SYNOPSIS
Objectives
Approximately 13% of all births occur prior to 37 weeks gestation in the U.S. Some established risk factors exist for preterm birth, but the etiology remains largely unknown. Recent studies have suggested an association with environmental exposures. We examined the relationship between preterm birth and exposure to a commonly used herbicide, atrazine, in drinking water.
Methods
We reviewed Kentucky birth certificate data for 2004–2006 to collect duration of pregnancy and other individual-level covariates. We assessed existing data sources for atrazine levels in public drinking water for the years 2000–2008, classifying maternal county of residence into three atrazine exposure groups. We used logistic regression to analyze the relationship between atrazine exposure and preterm birth, controlling for maternal age, race/ethnicity, education, smoking, and prenatal care.
Results
An increase in the odds of preterm birth was found for women residing in the counties included in the highest atrazine exposure group compared with women residing in counties in the lowest exposure group, while controlling for covariates. Analyses using the three exposure assessment approaches produced odds ratios ranging from 1.20 (95% confidence interval [CI] 1.14, 1.27) to 1.26 (95% CI 1.19, 1.32), for the highest compared with the lowest exposure group.
Conclusions
Suboptimal characterization of environmental exposure and variables of interest limited the analytical options of this study. Still, our findings suggest a positive association between atrazine and preterm birth, and illustrate the need for an improved assessment of environmental exposures to accurately address this important public health issue.
Preterm birth is an important public health problem in the United States, with approximately 13% of all births occurring prior to 37 weeks gestation.1 Preterm birth is associated with excess infant morbidity and mortality.2–7 Preterm infants who survive the neonatal period are more likely to require special medical and educational attention in the future.4,6 Established risk factors for preterm births include low socioeconomic status, black race/ethnicity, and maternal smoking.8 Other toxicology research demonstrates that environmental contaminants may act as endocrine disruptors and play a role in fetal development and consequently preterm birth.9
Atrazine (6-chloro-N-ethyl-N-(1-methylethyl)-1,3,5-triazine-2,4-diamine), a triazine, is an herbicide used worldwide to control broadleaf and grassy weeds. Triazines in general are considered to be endocrine disrupters,10 and current research shows that atrazine exposure may pose a threat to human health, with drinking water providing the most widespread route of exposure.11 The U.S. Environmental Protection Agency (EPA) estimates that approximately 76.5 million pounds of atrazine are applied annually within the United States, making it one of the most widely used herbicides in the country.12,13 Atrazine is most commonly applied to corn, sugarcane, and sorghum crops, but is sometimes used for other crops, maintenance of golf courses, rangeland, forests, and recreational areas, as well as near high-voltage power lines. Due to the direct application of atrazine to crops, there is opportunity for the substance to contaminate soil and, consequently, water sources via runoff.14,15 Atrazine's primary degradation can occur through soil bacteria and abiotic processes with an environmental half-life of a few weeks to several months.16 Atrazine is transported to bodies of water by runoff from fields, which is a concern when those water bodies happen to be sources of drinking water.
Myriad animal studies have reported links between atrazine and a wide range of adverse health effects, including reproductive outcomes and cancer.10,14,17 Based on the available evidence, the European Union banned the use of atrazine in 2004.18 In the U.S., the EPA conducted a reassessment of atrazine in 2003 and concluded that “there is a reasonable certainty that no harm will result to the general U.S. population, infants, children, or other major identifiable subgroups of consumers from aggregate exposure (from food, drinking water, and nonoccupational sources) to cumulative residues of atrazine and the other chlorinated triazine pesticides,” and atrazine and atrazine products were deemed eligible for re-registration and use as herbicides.19 The EPA does continue to classify atrazine as a restricted-use pesticide, thus mandating that all public drinking water supplies maintain a level below the maximum contaminant level (MCL) of 3 micrograms per liter (μg/L).14,20
Few studies regarding the consequences of human exposure to relatively low levels of atrazine exist, and those that do rely solely on ecologic-level data that do not allow for the determination of individual-level exposures.10–12,21–29 Additionally, the quality of the exposure data remains in question as the regulations for testing public water sources differ based on characteristics of the water system, and there is no consistent monitoring of atrazine contamination of private wells or other water sources. Although using the data currently available is problematic in many ways, it represents the current state of our knowledge and illustrates the need for collecting more accurate data regarding population-wide exposure to atrazine or other contaminants.
We examined the existing data to see if evidence exists for a relationship between exposure to levels of atrazine found in drinking water and preterm birth and, if so, to encourage further epidemiologic investigation.
