Abstract
Parkinson’s disease (PD) is a neurological movement disorder primarily resulting from damage to the nigrostriatal dopaminergic pathway. To elucidate the pathogenesis, mechanisms of cell death, and to evaluate therapeutic strategies for PD, numerous animal models have been developed. Understanding the strengths and limitations of these models can significantly impact the choice of model, experimental design, and data interpretation. The primary objectives of this article are twofold: First, to assist new investigators who are contemplating embarking on PD research to navigate through the available animal models. Emphasis will be placed on common neurotoxic murine models in which toxic molecules are used to lesion the nigrostriatal dopaminergic system. And second, to provide an overview of basic technical requirements for assessing the pathology, structure, and function of the nigrostriatal pathway.
Genetic models of Parkinson’s disease do not display appreciable neurodegeneration. Neurotoxic models, in which different molecules are used to damage the nigrostriatal dopaminergic pathway, are often suitable alternatives.
Parkinson’s disease (PD) is the second most common chronic neurodegenerative disorder, after Alzheimer’s disease. Currently, there is no cure for PD and additional effective treatments for this devastating disease are urgently needed. To achieve this goal, it is critical to understand its etiology and the underlying mechanisms of neurodegeneration and neuronal dysfunction. However, the cause(s) of the majority of PD cases remains unknown. Less than 10% of PD cases can be directly linked to monogenic mutations. Environmental factors, or a combination of both environment and genetic susceptibility, have been proposed to play a role in sporadic PD. Accordingly, experimental models utilizing exposure to exogenous neurotoxicants, mutations in genes linked to PD, or a combination of both, have been created to study PD and to screen therapeutic strategies. The success rate of translating this basic research into clinical relevance for PD relies heavily on the extent to which these experimental models accurately recapitulate the pathology, symptoms, and pathogenic mechanism as seen in PD patients.
Pathologically, the hallmarks of PD are the loss of dopaminergic neurons in the substantia nigra pars compacta and the presence of cytoplasmic protein aggregates, known as Lewy bodies, in remaining dopaminergic cells (Dauer and Przedborski 2003). When degeneration in these neurons results in a threshold reduction of ∼80% dopamine in the striatum (Dauer and Przedborski 2003), motor symptoms of PD emerge. Mechanistically, the death of dopaminergic neurons has been linked to mitochondrial dysfunction, oxidative stress, neuroinflammation, and insufficient autophagic or proteasomal protein degradation (Dauer and Przedborski 2003; Hirsch and Hunot 2009; Martin et al. 2010). In addition to the loss of nigrostriatal dopaminergic structures and function, PD also affects many other areas of the central nervous system, such as the dorsal motor nucleus of the vagus, the nucleus basalis of Meynert, the locus coeruleus, and the hypothalamus (Hornykiewicz and Kish 1987; Braak et al. 2004). Furthermore, the pathology of PD extends well beyond the central nervous system because Lewy bodies have been detected in the myenteric plexus (Kupsky et al. 1987). Together, these extranigrostriatal regions may account for the observed nonmotor symptoms such as sleep disturbances, depression, cognitive impairment, anosmia, constipation, incontinence, and autonomic dysfunctions (Chaudhuri et al. 2005; Langston 2006; Jain 2011).
An ideal model of PD should consist of pathological and clinical features of PD involving both dopaminergic and nondopaminergic systems, the central and peripheral nervous systems, plus motor and nonmotor symptoms. Additionally, the age-dependent onset and progressive nature of PD should be reflected. Unfortunately, none of the current models displays all of these PD features. Despite these limitations, animal models have contributed significantly to our current understanding of the disease processes and potential therapeutic targets in PD.
Current animal models of PD can be broadly divided into two categories: genetic and neurotoxic models. Each group has strengths and weaknesses. One strength of the genetic models is that they are created primarily based on identified targets associated with potential mechanisms known to cause PD in humans (Meredith et al. 2008; Bezard and Przedborski 2011). However, currently available models do not display appreciable neurodegeneration and phenotypes (Dawson et al. 2010). This limitation of genetic models, however, can be complemented by the neurotoxic models in which different molecules are used to damage the nigrostriatal pathway. Faced with a wide variety of PD models, a new investigator may find selecting the appropriate one to be a daunting task. Another challenging step is knowing what basic techniques and equipment are required for assessing neurodegeneration and dysfunction in these models. To address these issues, this article will provide both an overview of the strengths and limitations of some commonly used animal models, as well as a general guide to assessing nigrostriatal damage. Because genetic models of PD and other vertebrate species such as nonhuman primates are discussed by Dawson and Bezard, respectively, elsewhere in this collection, this article will focus on neurotoxic rodent models.
6-HYDROXYDOPAMINE
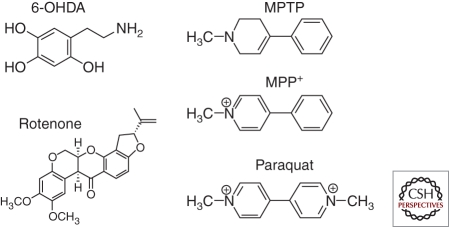
6-Hydroxydopamine (6-OHDA), a hydroxylated analog of dopamine (Fig. 1; see also Table 1), was first identified more than 50 years ago (Senoh and Witkop 1959; Senoh et al. 1959). Initially, 6-OHDA was reported to cause depletion of noradrenaline in the mouse heart (Porter et al. 1963, 1965). The subsequent discovery that 6-OHDA could induce selective degeneration in sympathetic adrenergic nerve terminals (Tranzer and Thoenen 1968, 1973) led to the novel concept of “chemical denervation” in neurobiology, in which a neurotoxic molecule is used to target a specific cell population (Jonsson 1980). To date, 6-OHDA is widely used to lesion the nigrostriatal dopaminergic system as a model of PD. The evidence that this molecule can be found endogenously in human brain (Curtius et al. 1974) and urine samples (Andrew et al. 1993) lends additional credence to this model.
Figure 1.
Structures of neurotoxic molecules used to induce nigrostriatal damage in some common animal models of PD.
Table 1.
Key features of common neurotoxic models of PD
| Models | Pathology |
Behavioral phenotypes |
|||||
|---|---|---|---|---|---|---|---|
| Nigrostriatal damage |
Extranigral pathology | LB | Motor (l-dopa or apomorphine responsive) | Nonmotor | |||
| SN cell body | Str. terminals | Str. DA | |||||
| 6-OHDA | |||||||
| Rat | |||||||
| Stereotactic injection to SN, MFB, striatum | Yes | Yes | Yes | No | No | Yes | Cognitive, psychiatric, and GI disorders |
| MPTP | |||||||
| Nonhuman primates | |||||||
| i.p, i.m, intracarotid infusion | Yes | Yes | Yes | LC | Yes | Yes | Numerous (see Fox and Brotchie 2010; Bezard 2011) |
| Mouse | |||||||
| Acute, subacute (i.p.) | Yes | Yes | Yes | No | No | Yes | Transient ↑colon motility |
| Chronic (osmotic minipumps) | Yes | Yes | Yes | LC | Yes | Yes | ND |
| PQ | |||||||
| Mouse | |||||||
| i.p. | Yes | Conflicting | No | LC | Yes | ND | ND |
| PQ/Maneb | |||||||
| Mouse | |||||||
| i.p. | Yes | ND | Yes | ND | Yes | ND | ND |
| Rotenone | |||||||
| Rat | |||||||
| Infusion via osmotic minipumps | Yes | Yes | Yes | ND | Yes | Yes | ↓GI motility |
| i.p. injection | Yes | Yes | Yes | GI | Yes | Yes | ↓GI motility |
Abbreviations: SN, substantia nigra; Str., striatal; LB, Lewy bodies; LC, locus coeruleus; GI, gastrointestinal; MFB, medial forebrain bundle; ND, not determined.
In the brain, 6-OHDA is capable of inducing degeneration of both dopaminergic and noradrenergic neurons (Ungerstedt 1968). These types of neurons are particularly vulnerable to 6-OHDA because their plasma membrane transporters, the dopamine transporter and noradrenergic transporter, respectively, have high affinity for this molecule (Luthman et al. 1989). Once taken up into neurons, 6-OHDA accumulates in the cytosol where it is readily oxidized leading to the generation of reactive oxygen species and ultimately, oxidative stress-related cytotoxicity (Saner and Thoenen 1971; Graham 1978; Jonsson 1983; Cohen and Werner 1994; Blum et al. 2001). Accordingly, the locus coeruleus and the nigrostriatal region are highly sensitive to this neurotoxin (Jonsson 1980). To target specific neurons and to bypass the blood–brain barrier, 6-OHDA is typically injected stereotactically into the brain region of interest. Because of the difficulty in targeting small brain structures such as the substantia nigra or medial forebrain bundle, 6-OHDA is more commonly used in rats than in mice to model PD (Jonsson 1983). Although uncommon, 6-OHDA has also been used in cats, guinea pigs, dogs, and monkeys (Bezard et al. 1998; Bezard and Przedborski 2011).
