J, Ferencz C. White-black differences in cardiovascular malformations in infancy and socioeconomic factors. The Baltimore-Washington Infant Study Group. Am J Epidemiol. 1991;13:393–402. doi: 10.1093/oxfordjournals.aje.a116101. [DOI] [PubMed] [Google Scholar]
- 13.Smedley BD, Stith AY, Nelson AR, editors. Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. Washington: National Academies Press; 2002. [PubMed] [Google Scholar]
- 14.Alderman BW, Lammer EJ, Joshua SC, Cordero JF, Ouimette DDR, Wilson MJ, et al. An epidemiologic study of craniosynostosis: risk indicators for the occurrence of craniosynostosis in Colorado. Am J Epidemiol. 1988;128:431–8. doi: 10.1093/oxfordjournals.aje.a114983. [DOI] [PubMed] [Google Scholar]
- 15.Boulet SL, Rasmussen SA, Honein MA. A population-based study of craniosynostosis in metropolitan Atlanta, 1989–2003. Am J Med Genet A. 2008;146A:984–91. doi: 10.1002/ajmg.a.32208. [DOI] [PubMed] [Google Scholar]
- 16.Croen LA, Shaw GM, Wasserman CR, Tolarová MM. Racial and ethnic variations in the prevalence of orofacial clefts in California, 1983–1992. Am J Med Genet. 1998;79:42–7. doi: 10.1002/(sici)1096-8628(19980827)79:1<42::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Carmichael SL, Shaw GM, Laurent C, Olney RS, Lammer EJ. National Birth Defects Prevention Study. Maternal reproductive and demographic characteristics as risk factors for hypospadias. Paediatr Perinat Epidemiol. 2007;21:210–8. doi: 10.1111/j.1365-3016.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- 18.Chung CS, Myrianthpoulos NC. Racial and prenatal factors in major congenital malformations. Am J Hum Genet. 1968;20:44–60. [PMC free article] [PubMed] [Google Scholar]
- 19.Beals RK. Club foot in the Maori: a genetic study of 50 kindreds. N Z Med J. 1978;88:144–6. [PubMed] [Google Scholar]
- 20.Cartlidge I. Observations on the epidemiology of club foot in Polynesian and Caucasian populations. J Med Genet. 1984;21:290–2. doi: 10.1136/jmg.21.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorthi RN, Hashmi SS, Langois P, Canfield M, Waller DK, Hecht JT. Idiopathic talipes equinovarus (ITEV) (clubfeet) in Texas. Am J Med Genet A. 2005;132:376–80. doi: 10.1002/ajmg.a.30505. [DOI] [PubMed] [Google Scholar]
- 22.Carmichael SL, Ma C, Shaw GM. Socioeconomic measures, orofacial clefts, and conotruncal heart defects in California. Birth Defects Res A Clin Mol Teratol. 2009;85:850–7. doi: 10.1002/bdra.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grewal J, Carmichael SL, Song J, Shaw GM. Neural tube defects: an analysis of neighbourhood- and individual-level socio-economic characteristics. Paediatr Perinat Epidemiol. 2009;23:116–24. doi: 10.1111/j.1365-3016.2008.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Carmichael SL, Canfield M, Song J, Shaw GM. National Birth Defects Prevention Study. Socioeconomic status in relation to selected birth defects in a large multicentered US case-control study. Am J Epidemiol. 2008;167:145–54. doi: 10.1093/aje/kwm283. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman CR, Shaw GM, Selvin S, Gould JB, Syme SL. Socioeconomic status, neighborhood social conditions, and neural tube defects. Am J Public Health. 1998;88:1674–80. doi: 10.2105/ajph.88.11.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramadhani T, Short V, Canfield MA, Waller DK, Correa A, Royle M, et al. ; National Birth Defects Prevention Study (NBDPS). Are birth defects among Hispanics related to maternal nativity or number of years lived in the United States? Birth Defects Res A Clin Mol Teratol. 2009;85:755–63. doi: 10.1002/bdra.20584. [DOI] [PubMed] [Google Scholar]
- 27.Salemi JL, Pierre M, Tanner JP, Kornosky JL, Hauser KW, Kirby RS, et al. Maternal nativity as a risk factor for gastroschisis: a population-based study. Birth Defects Res A Clin Mol Teratol. 2009;85:890–6. doi: 10.1002/bdra.20612. [DOI] [PubMed] [Google Scholar]
- 28.Zhu M, Druschel C, Lin S. Maternal birthplace and major congenital malformations among New York Hispanics. Birth Defects Res A Clin Mol Teratol. 2006;76:467–73. doi: 10.1002/bdra.20270. [DOI] [PubMed] [Google Scholar]
- 29.Riehle-Colarusso T, Strickland MJ, Reller MD, Mahle WR, Botto LD, Siffel C, et al. Improving the quality of surveillance data on congenital heart defects in the Metropolitan Atlanta Congenital Defects Program. Birth Defects Res A Clin Mol Teratol. 2007;79:743–53. doi: 10.1002/bdra.20412. [DOI] [PubMed] [Google Scholar]
- 30.Braveman P, Cubbin C, Marchi K, Egerter S, Chavez G. Measuring socioeconomic status/position in studies of racial/ethnic disparities: maternal and infant health. Public Health Rep. 2001;116:449–63. doi: 10.1093/phr/116.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee PR, Moss N, Krieger N. Measuring social inequalities in health. Report on the Conference of the National Institutes of Health. Public Health Rep. 1995;110:302–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Siffel C, Correa A, Cragan J, Alverson CJ. Prenatal diagnosis, pregnancy terminations and prevalence of Down syndrome in Atlanta. Birth Defects Res A Clin Mol Teratol. 2004;70:565–71. doi: 10.1002/bdra.20064. [DOI] [PubMed] [Google Scholar]
- 33.Cragan JD, Gilboa SM. Including prenatal diagnoses in birth defects monitoring: experience of the Metropolitan Atlanta Congenital Defects Program. Birth Defects Res A Clin Mol Teratol. 2009;85:20–9. doi: 10.1002/bdra.20508. [DOI] [PubMed] [Google Scholar]
- 34.Langlois PH, Schuerle A. Using registry data to suggest which birth defects may be more susceptible to artifactual clusters and trends. Birth Defects Res A Clin Mol Teratol. 2007;79:798–805. doi: 10.1002/bdra.20407. [DOI] [PubMed] [Google Scholar]
- 35.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–81. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen SA, Wong LY, Correa A, Gambrell D, Friedman JM. Survival in infants with Down syndrome, Metropolitan Atlanta, 1979–1998. J Pediatr. 2006;148:806–12. doi: 10.1016/j.jpeds.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Shin M, Kucik JE, Correa A. Causes of death and case fatality rates among infants with down syndrome in metropolitan Atlanta. Birth Defects Res A Clin Mol Teratol. 2007;79:775–80. doi: 10.1002/bdra.20414. [DOI] [PubMed] [Google Scholar]