METHODS
We used a cross-sectional design to examine already existing data sources. Levels of atrazine detected in public drinking water were determined for each county in Kentucky. Birth outcomes and covariates were assessed at the individual level using birth certificate data.
Kentucky has a population of approximately 4.2 million people distributed across 120 counties.30 During the three-year period 2004–2006, the state recorded 168,792 live births, with 154,447 being singleton births to women who were current residents of the state. Of the study population, 98.3% reported their race or ethnicity to be non-Hispanic white, non-Hispanic black, or Hispanic. As race is a major risk factor for preterm birth, the small proportion (1.7%) of individuals who did not identify as one of these racial or ethnic groups were excluded from further analyses due to small numbers, leaving 151,784 births.
Many differences exist between the state's Appalachian region, found predominantly in Eastern Kentucky, and the population residing in Western Kentucky. The Appalachian/Eastern Kentucky region does not depend greatly on agriculture, relies quite heavily on private well water sources, which are not subject to the EPA's drinking water regulations, and has a lower socioeconomic status and higher rates of important risk factors for preterm birth. As a result of these important regional differences, the Appalachian region was excluded from the final study population. Additionally, to better control for race and urban residence, we excluded Fayette and Jefferson counties, which include the major urban centers of Lexington and Louisville, respectively, and in which most people belonging to racial/ethnic minority groups (a relatively low percentage of the population in Kentucky) reside. After all exclusions, the final eligible study population consisted of 71,768 singleton, live births.
The EPA requires public water supply companies to test drinking water supplies quarterly for the presence of atrazine in a one-year period.20 Under the Safe Drinking Water Act, community water systems are required to monitor for atrazine and maintain sampling levels below the MCL of 3 μg/L.31 If atrazine is detected at levels greater than 1 μg/L, the company is required to continue to test its supply quarterly. Alternatively, if atrazine measurements are below the detectable limit, the company may follow protocol dependent on the size of the population served to test less than quarterly.20
We obtained from the Kentucky Division of Water the measured atrazine levels in public, community drinking water supplies from 2000–2008 and the method used to test each sample, as well as the county and population served. The Kentucky Geological Survey (KGS) has sampled a select number of private drinking water sources, but there is no consistent monitoring or sampling conducted. We obtained available KGS data for private wells within the state. The KGS data showed that private drinking water sources exhibited slightly higher levels of atrazine compared with public water sources; however, the highest levels in private water were found in the same counties that had the highest levels in public water. Additionally, most counties in Western Kentucky report that a majority of their residents use public water. Therefore, we used only public water data to assign exposure to atrazine from drinking water in each county.
We derived mean atrazine levels for each county. Using the population served by each water system, we determined population-weighted means for counties served by multiple water systems. We included all samples available from 2000–2008, as an unequal number of samples were available for each water supplier, and restricting the data to the study period would have caused some counties to have very few samples.
Outcome assessment
We determined gestational age using birth certificate records obtained from the Vital Statistics Branch of the Division of Epidemiology and Health Planning at the Kentucky Department for Public Health. We calculated gestational age from the mother's reported date of last menstrual period (LMP) and the infant's date of birth. If the mothers' estimated date of LMP was not present or the calculated gestational age was greater than 42 weeks, rendering the date of LMP improbable, the clinical estimation of gestational age was substituted. We defined preterm birth as a birth occurring before 37 completed weeks gestation.32
Demographic and health-related covariates
We assessed important covariates at the individual level using the birth certificate records. We examined maternal factors including age, race/ethnicity, education, rural or urban residence, marital status, smoking and alcohol consumption during pregnancy, parity, gestational hypertension and diabetes, previous preterm births, and infant factors, including delivery by Cesarean section and birthweight. We grouped maternal age into three categories (<20 years, 20–34 years, and ≥35 years of age), education into two categories (<high school and ≥high school diploma), and parity into nulliparous (i.e., having no previous births) and parous (i.e., having had at least one previous birth) women. We used the 2003 Rural-Urban Continuum Codes to classify county of residence into three groups: (1) counties in a metropolitan area, (2) nonmetropolitan counties with an urban population >2,500, and (3) nonmetropolitan counties with an urban population <2,500.33 In addition, we assessed the adequacy of prenatal care using the Kessner scale, which considers the trimester in which prenatal care began and the number of prenatal visits reported, creating four groups: adequate, intermediate, inadequate, and unknown.34
Statistical methods
Because the EPA monitors atrazine in drinking water and levels are generally low, a large proportion (90%) of the readings was reported as below the limit of detection (LOD) of the respective tests used. To account for the large amount of readings <LOD, we examined the results produced using three methods.