The magnitude and characteristics of neurodegeneration induced by 6-OHDA are significantly affected by the site of injection (Agid et al. 1973; Przedborski et al. 1995; Przedborski and Tieu 2006). 6-OHDA is most commonly injected unilaterally to the substantia nigra, medial forebrain bundle, or striatum. Although generally the nerve terminals are more sensitive to 6-OHDA toxicity than the axon and cell body (Malmfors and Sachs 1968; Jonsson 1983), when injected into the nigra or the medial forebrain bundle, 6-OHDA produces a complete and rapid lesion in the nigrostriatal pathway. When injected into the nigra, degeneration of dopaminergic neurons takes place within 12 h preceding a significant loss of striatal terminals, which occurs 2–3 d later (Faull and Laverty 1969; Jeon et al. 1995). When injected into the medial forebrain bundle, however, 6-OHDA induces degeneration in striatal terminals before dopaminergic cell death occurs (Sarre et al. 2004). In contrast to the nigra and medial forebrain bundle, when delivered to the striatum, 6-OHDA induces slow, progressive, and partial damage to the nigrostriatal structure in a retrograde fashion over a period of up to 3 weeks (Sauer and Oertel 1994; Przedborski et al. 1995). This latter route of administration offers three advantages: First, the progressive and less extensive lesion is more relevant to PD. Second, this regimen has been shown to produce nonmotor symptoms of PD, including cognitive, psychiatric, and gastrointestinal dysfunction (Branchi et al. 2008; Tadaiesky et al. 2008; Cannon and Greenamyre 2010). Third, the ease of stereotactically injecting a large structure such as the striatum enhances the likelihood of success in mice.
The major advantage of using the 6-OHDA model is its rather unique effect on quantifiable circling motor abnormality in animals (Ungerstedt and Arbuthnott 1970). Typically, unilateral injection into one hemisphere (hemiparkinsonian model) is performed, leaving the unlesioned side as an internal control. Subsequent to this unilateral lesioning, systemic injection of dopamine receptor agonists (such as apomorphine), l-3,4-dihydroxyphenylalanine (l-dopa, a dopamine precursor), or dopamine releasing compounds (such as amphetamine) induces asymmetrical rotation (Ungerstedt and Arbuthnott 1970; Hefti et al. 1980). The magnitude of nigrostriatal lesions correlates with the circling motor behavior (Ungerstedt 1968; Ungerstedt and Arbuthnott 1970; Hefti et al. 1980; Przedborski et al. 1995). Overall, the unilateral 6-OHDA rat model has been used extensively as a preclinical model to assess the antiparkinsonian effects and neuroprotection of new pharmacological therapies (Jiang et al. 1993; Chan et al. 2010; Ilijic et al. 2011), as well as the clinical improvement of cell transplantation (Bjorklund et al. 2002; Kirik et al. 2002; Roy et al. 2006). However, like many other neurotoxic models of PD, the acute neurodegenerative property of the 6-OHDA model lacks the progressive, age-dependent effects of PD. Additionally, Lewy bodies are not present in this model.
1-METHYL-4-PHENYL-1,2,3,6-TETRAHYDROPYRIDINE
The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model originates from discoveries in the early 1980s when several Californian intravenous drug users were admitted to hospitals showing severe symptoms similar to PD (Davis et al. 1979; Langston et al. 1983). Further investigations uncovered that these patients had self-administered synthetic meperidine contaminated with MPTP (Langston et al. 1983). The movement abnormalities in these patients were successfully treated with l-dopa, a cornerstone treatment of PD, suggesting similar underlying neuropathological and biochemical features to those seen in PD patients. Postmortem studies confirmed the loss of nigrostriatal structures in these patients (Davis et al. 1979; Langston et al. 1999). Recently, one of the surviving patients showed a significant clinical improvement when treated with deep brain stimulation (Christine et al. 2009), further affirming the damage induced by MPTP in the basal ganglia resembles that in human PD. Since its discovery, MPTP has drastically altered the course of PD research by providing insights into potential pathogenesis and mechanisms for cell death in PD. Studies using this model have led to the concepts such as environmental toxicity as a potential culprit in sporadic PD, and mitochondrial dysfunction as a potential pathogenic mechanism. Additionally, work with this model has enabled the development of some current treatments for PD (Fox and Brotchie 2010).
The mechanism of MPTP toxicity has been extensively studied and characterized (Dauer and Przedborski 2003; Rappold and Tieu 2010). Because MPTP is lipophilic, it can rapidly cross the blood–brain barrier. In astrocytes, MPTP is metabolized by monoamine oxidase-B, and subsequently converted to the active toxic cation 1-methyl-4-phenylpyridinium (MPP+; Fig. 1). MPP+ is released from the nigral and striatal astrocytes through the organic cation transporter 3 into the extracellular space (Cui et al. 2009; Rappold and Tieu 2010) where it is taken up by the neighboring dopaminergic neurons and terminals through the dopamine transporter. Once accumulated in dopaminergic neurons, MPP+ induces neurotoxicity primarily by inhibiting complex I of the mitochondrial electron transport chain, resulting in ATP depletion and increased oxidative stress (Nicklas et al. 1985; Mizuno et al. 1987).
Various mammalian species, including sheep, dogs, guinea pigs, cats, mice, rats, and monkeys, have been treated with MPTP to model PD (Bezard et al. 1998; Przedborski et al. 2001). To date, the MPTP-monkey model remains the gold standard for preclinical testing of therapeutic strategies for PD (for a detailed review of the MPTP-monkey model, please refer to Bezard [2011]). For many researchers, however, the mouse remains a popular choice owing to a lack of resources and trained personnel for the monkey model. Additionally, available genetic mouse models allow investigators to assess the roles of certain genetic mutations in response to MPTP neurotoxicity. Rats are less sensitive to MPTP toxicity than mice (Giovanni et al. 1994).
In both monkeys and mice, MPTP primarily causes damage to the nigrostriatal dopaminergic pathway (Forno et al. 1993; Dauer and Przedborski 2003; Fox and Brotchie 2010). This specific and reproducible neurotoxic effect to the nigrostriatal system is a strength of this model. Although intraneuronal inclusions reminiscent of Lewy bodies have been described in MPTP-injected monkeys (Forno et al. 1986), in general, this pathological feature is absent in mice. However, it has been claimed that when mice are infused with a chronic low dose of MPTP over a period of 30 d using osmotic minipumps, inclusions immunoreactive for both ubiquitin and α-synuclein can be detected (Fornai et al. 2005). Additionally, with this regimen, Lewy bodies and degeneration of noradrenergic neurons are detected in the locus coeruleus. However, the reproducibility of this regimen needs to be confirmed by other investigators. Behaviorally, motor deficits induced by MPTP have been extensively characterized in both monkeys and mice. These abnormal phenotypes are reversible by l-dopa or a dopamine agonist, confirming a connection between these symptoms and damage in the nigrostriatal system (Ogawa et al. 1985; Fredriksson and Archer 1994; Rozas et al. 1998; Fernagut et al. 2002). In general, parkinsonian symptoms are better reproduced in monkeys than in mice, and a more profound loss of striatal dopamine is required in mice to produce some such deficits. In addition to the nigrostriatal pathway, MPTP has also been reported to induce loss of dopaminergic neurons in the enteric nervous system and alter colon motility in mice (Anderson et al. 2007). However, the relevance of this observation to the commonly observed gastrointestinal dysfunction in PD patients requires further studies.
In summary, with the caveat of its acute toxic property as seen with other neurotoxic PD models, MPTP will continue to play a major role in PD research based on its ability to produce PD-like effects in humans and nonhuman primates, its reproducible l-dopa-responsive lesion on the nigrostriatal system, and its ease of administration with the typical intraperitoneal (i.p.) injection.
PARAQUAT
After the discovery of MPTP (Langston et al. 1983) and the subsequent characterization of MPP+ as an active metabolite of MPTP (Nicklas et al. 1985), a search for environmental contaminants with a similar molecular structure to MPP+ was initiated. The widely use herbicide paraquat (N,N'-dimethyl-4-4'-bipiridinium) was identified as such an agent (Fig. 1) (Snyder and D’Amato 1985). Since then, this divalent cation has been used to model PD in mice.
Despite a similar structure to MPP+, paraquat shows different transport properties and mechanism(s) of toxicity. First, despite both being cations, in contrast to MPP+, paraquat appears to have the ability to penetrate the blood–brain barrier. This property is proposed to be mediated by the neutral amino acid transporter (Shimizu et al. 2001; McCormack and Di Monte 2003). However, the extent to which paraquat accumulates in the brain is age dependent, with the highest levels detected in 2-wk-old or >12-mo-old rats—suggesting the blood–brain barrier does play a role (Corasaniti et al. 1991), as young and old animals have higher blood–brain-barrier permeability, in general. Second, MPP+ is an excellent substrate for the dopamine transporter, but paraquat is not (Richardson et al. 2005). Hence, it is still uncertain how paraquat enters dopaminergic neurons to induce neurotoxicity. Third, toxicity induced by paraquat is primarily mediated by redox cycling with cellular diaphorases such as NADPH oxidase and nitric oxide synthase (Day et al. 1999), leading to the generation of superoxide. Fourth, inside mitochondria, paraquat is not a complex I inhibitor per se (Richardson et al. 2005), although this is the presumed site where it is reduced to form superoxide (Cocheme and Murphy 2008).
When injected into mice, paraquat was reported to induce motor deficits and loss of nigral dopaminergic neurons in a dose- (Brooks et al. 1999; McCormack et al. 2002) and age- (McCormack et al. 2002; Thiruchelvam et al. 2003) dependent manner. The effects of paraquat on nigral dopaminergic neurons appear to be specific as γ-amino butyric acid (GABA) neurons in the nigral and striatal regions, glutamate neurons in the hippocampus, and dopaminergic neurons in the ventral tegmental area are not affected (Thiruchelvam et al. 2000b; McCormack et al. 2002). However, the damage induced by paraquat in dopaminergic cell bodies and terminals has not been consistently observed (Thiruchelvam et al. 2000b; Cicchetti et al. 2005). Furthermore, even in studies in which a loss in nigral dopaminergic neurons is detected, paraquat does not have an effect on striatal dopamine levels (Thiruchelvam et al. 2000b; McCormack et al. 2002). This lack of dopamine reduction might be related to the compensatory up-regulation of tyrosine hydroxylase activity in the striatum after paraquat injection (Thiruchelvam et al. 2000b; McCormack et al. 2002; Ossowska et al. 2005). When combined with manganese ethylenebisdithiocarbamate (Maneb), a more significant loss in dopaminergic neurons and a trend reduction (∼20%, not statistically significant) of striatal dopamine are produced (Thiruchelvam et al. 2000a,b). Even with this revised paraquat/Maneb protocol, however, it remains unclear how the motor deficits reported in this model can be attributed to such a modest loss of striatal dopamine. A similar argument can be made in a rat model in which paraquat is injected weekly for 24 wks but only ∼30% reduction in dopamine is detected (Ossowska et al. 2005). Given that paraquat is known to cause pulmonary toxicity (Clark et al. 1966; Smith and Heath 1976; Cicchetti et al. 2005; Saint-Pierre et al. 2006), which could affect motor performance, it may be necessary to assess whether l-dopa can alleviate motor deficits induced by paraquat. As discussed, this strategy has been used in the MPTP and 6-OHDA animal models to confirm that the observed motor deficit is linked to the damage of the nigrostriatal pathway.
The strength of the paraquat or paraquat/Maneb model is its potential relevance to environmental toxicants as a risk factor for developing PD. Both of these chemicals have been used in overlapping geographical areas. Epidemiological studies have suggested an increased risk for PD after paraquat exposure (Hertzman et al. 1990; Liou et al. 1997; Kamel et al. 2007; Ritz et al. 2009; Tanner et al. 2011). Additionally, paraquat treatment increases α-synuclein aggregates reminiscent of Lewy bodies in PD (Manning-Bog et al. 2002; Fernagut et al. 2007; Mak et al. 2010). Paraquat is also reported in one study to reduce noradrenergic neurons in the locus coeruleus (Fernagut et al. 2007). However, the lack of significant effect of paraquat on striatal dopamine depletion in the commonly used regimens may limit the use of this model to assess neuroprotective therapies for PD.
ROTENONE
Rotenone is a pesticide that is widely used to kill insects and nuisance fish in lakes. This chemical is found naturally in plants that belong to the family of Leguminosa. Because this is a natural product, it has also been used in organic farming. Due to its high lipophilicity, rotenone can readily cross the blood–brain barrier and enter all cells without being dependent on a specific transporter. The mechanism of toxicity of rotenone is primarily mediated by its potent complex I inhibition.
Based on the observations that MPP+ is a complex I inhibitor and that reduced function of this mitochondrial subunit has been reported in PD patients (Parker et al. 1989; Schapira et al. 1989), there has been an interest in using rotenone to model PD. In initial studies in which high doses of rotenone were used, widespread lesions beyond the nigrostriatal system were reported (Heikkila et al. 1985; Ferrante et al. 1997; Rojas et al. 2009). The rotenone model thus received little attention until Greenamyre and colleagues developed a chronic low-dose regimen (Betarbet et al. 2000). When infused continuously via a jugular vein cannula attached to a subcutaneous osmotic minipump, rotenone produces selective nigrostriatal neurodegeneration and α-synuclein-positive cytoplasmic inclusions. This study generated significant interest in the field because, first, despite widespread complex I inhibition in the brain, selective neurodegeneration occurs in the nigrostriatal pathway, strengthening the notion that nigral dopaminergic neurons are inherently more vulnerable. Second, the relationship between complex I inhibition and the pathogenic mechanism of cell death in PD is reinforced. Third, it suggests that a chronic low-dose neurotoxic regimen may be required to produce Lewy bodies. This rationale was subsequently applied in the chronic model of MPTP, in which continuous delivery of MPTP for 30 d using osmotic minipumps produces α-synuclein aggregates (Fornai et al. 2005), although the reproducibility of this observation has not been reported by other laboratories. Fourth, the rotenone model reinforces the theory that environmental agents may play a role in the pathogenesis of sporadic PD.
Despite its positive features, the rotenone infusion model has not been widely adopted. The primary concern is related to the high variability in animal sensitivity and the inability of other investigators to consistently reproduce the parkinsonian neuropathology and phenotype of this model (Hoglinger et al. 2003; Fleming et al. 2004b; Lapointe et al. 2004; Zhu et al. 2004). To address these concerns, a revised rotenone model has been developed (Cannon et al. 2009). With this protocol, daily i.p. injection using medium chain fatty acid as a specialized vehicle, rotenone is reported to produce a more consistent lesion in the nigrostriatal pathway, accompanied by the presence of α-synuclein and ubiquitin-positive inclusions and motor deficits that are reversed by apomorphine (Cannon et al. 2009). In a separate study, these investigators also report loss of neurons and the appearance of α-synuclein aggregation in the myenteric plexus, as well as a decrease in gastrointestinal motility (Drolet et al. 2009). Although promising, as with other newly developed models, the results of this modified rotenone model needs to be replicated by other laboratories.
OTHER NIGROSTRIATAL NEUROTOXIC MODELS
The following models have also been used to deplete striatal dopamine and induce neurotoxicity in the nigrostriatal pathway. However, unless the nature of study requires their specific effects, these models are not commonly used currently.
RESERPINE
Reserpine represents the earliest recognized pharmacological PD model. In their seminal work, Carlsson et al. (1957, 1959) showed that when rodents were treated with reserpine to deplete dopamine and other catecholamines in the brain, they developed akinesia, which could be successfully reversed by l-dopa. These early observations have led to our current appreciation for the role of striatal dopamine in motor function, to the discovery and current use of l-dopa as the cornerstone treatment of PD, as well as to the revolutionized strategy of generating PD animal models.
The depletion of monoamines and motor deficits induced by reserpine have been replicated in rabbits, guinea pigs, cats, and monkeys (Bezard et al. 1998). Reserpine is believed to mediate its effects by temporarily interfering with the storage of catecholamines in synaptic vesicles via magnesium- and ATP-dependent mechanisms (Bezard et al. 1998). Because the effects are transient, nonspecific to dopamine, and not neurotoxic to the nigrostriatal pathway, reserpine is no longer a popular choice for a PD model.