Method 1.
We substituted the value of zero for all readings recorded as <LOD.
Method 2.
Based on information included in the water database, we found that different laboratories had used three distinct water-testing methods. Each method had a different LOD: the EPA 507 method had an LOD of 0.015 μg/L, the EPA 508.1 method had an LOD of 0.003 μg/L, and the EPA 525.2 method had an LOD of 0.081 μg/L. For all values recorded as <LOD, we substituted one-half of the LOD of the respective test used. In addition, a few readings were recorded generally as “gas chromatographic” or as an unspecified method. In these cases, we assigned the value of the lowest LOD. This method may have had the unintended effect of introducing a bias, so that areas using a testing method with a higher LOD were assigned a higher level of atrazine for measurements recorded as <LOD.
Method 3.
As a way to avoid the bias described in Method 2, we substituted one-half of the lowest LOD (i.e., one-half of 0.003, or 0.0015 μg/L) of the three testing methods used for all results recorded as <LOD.
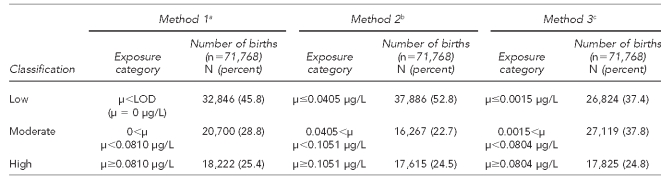
We present the results of all three analyses in this article. For each method, we first calculated the mean atrazine level for each water company. Because more than one company could serve a county, but water companies did not serve multiple counties, we then calculated the population-weighted mean level of atrazine by county using the mean level of atrazine for each company and the population served by each company. Counties were grouped into three exposure levels based on the distribution of the data (Table 1). Maternal atrazine exposure was consequently determined based on the maternal county of residence as recorded on the birth certificate.
Table 1.
Cut points used to determine categories for three methods of atrazine exposure classification in a study of atrazine exposure in public drinking water and preterm birth in central and western Kentucky
aAll values recorded as <LOD replaced with 0 μg/L
bAll values recorded as <LOD replaced with one-half of the LOD of the respective test
cAll values recorded as <LOD replaced with one-half of the lowest LOD
μ = mean
LOD = limit of detection
μg/L = microgram per liter
We used logistic regression to assess the relationship between exposure to atrazine via drinking water and preterm birth. A full model was fit including all potential confounders. We also examined effect measure modification by season of birth in two ways. We included an interaction term between the atrazine exposure and birth month, and also explored an interaction between birth quarter and atrazine exposure. We used a backward elimination approach to fit the model.35 No interaction term was significant, and none was retained. Covariates were included based on previous literature or if their exclusion caused a 10% or greater change in the coefficient of interest.35 The final model included six categorical variables (atrazine exposure level, maternal age, maternal race, maternal education, prenatal care, and maternal smoking). In addition, we conducted a mixed-model analysis to account for county-level effects. This analysis produced similar results to the logistic regression and, therefore, we did not report the results of the mixed models in this article. We performed all analyses using SAS® version 9.1.36
RESULTS
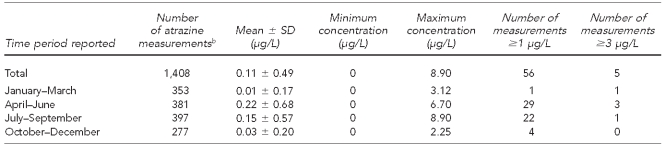
The Kentucky Division of Water database had 1,408 atrazine measurements from 142 public water supplies in the counties included in this study. The number of measurements per water supply ranged from one to 49, with a median number of seven measures per water supply. Samples ranged from <LOD to 8.9 μg/L (Table 2). A total of 56 readings in 17 counties had measurements >1 μg/L and five readings in four counties had measurements ≥3 μg/L. Atrazine levels were highest in April–June.
Table 2.
Description of atrazine levels reported in public water samples between 2000 and 2008,a in a study of atrazine exposure in public drinking water and preterm birth in central and western Kentucky
aMean, SD, and minimum and maximum concentrations are provided for Method 1.
bData are from the Kentucky Division of Water database.