α-METHYL-PARA-TYROSINE
As an inhibitor of tyrosine hydroxylase, an enzyme involved in dopamine synthesis, α-methyl-p-tyrosine is another pharmacological agent used to deplete dopamine (Spectors et al. 1965; Corrodi and Hanson 1966). Similar to reserpine, the effect of α-methyl-p-tyrosine on dopamine depletion is transient and without neurodegeneration in the nigrostriatal structure. Thus this molecule shares the same limitations as the reserpine model. Occasionally, α-methyl-p-tyrosine is used in combination with reserpine to potentiate the effect of dopamine depletion (Carlsson and Carlsson 1989).
AMPHETAMINES
Amphetamines are widely abused psychostimulants. Besides their addictive properties, amphetamine derivatives such as p-chloroamphetamine (PCA), methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA), and fenfluramine are also highly neurotoxic. In the context of PD models, methamphetamine is more commonly used than other amphetamine derivatives. For brevity, only methamphetamine will be discussed; however, there are many overlapping effects among amphetamine derivatives (Przedborski and Tieu 2006).
The neurotoxicity of methamphetamine has been extensively studied in nonhuman primates, rats, and mice (Krasnova and Cadet 2009). Methamphetamine is toxic to both serotoninergic and dopaminergic terminals. Regarding the latter effect, methamphetamine primarily destroys dopaminergic nerve terminals in the striatum, nucleus accumbens, and frontal cortex, but not their cell bodies in the substantia nigra and ventral tegmental area (Axt et al. 1994; Krasnova and Cadet 2009), unless high-dose regimens are used (Trulson et al. 1985; Sonsalla et al. 1996). This feature of selective cell-part toxicity is strikingly different from those seen with paraquat, which primarily affects the cell bodies, and with those seen with MPTP and 6-OHDA, which are toxic to both cell bodies and terminals. This neurotoxic property of methamphetamine reinforces the notion that degeneration of the terminals and cell bodies can be governed by different processes.
Methamphetamine can enter dopaminergic neurons through both active uptake by the dopamine transporter (Zaczek et al. 1991a,b; Fumagalli et al. 1998) and simple diffusion across the plasma membrane. Inside dopaminergic neurons, methamphetamine induces dopamine release from the synaptic vesicles into the cytosol (Sulzer et al. 1995) where it is reversely transported by the dopamine transporter into the synaptic cleft (Sulzer et al. 1993). Through these actions, in combination with blocking dopamine degradation by monoamine oxidase (Scorza et al. 1997), methamphetamine induces dramatic release of dopamine into extracellular space. In vivo microdialysis experiments demonstrate that this release peaks within 1 h after systemic injection (Clausing and Bowyer 1999; Kita et al. 2000; Cui et al. 2009), leading to acute behavioral changes such as increased locomotor activity (Ricaurte et al. 1994; Segal and Kuczenski 1994).
The mechanism by which methamphetamine induces in vivo neurotoxicity remains controversial. There are two major schools of thought on this topic. One proposed mechanism is related to the dramatic increase in dopamine levels in the cytosol, resulting in oxidative stress (Cubells et al. 1994; Fumagalli et al. 1999; Guillot et al. 2008). Supporting this view are studies that show depleting intracellular dopamine with reserpine and α-methyl-p-tyrosine reduces (Gibb and Kogan 1979; Schmidt et al. 1985), and those that replenish dopamine, such as l-dopa, reproduce (Gibb and Kogan 1979; Thomas et al. 2008) methamphetamine toxicity. On the other hand, hyperthermia induced by methamphetamine has been suggested to be the culprit of its neurotoxicity. There is a significant body of evidence demonstrating that hyperthermia correlates with neurotoxicity (Ali et al. 1994; Albers and Sonsalla 1995; Yuan et al. 2010), and that neuroprotective effects of treatments such as reserpine and α-methyl-p-tyrosine are related to their hypothermic effects (Albers and Sonsalla 1995; Yuan et al. 2001, 2002). In a recent study in which mice are genetically modified to be deficient in brain dopamine synthesis either globally or unilaterally without altering the core temperature, methamphetamine neurotoxicity is comparable with control counterparts that have normal dopamine levels, suggesting dopamine is not necessary for methamphetamine neurotoxicity (Yuan et al. 2010). In light of these studies, core temperature should be assessed when neurotoxicity or neuroprotection is conducted in the methamphetamine animal models. The lack of PD-like brain pathology and the potential confounding factor of body temperature make methamphetamine a less than ideal model of PD.
ISOQUINOLINE DERIVATIVES
Isoquinoline and its derivatives can be found in the environment (Rommelspacher and Susilo 1985), in plants (Rommelspacher and Susilo 1985), in foodstuffs (Niwa et al. 1989), and in mammalian organs, including the brain (Nagatsu 1997; DeCuypere et al. 2008). In the brain, these molecules can be formed endogenously either enzymatically or nonenzymatically from dopamine and its metabolites (Nagatsu 1997; Naoi et al. 2002). Hence, these isoquinoline derivatives have been found in brain regions that are rich in dopamine (Musshoff et al. 2005). Because some isoquinoline derivatives are permeable to the blood–brain barrier (Niwa et al. 1988; Kikuchi et al. 1991; Thumen et al. 2002), exogenous sources may also account for accumulation of these molecules in the brain. The fact that these naturally occurring compounds bear structural similarity to MPTP/MPP+ makes them attractive candidates for being involved in the pathogenesis of PD. Indeed, the mechanisms of toxicity induced by isoquinolines have been shown to be very similar to those of MPP+, including their affinity for the dopamine transporter and complex I inhibition (Nagatsu 1997).
Despite these common features, however, conflicting neurotoxic properties have been reported between isoquinoline derivatives and between animal species when the same molecule is used. For example, subcutaneous injection of 1,2,3,4-tetrahydroisoquinoline (Nagatsu and Yoshida 1988; Yoshida et al. 1990) induces parkinsonian phenotypes and a reduction in dopamine level in monkeys, but not in mice—even at a high cumulative dose of 2.1g/kg (Perry et al. 1988). Additionally, the effects of 1,2,3,4-tetrahydroisoquinoline in monkeys are not reproduced by other isoquinoline derivatives that have similar properties to 1,2,3,4-tetrahydroisoquinoline (Yoshida et al. 1993). Furthermore, some tetrahydroisoquinoline derivatives such as 1-methyl-1,2,3,4-tetrahydroisoquinoline even have neuroprotective effects (Antkiewicz-Michaluk et al. 2004; Abe et al. 2005; Okuda et al. 2006). Because one isoquinoline derivative can be transformed to another one, for example, 1,2,3,4-tetrahydroisoquinoline (neurotoxic) can be converted by N-methyltransferase in vivo to 1-methyl-1,2,3,4-tetrahydroisoquinoline (neuroprotective), which is then further oxidized by monoamine oxidase to N-methyl-isoquinolinium ion (neurotoxic) (Nagatsu 1997), the overall neurotoxic properties of a particular isoquinoline isoform can be difficult to interpret or predict. Adding another layer of complexity to the isoquinoline-induced toxicity is the claim that these molecules down-regulate the expression of tyrosine hydroxylase rather than inducing the loss of dopaminergic structure (Lorenc-Koci et al. 2004), thus questioning the validity of previous studies that used reduced immunoreactivity of this enzyme as a marker for dopaminergic neurodegeneration. Together, these studies raise the question of the reliability of using isoquinolines to model PD. Although there was an interest in the isoquinoline models in the 1980s–1990s, a search of the literature reveals that the use of this model has declined over the last decade.
LIPOPOLYSACCHARIDE
Neuroinflammation has been proposed to be involved in PD (Przedborski 2007; Hirsch and Hunot 2009). Although all the neurotoxic molecules described above can elicit an inflammatory response, it is difficult to delineate whether neuroinflammation is the cause or consequence of injured dopaminergic neurons. The consensus appears to be that neuroinflammation is not the primary cause of cell death in these models, but rather, it may be a secondary event that perpetuates the vicious cycle of neurotoxicity. To study the role of neuroinflammation in causing cell death, the lipopolysaccharide (LPS) model is probably more appropriate.
LPS is a gram-negative bacterial endotoxin that activates microglia through the toll-like receptor-4 receptor, leading to the production of inflammatory cytokines and chemokines. LPS has been administered under various routes to induce nigrostriatal damage. It can be delivered stereotactically into the substantia nigra, medial forebrain bundle, or striatum to produce acute damage (Herrera et al. 2000). When injected unilaterally to the rat nigra, microglia activation and stable loss of nigral dopamine neurons occurs within 24 h (Iravani et al. 2005) along with a stable reduction in striatal dopamine to ∼60% of the contralateral side (Herrera et al. 2000). To induce a more chronic and progressive cell loss, LPS is infused into the rat supranigra for 2 wk using osmotic minipumps (Gao et al. 2002). Microglia activation can be detected within 3 d and reaches a sustained plateau from 2 to 8 wk after infusion of LPS. Significant loss of dopamine neurons (∼39%) was detectable at 6 wk and progressed to 69% loss at 10 wk after the initiation of LPS infusion. To avoid the technical challenges of stereotactic surgery, a single systemic i.p. injection of LPS has been described in mice (Qin et al. 2007). Although microglial activation occurs as early as 3 h after this peripheral injection, it takes 7 mo to observe a modest (23%) and 10 mo to have significant (47%) nigral dopaminergic cell loss.
Overall, the LPS model has not been widely used. Several reasons may contribute to this lack of popularity. First, stereotactic injection is a technical deterrent for many laboratories. Second, it takes too long to detect nigrostriatal damage with i.p. injection. Although this progressive cell loss can be an attractive feature, it is not feasible for most neuroprotective studies. Third, protein aggregation and extranigral pathology have not been reported. Fourth, despite it being a useful tool to establish that neuroinflammation can cause nigral cell loss, its relevance to PD remains uncertain as it is controversial that neuroinflammation is the primary cause of cell death in this disorder.
BASIC ASSESSMENTS OF THE NIGROSTRIATAL DOPAMINERGIC STRUCTURE AND FUNCTION
Depending on the nature of the study and the selected PD model, the methods required for the analysis of neuropathology and function may vary. However, whether planning to study neurodegeneration or neuroprotection using neurotoxic models, it is common to assess the integrity and function of the nigrostriatal pathway. Over the years, there have been some well-developed and accepted techniques that are commonly used for these purposes. This section will highlight such basic methods and equipment with an emphasis on murine models. Detailed step-by-step procedures are beyond the scope of this article and readers are encouraged to consult the recommended references.
Quantification of Dopaminergic Neurons in the Substantia Nigra Par Compacta
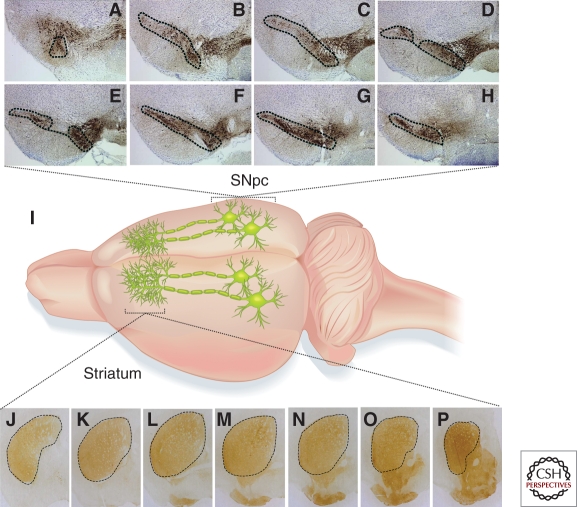
In the mesencephalon, there are three groups of dopaminergic neurons classified historically as A8, A9, and A10 (Dahlstrom and Fuxe 1964; Hokfelt et al. 1984; Smith and Kieval 2000; Zaborszky and Vadasz 2001). The A8 group resides in the retrorubral area, whereas the A9 and A10 groups belong to the substantia nigra and ventral tegmental area, respectively (Fig. 2). The estimated average number of dopaminergic neurons in an adult mouse nigra is commonly reported to be ∼8000–14,000. The number may vary slightly depending on the strain (Zaborszky and Vadasz 2001). The distribution of dopaminergic neurons in the nigra is not homogenous. As illustrated in Figure 2, when the nigra is sectioned coronally, there is a significant difference in the density of dopaminergic neurons between the caudal and rostral regions. It is necessary, therefore, to sample the population of dopaminergic neurons at different levels throughout the entire nigra. The past practice of comparing the number of dopaminergic neurons from only one nigral section between animals should be avoided. One way to sample the entire nigra efficiently is to count dopaminergic neurons systematically at a regular section interval, as illustrated in Figure 2. To count these neurons, the gold standard is to use an unbiased stereological cell counting with optical dissector system (West et al. 1991, 1993, 1996; Tieu et al. 2003). Major components required for this method are computerized stereology software and a microscope with a motorized stage.
Figure 2.
Topographical distribution of dopaminergic neurons in the nigral and striatal regions. The cell bodies of dopaminergic neurons that reside in the substantia nigra pars compacta (SNpc) project their terminals to the dorsal striatum where dopamine is released (I). To illustrate the heterogeneous distribution of dopaminergic neurons in the entire SNpc (outlined) spanning from the caudal (A) to rostral regions (H), coronal sections (30 µm) are presented at fourth section intervals. Similarly, the distribution of dopaminergic terminals is not homogenous in the striatum (J–P). The density is relatively low in the caudal (J) as compared to the rostral region (P). Images are captured at eight section intervals of the striatum. Dopaminergic neurons and terminals are immunostained with an antibody against tyrosine hydroxylase and visualized using 3,3′-diaminobenzidine.
Quantification of Dopaminergic Terminals in the Striatum
The projection of terminals to the striatum from the nigral dopaminergic neurons has been well established (Joel and Weiner 2000). Although the total density of striatal dopaminergic terminals frequently correlates with the number of their cell bodies in the nigra, it is not uncommon to observe differential damage or protection between these two structures. For example, as discussed, in the paraquat model, the dopaminergic cell bodies are more vulnerable than their terminals. On the other hand, striatal terminals are more sensitive to methamphetamine, MPTP, or 6-OHDA toxicity. Quantifying striatal dopaminergic terminals is therefore also informative.
Similar to the uneven distribution of dopaminergic neurons throughout the nigra, the density of dopaminergic terminals is also not homogenous in the striatum. As illustrated in Figure 2 and previous studies (Tassin et al. 1976; Scally et al. 1978; Widmann and Sperk 1986), dopaminergic innervation increases from the caudal to rostral part. Accordingly, when quantifying dopaminergic terminals, representative regions of the striatum should be assessed (Fig. 2). The density of striatal dopaminergic terminals can be digitized and then quantified based on optical density or fiber density of tyrosine hydroxylase immunoreactivity (Tieu et al. 2003; Fernagut et al. 2007). As an alternative to immunohistochemistry, fresh striatal tissues can be isolated for immunoblotting using tyrosine hydroxylase or dopamine transporter as markers to assess for the levels of striatal dopaminergic terminals. Some potential disadvantages of this later approach are the limited quantity of striatal tissue and the lack of resolution of the size and pattern of the lesion.
Quantification of Dopamine Content in the Striatum
In addition to structural analysis (cell body and terminal), it is also critical to assess the function of the nigrostriatal pathway. Measuring the amount of dopamine produced in the striatum and assessing motor movement (see below) are some such functional studies. The best way to measure dopamine is to use reverse-phase high-performance liquid chromatography (HPLC) coupled with an electrochemical detector. With this method, samples prepared from striatal tissues are injected into HPLC. Figure 3 provides an example of what to expect when a striatal sample is analyzed using HPLC. To maximize efficiency and consistency, as well as to minimize the number of required animals, the brain can be processed in such a way that data for striatal dopamine content, striatal terminal density, and nigral cell counts can be obtained within the same animal. For this approach, freshly removed brains are coronally sectioned at ∼1–2 mm caudal to the optic chiasm. The caudal half containing the substantia nigra is used for stereological cell counts, whereas the rostral half is divided midsagittally with one hemisphere used for striatal density and the other hemisphere used for HPLC measurement of dopamine.
Figure 3.
High-performance liquid chromatography (HPLC) chromatogram of catecholamines in striatal tissue. The striatum was freshly dissected and processed for HPLC measurement as described (Cui et al. 2009). Samples were eluted on a narrow-bore (inner diameter: 2 mm) reverse-phase C18 column (MD-150, ESA Inc) using a 12-channel CoulArray 5600A (ESA). A highly sensitive amperometric microbore cell (model 5041, ESA Inc.) was used to analyze the content of dopamine with a potential set at +220 mV. The striatum is a major “input” structure of the basal ganglia. In addition to receiving extensive dopaminergic terminals from the substantia nigra, the striatum also contains serotonergic terminals from the dorsal raphe nuclei. Dopamine and serotonin, as well as their metabolites, therefore, are often detectable in the same striatal samples. Abbreviations: DA (dopamine), DOPAC (3,4-dihydroxyphenylacetic acid, a metabolite of DA), HVA (homovanillic acid, a metabolite of DA), 3-MT (3-methoxytyramine, a metabolite of DA), 5-HT (serotonin), 5-HIAA (5-hydroxyindoleacetic acid, a metabolite of 5-HT), DHBA (3,4-dihydroxybenzylamine, internal control for the measurement of catecholamines).