SD = standard deviation
μg/L = microgram per liter
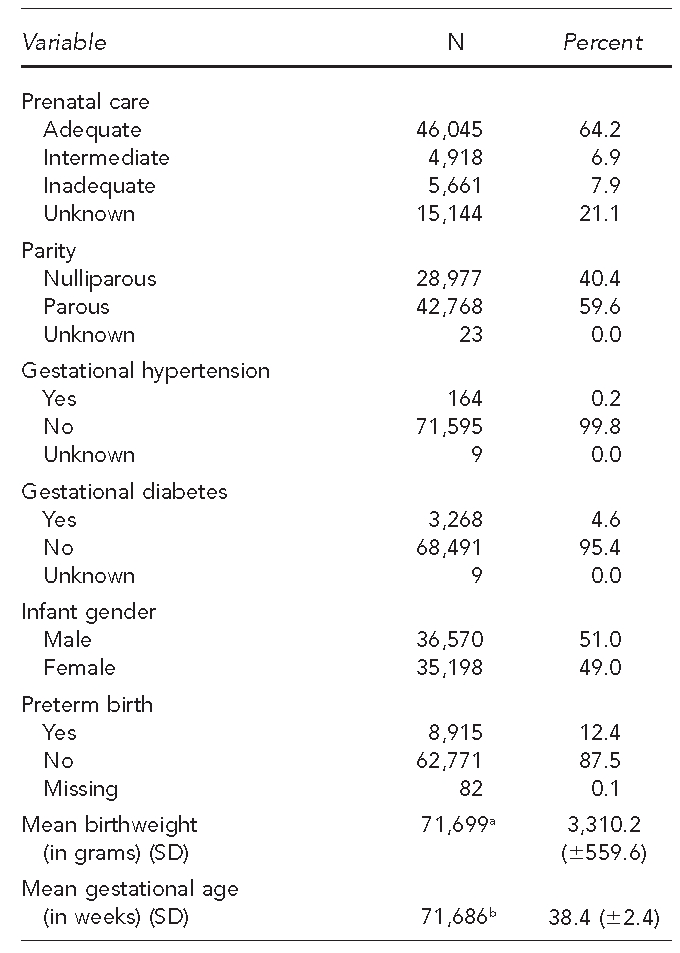
Of the 71,768 births included in the study, a majority of the mothers were non-Hispanic white (89.6%) and had completed at least a high school education (79.5%) (Table 3). In addition, most were married (64.7%) and parous (59.6%). The reported maternal smoking rate was high (32.1%), while the reported alcohol consumption was low (0.6%). A majority of mothers had an adequate level of prenatal care (64.2%); however, 21.1% had an unknown level of prenatal care. The mean weight of the infants was 3,310.2 6 559.6 grams and their mean gestational age was 38.4 6 2.4 weeks. Approximately 12.4% of births qualified as preterm.
Table 3.
Maternal and infant characteristics for births (n=71,768) occurring between 2004 and 2006, in a study of atrazine exposure in public drinking water and preterm birth in central and western Kentucky
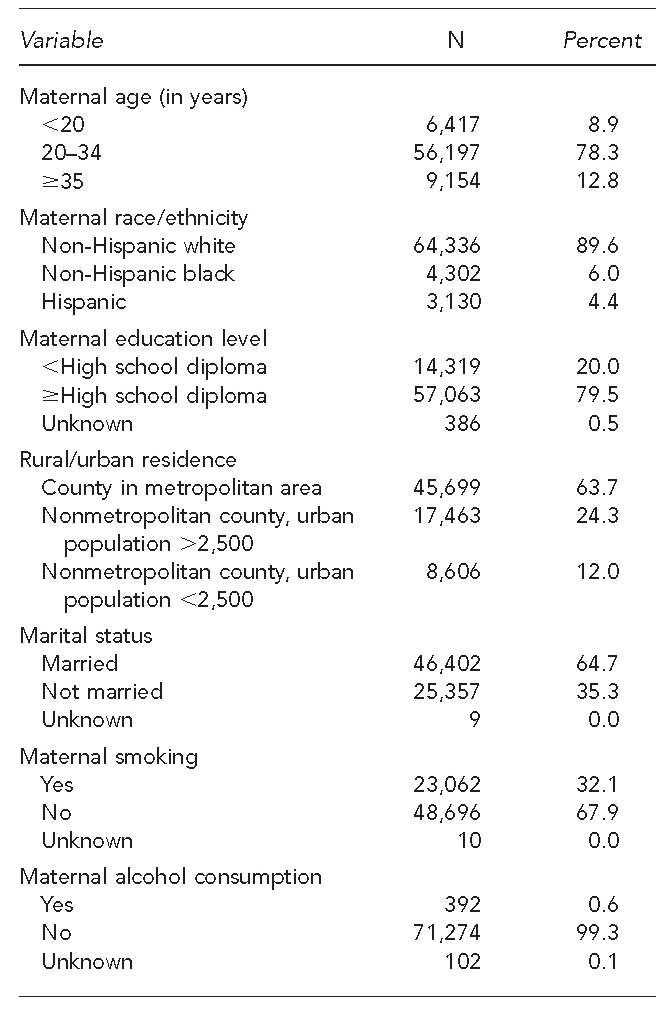
aBirthweight was missing for 69 infants.
bGestational age was missing for 82 infants.