Detection of Lewy Body-like Aggregation
As discussed, Lewy bodies are a neuropathological hallmark in PD. Although the neurotoxic property of this feature is not fully established, it does suggest the presence of misfolded protein and inefficiency in protein processing or degradation. Whether studying neurodegenerative or neuroprotective processes in PD models, it may be desirable to determine the presence or absence of protein aggregations. Several methods have been used. For example, thioflavin S can be used to identify the presence of fibrillary protein (Conway et al. 2000; Manning-Bog et al. 2002). However, it is more common to use an antibody to assess the pattern and level of intracellular α-synuclein (Betarbet et al. 2000; Vila et al. 2000; Fornai et al. 2005). Because misfolded or fibrillary α-synuclein is relatively resistant to proteolysis (Conway et al. 2000; Giasson et al. 2001; Miake et al. 2002), one simple way to detect the presence of α-synuclein aggregates is to treat brain sections with proteinase K, followed by immunohistochemistry using an antibody against α-synuclein (Fernagut et al. 2007; Beach et al. 2008).
Assessment of Motor Function
The functional relationship between the depletion of striatal dopamine and the motor deficits in PD was discovered more than 50 years ago (Carlsson et al. 1957; Carlsson 1959). Since this discovery, subsequent PD animal models have been generated with the objectives of inducing a loss in nigrostriatal dopaminergic structure and dopamine content to accurately reproduce PD pathology and l-dopa responsive motor deficits as seen in PD. Many behavioral tests are therefore designed to assess motor phenotypes linked to the nigrostriatal function.
Behavioral tests routinely used to quantify locomotor activities in animal models of PD include locomotor activity, rotation, rotarod, stride length of the paws, and pole test. For a more in-depth discussion of these methods, including their strengths and weaknesses, readers are encouraged to consult other reviews (Sedelis et al. 2001; Brooks and Dunnett 2009; Taylor et al. 2010). Briefly, one simple and basic way to assess locomotor activity is to use automated open-field chambers. Typically, mice are placed in transparent chambers that are equipped with horizontal and vertical infrared photobeams. The movement of each animal is tracked and quantified based on photobeam breaks that are registered in a computer and processed for parameters such as jumps, traveled distance, and vertical, ambulatory, or stereotypical movements in different bin sizes over different periods of time. Most PD models have reduced locomotor activity (Taylor et al. 2010). The rotation test has been used for decades to assess the motor asymmetry in the unilateral 6-OHDA lesion rat, and more recently in mice (Nishimura et al. 2003; Iancu et al. 2005). As discussed previously, subsequent to the 6-OHDA injection, the magnitude of the nigrostriatal lesion correlates with the circling motor behavior (Ungerstedt 1968; Ungerstedt and Arbuthnott 1970; Hefti et al. 1980; Przedborski et al. 1995). The direction of rotation is ipsilateral to the lesion if dopamine released from the intact terminals is induced by compounds such as amphetamine; however, an agonist such as apomorphine stimulates the sensitized dopaminergic receptors on the lesioned side and will lead to contralateral rotation. This rotation can be quantified using a rotameter test bowl (Brooks and Dunnett 2009). To measure abnormal movement that is analogous to the shuffling gait in PD patients, the “footprint” test can be used to measure the stride length of the paws. With this test, the fore and hind limbs of the animal are inked with different colors and the stride length is quantified after a walk down the narrow corridor (Fernagut et al. 2002; Brooks and Dunnett 2009). To measure body coordination and balance, either the rotarod or pole test can be used. In the rotarod test (Dunham and Miya 1957; Rozas et al. 1998; Brooks and Dunnett 2009), the most commonly used behavioral test, a mouse is placed on a rod that can rotate at a fixed or an accelerating speed. The length of time an animal can maintain balance and stay on the rotating rod is recorded. In the pole test (Ogawa et al. 1985; Fleming et al. 2004a), the animal is placed facing upward on top of a vertical wooden or wire-mesh pole. The time that it takes the animal to orient downward and the total time that it takes to descend down to the base of the pole are recorded.
CONCLUDING REMARKS
Since the initial discovery by Carlsson and colleagues in the 1950s that l-dopa restored motor deficits induced by reserpine (Carlsson et al. 1957; Carlsson 1959), many animal models of PD have been developed with the primary objective of improving PD-like pathology and phenotypes in existing ones for bettering the utility of these models for developing treatments. Together, these models have contributed to the development of some current PD treatments such as l-dopa, dopamine agonists, and monoamine oxidase-B inhibitors. Our current understanding of the basal ganglia circuitry, pathogenic mechanisms, and potential therapeutic targets for PD also greatly benefited from these models. However, despite these accomplishments, current models still have significant shortcomings. Some such limitations are highlighted in the models discussed in this article.
Confronted with the pros and cons of numerous PD models, a new investigator may find it challenging to make a selection. Because there is no perfect model, the decision should be carefully balanced by considerations such as the following: (1) What is the nature and the goal of the study? If the loss of the nigrostriatal pathway is required to address the question, then a neurotoxic model is necessary. However, if a target known to cause PD is the primary interest, then perhaps a genetic model is relevant with the caveats that these animals do not display appreciable neurodegeneration, and require time to develop motor deficits. (2) How technically involved is the model? i.p. injection certainly is more convenient and consistent than stereotactic delivery into the brain or the implantation of osmotic minipumps. (3) How reproducible is the model? Once an investigator has navigated through such questions and arrived at a decision, the technical aspects will follow that particular model. By providing an overview of the methods commonly used to assess the nigrostriatal structure and function, this article may also provide a foundation to guide a new investigator through the next step of entering the PD research arena.
ACKNOWLEDGMENTS
This work was supported in part by the U.S. National Institutes of Health Grants No. ES014899 and No. ES17470. I would like to thank Phillip M. Rappold and Adrianne Chesser for proofreading this manuscript. I am also thankful for the illustrations prepared by Meghan Shoemaker.
Footnotes
Editor: Serge Przedborski
Additional Perspectives on Parkinson's Disease available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abe K, Saitoh T, Horiguchi Y, Utsunomiya I, Taguchi K 2005. Synthesis and neurotoxicity of tetrahydroisoquinoline derivatives for studying Parkinson’s disease. Biol Pharm Bull 28: 1355–1362 [DOI] [PubMed] [Google Scholar]
- Agid Y, Javoy F, Glowinski J, Bouvet D, Sotelo C 1973. Injection of 6-hydroxydopamine into the substantia nigra of the rat. II. Diffusion and specificity. Brain Res 58: 291–301 [DOI] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK 1995. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: Pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther 275: 1104–1114 [PubMed] [Google Scholar]
- Ali SF, Newport GD, Holson RR, Slikker W Jr, Bowyer JF 1994. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res 658: 33–38 [DOI] [PubMed] [Google Scholar]
- Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, Srinivasan S, Greene JG 2007. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp Neurol 207: 4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R, Watson DG, Best SA, Midgley JM, Wenlong H, Petty RK 1993. The determination of hydroxydopamines and other trace amines in the urine of parkinsonian patients and normal controls. Neurochem Res 18: 1175–1177. [DOI] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L, Wardas J, Michaluk J, Romaska I, Bojarski A, Vetulani J 2004. Protective effect of 1-methyl-1,2,3,4-tetrahydroisoquinoline against dopaminergic neurodegeneration in the extrapyramidal structures produced by intracerebral injection of rotenone. Int J Neuropsychopharmacol 7: 155–163 [DOI] [PubMed] [Google Scholar]
- Axt KJ, Mamounas LA, Molliver ME 1994. Structural features of amphetamine neurotoxicity in the brain. In Amphetamine and its analogs: Psychopharmacology, toxicology, and abuse (ed. Cho AK, Segal DS), pp. 315–367 Academic, New York [Google Scholar]
- Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Leverenz JB, Roncaroli F, Buttini M, Hladik CL, et al. 2008. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 116: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT 2000. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3: 1301–1306 [DOI] [PubMed] [Google Scholar]
- *.Bezard E 2011. Parkinson’s disease: Animal models—primates Park. Cold Spring Harb Perspect Med 10.1101/cshperspect.a009308 [DOI] [PMC free article] [PubMed]
- Bezard E, Przedborski S 2011. A tale on animal models of Parkinson’s disease. Mov Disord 26: 993–1002 [DOI] [PubMed] [Google Scholar]
- Bezard E, Imbert C, Gross CE 1998. Experimental models of Parkinson’s disease: From the static to the dynamic. Rev Neurosci 9: 71–90 [DOI] [PubMed] [Google Scholar]
- Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, et al. 2002. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci 99: 2344–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM 2001. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65: 135–172 [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del TK 2004. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318: 121–134 [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Armida M, Cassano T, Pezzola A, Potenza RL, Morgese MG, Popoli P, Alleva E 2008. Nonmotor symptoms in Parkinson’s disease: Investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J Neurosci Res 86: 2050–2061 [DOI] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB 2009. Tests to assess motor phenotype in mice: A user’s guide. Nat Rev Neurosci 10: 519–529 [DOI] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ 1999. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res 823: 1–10 [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT 2010. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res 184: 17–33 [DOI] [PubMed] [Google Scholar]
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT 2009. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis 34: 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A 1959. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev 11: 490–493 [PubMed] [Google Scholar]
- Carlsson M, Carlsson A 1989. Dramatic synergism between MK-801 and clonidine with respect to locomotor stimulatory effect in monoamine-depleted mice. J Neural Transm 77: 65–71 [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T 1957. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 180: 1200. [DOI] [PubMed] [Google Scholar]
- Chan H, Paur H, Vernon AC, Zabarsky V, Datla KP, Croucher MJ, Dexter DT 2010. Neuroprotection and functional recovery associated with decreased microglial activation following selective activation of mGluR2/3 receptors in a rodent model of Parkinson’s disease. Parkinsons Dis pii: 190450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Yates L, Martinez-Martin P 2005. The non-motor symptom complex of Parkinson’s disease: A comprehensive assessment is essential. Curr Neurol Neurosci Rep 5: 275–283 [DOI] [PubMed] [Google Scholar]
- Christine CW, Langston JW, Turner RS, Starr PA 2009. The neurophysiology and effect of deep brain stimulation in a patient with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism. J Neurosurg 110: 234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, Ficke BW, Gross RE 2005. Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiol Dis 20: 360–371 [DOI] [PubMed] [Google Scholar]
- Clark DG, McElligott TF, Hurst EW 1966. The toxicity of paraquat. Br J Ind Med 23: 126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausing P, Bowyer JF 1999. Time course of brain temperature and caudate/putamen microdialysate levels of amphetamine and dopamine in rats after multiple doses of d-amphetamine. Ann NY Acad Sci 890: 495–504 [DOI] [PubMed] [Google Scholar]
- Cocheme HM, Murphy MP 2008. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem 283: 1786–1798 [DOI] [PubMed] [Google Scholar]
- Cohen G, Werner P 1994. Free radicals, oxidative stress, and neurodegeneration. In Neurodegenerative diseases (ed. Calne DB), pp. 139–161 W.B. Saunders, Philadelphia [Google Scholar]
- Conway KA, Harper JD, Lansbury PT Jr 2000. Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry 39: 2552–2563 [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Defilippo R, Rodino P, Nappi G, Nistico G 1991. Evidence that paraquat is able to cross the blood-brain barrier to a different extent in rats of various age. Funct Neurol 6: 385–391 [PubMed] [Google Scholar]
- Corrodi H, Hanson LC 1966. Central effects of an inhibitor of tyrosine hydroxylation. Psychopharmacologia 10: 116–125 [DOI] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D 1994. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci 14: 2260–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, et al. 2009. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci 106: 8043–8048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtius HC, Wolfensberger M, Steinmann B, Redweik U, Siegfried J 1974. Mass fragmentography of dopamine and 6-hydroxydopamine. Application to the determination of dopamine in human brain biopsies from the caudate nucleus. J Chromatogr 99: 529–540 [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K 1964. Localization of monoamines in the lower brain stem. Experientia 20: 398–399 [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S 2003. Parkinson’s disease: Mechanisms and models. Neuron 39: 889–909 [DOI] [PubMed] [Google Scholar]
- Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ 1979. Chronic parkinsonism secondary to intravenous injection of meperidine analogs. Psychiatry Res 1: 249–254 [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL 2010. Genetic animal models of Parkinson’s disease. Neuron 66: 646–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS 1999. A mechanism of paraquat toxicity involving inclusions in MPTP-treated monkeys. Ann Neurol 20: 449–455 [Google Scholar]
- DeCuypere M, Lu Y, Miller DD, LeDoux MS 2008. Regional distribution of tetrahydroisoquinoline derivatives in rodent, human, and Parkinson’s disease brain. J Neurochem 107: 1398–1413 [DOI] [PubMed] [Google Scholar]
- Drolet RE, Cannon JR, Montero L, Greenamyre JT 2009. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol Dis 36: 96–102 [DOI] [PubMed] [Google Scholar]
- Dunham NW, Miya TS 1957. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 46: 208–209 [DOI] [PubMed] [Google Scholar]
- Faull RL, Laverty R 1969. Changes in dopamine levels in the corpus striatum following lesions in the substantia nigra. Exp Neurol 23: 332–340 [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Labattu B, Tison F 2002. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Methods 113: 123–130 [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF 2007. Behavioral and histopathological consequences of paraquat intoxication in mice: Effects of alpha-synuclein over-expression. Synapse 61: 991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Schulz JB, Kowall NW, Beal MF 1997. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res 753: 157–162 [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF 2004a. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci 24: 9434–9440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, Chesselet MF 2004b. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol 187: 418–429 [DOI] [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, et al. 2005. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci 102: 3413–3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA 1986. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann Neurol 20: 449–455 [DOI] [PubMed] [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Langston JW 1993. Similarities and differences between MPTP-induced parkinsonism and Parkinson’s disease: Neuropathologic considerations. Adv Neurol 60: 600–608 [PubMed] [Google Scholar]
- Fox SH, Brotchie JM 2010. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog Brain Res 184: 133–157 [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Archer T 1994. MPTP-induced behavioural and biochemical deficits: A parametric analysis. J Neural Transm Park Dis Dement Sect 7: 123–132 [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG 1998. Role of dopamine transporter in methamphetamine-induced neurotoxicity: Evidence from mice lacking the transporter. J Neurosci 18: 4861–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG 1999. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci 19: 2424–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B 2002. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson’s disease. J Neurochem 81: 1285–1297 [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IV, Trojanowski JQ, Lee VM 2001. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem 276: 2380–2386 [DOI] [PubMed] [Google Scholar]