SD = standard deviation
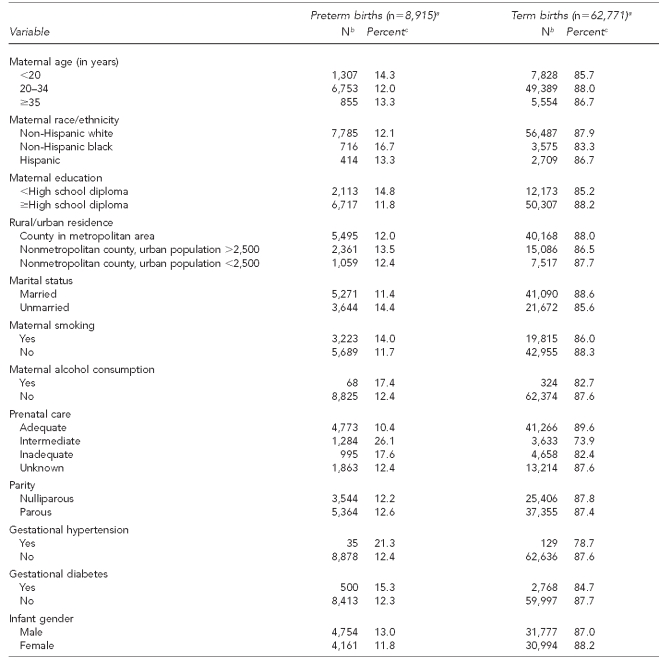
We further examined the relationship between covariates and birth outcomes of interest through bivariate analysis (Table 4). Mothers younger than 20 years of age and those with less than a high school education demonstrated elevated proportions of preterm birth compared with women older than 20 years of age and those who had a high school diploma or higher level of education. Non-Hispanic black mothers had the highest proportion of preterm birth (16.7%). Mothers who reported smoking had higher proportions of preterm birth (14.0%) compared with mothers who did not report smoking (11.7%). Those who had an adequate level of prenatal care also reported lower proportions of preterm birth (10.4%) than those with inadequate prenatal care (17.6%) or intermediate care (26.1%).
Table 4.
Characteristics of preterm births compared with term births occurring between 2004 and 2006, in a study of atrazine exposure in public drinking water and preterm birth in central and western Kentucky
aTotal n=71,686, as gestational age was missing for 82 births.
bNumber for preterm births may not total 8,915 and number for term births may not total 62,771 due to unknown or missing information for some variables.
cRow percentage based on numbers shown for each variable (does not include unknown or missing information).
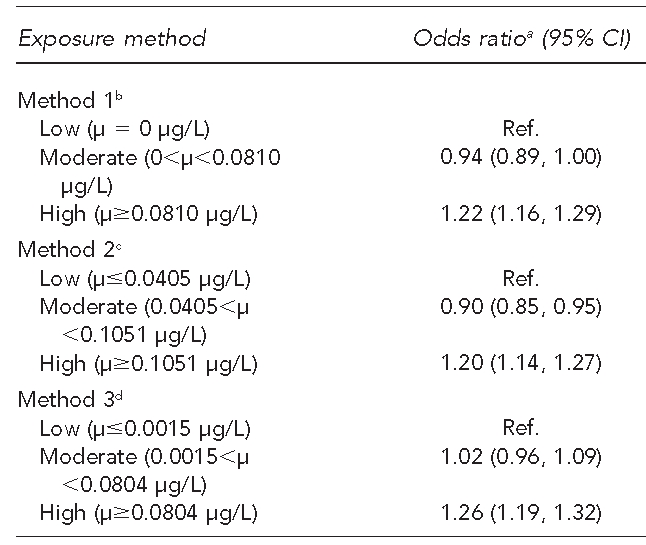
We performed a logistic regression analysis to determine the relationship between atrazine exposure level and preterm birth while controlling for important covariates. The three exposure classification methods resulted in similar results (Table 5). We calculated adjusted odds ratios (AORs) ranging from 0.90 (95% confidence interval [CI] 0.85, 0.95) to 1.02 (95% CI 0.96, 1.09) for mothers residing in moderate-exposure counties compared with those residing in low-exposure counties. For mothers residing in high-exposure counties, we calculated AORs ranging from 1.20 (95% CI 1.14, 1.27) to 1.26 (95% CI 1.19, 1.32) compared with mothers residing in low-exposure counties.
Table 5.
Results of logistic regression examining the effect of atrazine exposure (by exposure category) on preterm births occurring between 2004 and 2006 in central and western Kentucky, using three exposure methods
aAdjusted for maternal age, race/ethnicity, education, smoking status, and prenatal care
bAll values coded as <LOD replaced with 0 μg/L
cAll values coded as <LOD replaced with one-half of the LOD of the respective test
dAll values coded as <LOD replaced with one-half of the lowest LOD
CI = confidence interval
μ = mean
μg/L = microgram per liter
Ref. = reference group
LOD = limit of detection
DISCUSSION
We explored the relationship between preterm birth in the western region of Kentucky between 2004 and 2006 and exposure to atrazine via public drinking water using existing data. We found an increase in odds of preterm birth for mothers residing in counties in the highest exposure category compared with the lowest exposure category using three distinct exposure classification methods while controlling for maternal age, maternal race/ethnicity, maternal education, prenatal care, and maternal smoking. However, we found no elevation and, with Method 2, a slight reduction, in the odds of preterm birth for those residing in counties with moderate levels of exposure compared with counties with low exposure.
Few published studies have examined the relationship between exposure to atrazine in humans and adverse birth outcomes. Savitz et al. conducted a study examining paternal exposure to triazines among other exposures and several reproductive outcomes.26 They found that men reporting yard use of atrazine demonstrated elevated odds of having a child that was born preterm (OR=4.9, 95% CI 1.6, 14.5) compared with men not reporting exposure. The results of the present study are consistent with the findings from a study conducted in France by Villanueva et al., which suggested a possible, but not statistically significant, association between levels of atrazine in municipal drinking water and preterm birth. However, Villa-nueva et al. included a much smaller study sample, which may have interfered with the precision of the results.11 A study by Munger et al. conducted in Iowa did not find an association between atrazine exposure via drinking water and preterm birth, but did report an association with an increase in rates of intrauterine growth retardation.25
Similarly, a recently published study conducted in Indiana by Ochoa-Acuña et al. reported a positive association between atrazine exposure and small-for-gestational-age births, but not with preterm birth.29 Ochoa-Acuña et al. used a similar methodology to the present study; however, two important differences should be noted. The study population included in the Ochoa-Acuña et al. study had a preterm birth rate that was lower than the national average (7.4%), and most of the birth records (68.0%) were from one urban center. Conversely, Ochoa-Acuña et al. were able to present more detailed data on drinking water atrazine levels than were available in Kentucky.29 An association between exposure to atrazine or other pesticides and reproductive effects may work through many pathways. For example, a study of birth defects and levels of pesticides and nitrates in surface waters in the U.S. found elevations in the geometric mean atrazine exposure between April and June and a corresponding elevation in the overall rate of birth defects in pregnancies conceived during these months.37 Many of these birth defects may contribute to preterm birth; thus, it is possible that these two issues may be related.
Limitations
In our study, the main limitation was in the exposure assessment. Misclassification of exposure status may be present for several reasons. The monitoring of atrazine levels in drinking water supplies is inconsistent and varies among water systems, providing a poor picture of levels of atrazine present in drinking water. Specifically, in our study we noted a reduction in samples taken in June, a month with peak atrazine levels. This finding could have led to an underestimation of mean atrazine levels. Additionally, seasonality of exposure could affect the results presented in this article either by masking or underestimating an effect. However, due to limitations in our data, we were unable to include a measure of seasonality in the analysis. Another reason for potential misclassification is that we determined exposure level using maternal county of residence at the time of birth, with no information regarding levels of atrazine in the water at its point of use. We also did not have information regarding individual water intake or use of alternative water sources, such as bottled water. Systematic monitoring of atrazine in public and private drinking water and individual-level data concerning water consumption would produce a more accurate picture of exposure to atrazine through drinking water.
While classification of exposure may be affected for the reasons just discussed, it may also be affected by exposure through routes not measured in the present study. Even studies that attempt to measure individual exposure to atrazine through biomarkers must confront these issues of misclassification. For example, a study by Barr et al. determined that previous studies measuring atrazine mercapturate, a urinary metabolite, under-esti-mated exposure to atrazine, as several metabolites are required to present the full picture of exposure.38 Our inability to measure atrazine exposure accurately, including all potential sources, may explain why we observed elevated levels of preterm birth at what appear to be relatively low levels of exposure.
CONCLUSIONS
The results presented in this article illustrate the need for more accurate data regarding population-wide exposure to commonly used pesticides such as atrazine. The study design and exposure assessment issues raised in this article indicate the need for further research with comprehensive sampling and survey methods for measuring the total bioavailability of atrazine through individual-level exposure assessment to better determine the true relationship between atrazine exposure and preterm birth.
Footnotes
Funding for this work was provided by the Southeast Center for Agricultural Health and Injury Prevention, University of Kentucky, College of Public Health (National Institute for Occupational Safety and Health Cooperative Agreement #5U50OH007547-08). The authors thank the Kentucky Geological Survey for providing input on well-water usage and data regarding private water sources, Drs. Richard Clayton and Lori Chesnut for their useful comments, the Kentucky Department for Public Health for the birth certificate data, and the Kentucky Division of Water for the atrazine data.
The study protocol was reviewed and approved by the University of Kentucky and Western Kentucky University Institutional Review Boards.
REFERENCES
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2006. Natl Vital Stat Rep. 2009;57(7):1–101. [PubMed] [Google Scholar]
- 2.Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133(5 Suppl 2):1592S–6S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- 3.Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA. 2000;284:843–9. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- 4.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 5.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–8. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 6.Overpeck MD, Moss AJ, Hoffman HJ, Hendershot GE. A comparison of the childhood health status of normal birth weight and low birth weight infants. Public Health Rep. 1989;104:58–70. [PMC free article] [PubMed] [Google Scholar]
- 7.Zaw W, Gagnon R, da Silva O. The risks of adverse neonatal outcome among preterm small for gestational age infants according to neonatal versus fetal growth standards. Pediatrics. 2003;111(6 Pt 1):1273–7. doi: 10.1542/peds.111.6.1273. [DOI] [PubMed] [Google Scholar]
- 8.Farhang L, Weintraub JM, Petreas M, Eskenazi B, Bhatia R. Association of DDT and DDE with birth weight and length of gestation in the Child Health and Development Studies, 1959–1967. Am J Epidemiol. 2005;162:717–25. doi: 10.1093/aje/kwi276. [DOI] [PubMed] [Google Scholar]
- 9.Agency for Toxic Substances and Disease Registry. Atlanta: ATSDR; 2003. [cited 2010 Oct 4]. Toxicological profile for atrazine. Also available from: URL: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=338&tid=59. [PubMed] [Google Scholar]
- 10.Gammon DW, Aldous CN, Carr WC, Jr, Sanborn JR, Pfeifer KF. A risk assessment of atrazine use in California: human health and ecological aspects. Pest Manag Sci. 2005;61:331–55. doi: 10.1002/ps.1000. [DOI] [PubMed] [Google Scholar]
- 11.Villanueva CM, Durand G, Coutte MB, Chevrier C, Cordier S. Atrazine in municipal drinking water and risk of low birth weight, preterm delivery, and small-for-gestational-age status. Occup Environ Med. 2005;62:400–5. doi: 10.1136/oem.2004.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper RL, Laws SC, Das PC, Narotsky MG, Goldman JM, Lee Tyrey E, et al. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:98–112. doi: 10.1002/bdrb.20110. [DOI] [PubMed] [Google Scholar]
- 13.Environmental Protection Agency (US) Washington: EPA; 2008. [cited 2010 Oct 4]. Pesticides: topical & chemical fact sheets: atrazine interim reregistration eligibility decision (IRED) Q&A's—January 2003. Also available from: URL: http://www.epa.gov/opp00001/factsheets/atrazine.htm. [Google Scholar]
- 14.EXTOXNET (Extension Toxicology Network) Pesticide information profiles: atrazine. 1996. [cited 2010 Oct 4]. Available from: URL: http://extoxnet.orst.edu/pips/atrazine.htm.
- 15.Huber W. Ecotoxicological relevance of atrazine in aquatic systems. Environ Toxicol Chemistry. 1993;12:1865–81. [Google Scholar]
- 16.Mandelbaum RT, Wackett LP, Allan DL. Rapid hydrolysis of atrazine to hydroxyatrazine by soil bacteria. Environ Sci Technol. 1993;27:1943–6. [Google Scholar]
- 17.Hayes TB. Welcome to the revolution: integrative biology and assessing the impact of endocrine disruptors on environmental and public health. Integr Comp Biol. 2005;45:321–9. doi: 10.1093/icb/45.2.321. [DOI] [PubMed] [Google Scholar]
- 18.European Commission. Commission decision of 10 March 2004 concerning the non-inclusion of atrazine in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this active substance, 2004/248/EC. Official Journal of the European Union. 2004;L78:53–5. [Google Scholar]
- 19.Environmental Protection Agency (US), Office of Prevention, Pesticides and Toxic Substances. Decision documents for atrazine. Washington: EPA; 2006. [Google Scholar]
- 20.Environmental Protection Agency (US) Consumer factsheet on: atrazine. [cited 2010 Oct 4]. Available from: URL: http://www.epa.gov/ogwdw000/pdfs/factsheets/soc/atrazine.pdf.
- 21.Hessel PA, Kalmes R, Smith TJ, Lau E, Mink PJ, Mandel J. A nested case-control study of prostate cancer and atrazine exposure. J Occup Environ Med. 2004;46:379–85. doi: 10.1097/01.jom.0000121128.73921.a1. [DOI] [PubMed] [Google Scholar]
- 22.Hopenhayn-Rich C, Stump ML, Browning SR. Regional assessment of atrazine exposure and incidence of breast and ovarian cancers in Kentucky. Arch Environ Contam Toxicol. 2002;42:127–36. doi: 10.1007/s002440010300. [DOI] [PubMed] [Google Scholar]
- 23.International Agency for Research on Cancer. Lyon (France): IARC; 1999. [cited 2010 Oct 4]. Summaries and evaluations: atrazine (group 3) Also available from: URL: http://monographs.iarc.fr/ENG/Monographs/vol73/volume73.pdf. [Google Scholar]
- 24.McElroy JA, Gangnon RE, Newcomb PA, Kanarek MS, Anderson HA, Brook JV, et al. Risk of breast cancer for women living in rural areas from adult exposure to atrazine from well water in Wisconsin. J Expo Sci Environ Epidemiol. 2007;17:207–14. doi: 10.1038/sj.jes.7500511. [DOI] [PubMed] [Google Scholar]
- 25.Munger R, Isacson P, Hu S, Burns T, Hanson J, Lynch CF, et al. Intrauterine growth retardation in Iowa communities with herbicide-contaminated drinking water supplies. Environ Health Perspect. 1997;105:308–14. doi: 10.1289/ehp.97105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savitz DA, Arbuckle T, Kaczor D, Curtis KM. Male pesticide exposure and pregnancy outcome. Am J Epidemiol. 1997;146:1025–36. doi: 10.1093/oxfordjournals.aje.a009231. [DOI] [PubMed] [Google Scholar]
- 27.Young HA, Mills PK, Riordan DG, Cress RD. Triazine herbicides and epithelial ovarian cancer risk in central California. J Occup Environ Med. 2005;47:1148–56. doi: 10.1097/01.jom.0000177044.43959.e8. [DOI] [PubMed] [Google Scholar]
- 28.International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. volume 73. Lyon (France): IARC; 1999. p. 674. [Google Scholar]
- 29.Ochoa-Acuña H, Frankenberger J, Hahn L, Carbajo C. Drinking-water herbicide exposure in Indiana and prevalence of small-for-gestational-age and preterm delivery. Environ Health Perspect. 2009;117:1619–24. doi: 10.1289/ehp.0900784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Census Bureau (US) State & county quickfacts: Kentucky. Washington: Census Bureau; 2006. [Google Scholar]
- 31.Environmental Protection Agency (US) Overview of atrazine risk assessment. Washington: EPA; 2002. [Google Scholar]
- 32.Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;339:313–20. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- 33.Department of Agriculture (US), Economic Research Service. Washington: USDA; 2003. [cited 2009 Jan 23]. 2003 rural-urban continuum codes. Also available from: URL: http://www.ers.usda.gov/Data/RuralUrbanContinuumCodes. [Google Scholar]
- 34.Kessner DM, Singer J, Kalk CE, Schlesinger ER. Infant death: an analysis by maternal risk and health care. Washington: Institute of Medicine, National Academy of Sciences; 1973. [Google Scholar]
- 35.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–9. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SAS Institute, Inc. Cary (NC): SAS Institute, Inc.; 2004. SAS®: Version 9.1. [Google Scholar]
- 37.Winchester PD, Huskins J, Ying J. Agrichemicals in surface water and birth defects in the United States. Acta Paediatr. 2009;98:664–9. doi: 10.1111/j.1651-2227.2008.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barr DB, Panuwet P, Nguyen JV, Udunka S, Needham LL. Assessing exposure to atrazine and its metabolites using biomonitoring. Environ Health Perspect. 2007;115:1474–8. doi: 10.1289/ehp.10141. [DOI] [PMC free article] [PubMed] [Google Scholar]