- Gibb JW, Kogan FJ 1979. Influence of dopamine synthesis on methamphetamine-induced changes in striatal and adrenal tyrosine hydroxylase activity. Naunyn Schmiedebergs Arch Pharmacol 310: 185–187 [DOI] [PubMed] [Google Scholar]
- Giovanni A, Sieber B-A, Heikkila RE, Sonsalla PK 1994. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 1: Systemic administration. J Pharmacol Exp Ther 270: 1000–1007 [PubMed] [Google Scholar]
- Graham DG 1978. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol 14: 633–643 [PubMed] [Google Scholar]
- Guillot TS, Shepherd KR, Richardson JR, Wang MZ, Li Y, Emson PC, Miller GW 2008. Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J Neurochem 106: 2205–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F, Melamed E, Wurtman RJ 1980. Partial lesions of the dopaminergic nigrostriatal system in rat brain: Biochemical characterization. Brain Res 195: 123–137 [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Nicklas WJ, Vays I, Duvoisin RC 1985. Dopaminergic toxicity of rotenone and the MPTP ion after their stereotaxic administration to rats: Implication for the mechanism of MPTP toxicity. Neurosci Lett 62: 389–394 [DOI] [PubMed] [Google Scholar]
- Herrera AJ, Castano A, Venero JL, Cano J, Machado A 2000. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis 7: 429–447 [DOI] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Bowering D, Snow B, Calne D 1990. Parkinson’s disease: A case-control study of occupational and environmental risk factors. Am J Ind Med 17: 349–355 [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S 2009. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol 8: 382–397 [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Feger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC 2003. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem 84: 491–502 [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Martensson R, Bjorklund A, Kleinau S, Goldstein M 1984. Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In Handbook of chemical neuroanatomy. Classical transmitters in the CNS, Part I (ed. Bjorklund A, Hokfelt T), pp. 277–379 Elsevier, Amsterdam [Google Scholar]
- Hornykiewicz O, Kish SJ 1987. Biochemical pathophysiology of Parkinson’s disease. In Parkinson’s disease (ed. Yahr M, Bergmann KJ), pp. 19–34 Raven Press, New York: [PubMed] [Google Scholar]
- Iancu R, Mohapel P, Brundin P, Paul G 2005. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162: 1–10 [DOI] [PubMed] [Google Scholar]
- Ilijic E, Guzman JN, Surmeier DJ 2011. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis 10.1016/j.nbd.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, Leung CC, Sadeghian M, Haddon CO, Rose S, Jenner P 2005. The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur J Neurosci 22: 317–330 [DOI] [PubMed] [Google Scholar]
- Jain S 2011. Multi-organ autonomic dysfunction in Parkinson disease. Parkinsonism Relat Disord 17: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE 1995. 6-hydroxydopamine lesion of the rat substantia nigra: Time course and morphology of cell death. Neurodegeneration 4: 131–137 [DOI] [PubMed] [Google Scholar]
- Jiang H, Jackson-Lewis V, Muthane U, Dollison A, Ferreira M, Espinosa A, Parsons B, Przedborski S 1993. Adenosine receptor antagonists potentiate dopamine receptor agonist-induced rotational behavior in 6-hydroxydopamine-lesioned rats. Brain Res 613: 347–351 [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I 2000. The connections of the dopaminergic system with the striatum in rats and primates: An analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96: 451–474 [DOI] [PubMed] [Google Scholar]
- Jonsson G 1980. Chemical neurotoxins as denervation tools in neurobiology. Annu Rev Neurosci 3: 169–187 [DOI] [PubMed] [Google Scholar]
- Jonsson G 1983. Chemical lesioning techniques: Monoamine neurotoxins. In Handbook of chemical neuroanatomy Vol 1: Methods in chemical neuroanatomy (ed. Björklund A, Hökfelt T), pp. 463–507 Elsevier, Amsterdam [Google Scholar]
- Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, Comyns K, Goldman S, Korell M, Langston J, et al. 2007. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol 165: 364–374 [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Nagatsu Y, Makino Y, Mashino T, Ohta S, Hirobe M 1991. Metabolism and penetration through blood-brain barrier of parkinsonism-related compounds. 1,2,3,4-Tetrahydroisoquinoline and 1-methyl-1,2,3,4-tetrahydroisoquinoline. Drug Metab Dispos 19: 257–262 [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Burger C, Winkler C, Muzyczka N, Mandel RJ, Bjorklund A 2002. Reversal of motor impairments in parkinsonian rats by continuous intrastriatal delivery of L-dopa using rAAV-mediated gene transfer. Proc Natl Acad Sci 99: 4708–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Matsunari Y, Saraya T, Shimada K, O’Hara K, Kubo K, Wagner GC, Nakashima T 2000. Methamphetamine-induced striatal dopamine release, behavior changes and neurotoxicity in BALB/c mice. Int J Dev Neurosci 18: 521–530 [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL 2009. Methamphetamine toxicity and messengers of death. Brain Res Rev 60: 379–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ 1987. Parkinson’s disease and megacolon: Concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology 37: 1253–1255 [DOI] [PubMed] [Google Scholar]
- Langston JW 2006. The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann Neurol 59: 591–596 [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Irwin I 1983. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219: 979–980 [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D 1999. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 46: 598–605 [DOI] [PubMed] [Google Scholar]
- Lapointe N, St-Hilaire M, Martinoli MG, Blanchet J, Gould P, Rouillard C, Cicchetti F 2004. Rotenone induces non-specific central nervous system and systemic toxicity. FASEB J 18: 717–719 [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC 1997. Environmental risk factors and Parkinson’s disease: A case-control study in Taiwan. Neurology 48: 1583–1588 [DOI] [PubMed] [Google Scholar]
- Lorenc-Koci E, Antkiewicz-Michaluk L, Wardas J, Zapala M, Wieronska J 2004. Effect of 1,2,3,4,-tetrahydroisoquinoline administration under conditions of CYP2D inhibition on dopamine metabolism, level of tyrosine hydroxylase protein and the binding of [3H]GBR 12,935 to dopamine transporter in the rat nigrostriatal, dopaminergic system. Brain Res 1009: 67–81 [DOI] [PubMed] [Google Scholar]
- Luthman J, Fredriksson A, Sundstrom E, Jonsson G, Archer T 1989. Selective lesion of central dopamine or noradrenaline neuron systems in the neonatal rat: Motor behavior and monoamine alterations at adult stage. Behav Brain Res 33: 267–277 [DOI] [PubMed] [Google Scholar]
- Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA 2010. Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem 285: 13621–13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmfors T, Sachs C 1968. Degeneration of adrenergic nerves produced by 6-hydroxydopamine. Eur J Pharmacol 3: 89–92 [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA 2002. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: Paraquat and alpha-synuclein. J Biol Chem 277: 1641–1644 [DOI] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM 2010. The impact of genetic research on our understanding of Parkinson’s disease. Prog Brain Res 183: 21–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA 2003. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J Neurochem 85: 82–86 [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA 2002. Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 10: 119–127 [DOI] [PubMed] [Google Scholar]
- Meredith GE, Sonsalla PK, Chesselet MF 2008. Animal models of Parkinson’s disease progression. Acta Neuropathol 115: 385–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake H, Mizusawa H, Iwatsubo T, Hasegawa M 2002. Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem 277: 19213–19219 [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Sone N, Saitoh T 1987. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem 48: 1787–1793 [DOI] [PubMed] [Google Scholar]
- Musshoff F, Lachenmeier DW, Schmidt P, Dettmeyer R, Madea B 2005. Systematic regional study of dopamine, norsalsolinol, and (R/S)-salsolinol levels in human brain areas of alcoholics. Alcohol Clin Exp Res 29: 46–52 [DOI] [PubMed] [Google Scholar]
- Nagatsu T 1997. Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci Res 29: 99–111 [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Yoshida M 1988. An endogenous substance of the brain, tetrahydroisoquinoline, produces parkinsonism in primates with decreased dopamine, tyrosine hydroxylase and biopterin in the nigrostriatal regions. Neurosci Lett 87: 178–182 [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Akao Y, Yi H 2002. Dopamine-derived endogenous N-methyl-(R)-salsolinol: Its role in Parkinson’s disease. Neurotoxicol Teratol 24: 579–591 [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE 1985. Inhibition of NADH-linked oxidation in brain mitochondria by MPP+, a metabolite of the neurotoxin MPTP. Life Sci 36: 2503–2508 [DOI] [PubMed] [Google Scholar]
- Nishimura F, Yoshikawa M, Kanda S, Nonaka M, Yokota H, Shiroi A, Nakase H, Hirabayashi H, Ouji Y, Birumachi J, et al. 2003. Potential use of embryonic stem cells for the treatment of mouse parkinsonian models: Improved behavior by transplantation of in vitro differentiated dopaminergic neurons from embryonic stem cells. Stem Cells 21: 171–180 [DOI] [PubMed] [Google Scholar]
- Niwa T, Takeda N, Tatematsu A, Matsuura S, Yoshida M, Nagatsu T 1988. Migration of tetrahydroisoquinoline, a possible parkinsonian neurotoxin, into monkey brain from blood as proved by gas chromatography-mass spectrometry. J Chromatogr 452: 85–91 [DOI] [PubMed] [Google Scholar]
- Niwa T, Yoshizumi H, Tatematsu A, Matsuura S, Nagatsu T 1989. Presence of tetrahydroisoquinoline, a parkinsonism-related compound, in foods. J Chromatogr 493: 347–352 [DOI] [PubMed] [Google Scholar]
- Ogawa N, Hirose Y, Ohara S, Ono T, Watanabe Y 1985. A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol 50: 435–441 [PubMed] [Google Scholar]
- Okuda K, Kotake Y, Ohta S 2006. Parkinsonism-preventing activity of 1-methyl-1,2,3,4-tetrahydroisoquinoline derivatives in C57BL mouse in vivo. Biol Pharm Bull 29: 1401–1403 [DOI] [PubMed] [Google Scholar]
- Ossowska K, Wardas J, Smialowska M, Kuter K, Lenda T, Wieronska JM, Zieba B, Nowak P, Dabrowska J, Bortel A, et al. 2005. A slowly developing dysfunction of dopaminergic nigrostriatal neurons induced by long-term paraquat administration in rats: An animal model of preclinical stages of Parkinson’s disease? Eur J Neurosci 22: 1294–1304 [DOI] [PubMed] [Google Scholar]
- Parker WD Jr, Boyson SJ, Parks JK 1989. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26: 719–723 [DOI] [PubMed] [Google Scholar]
- Perry TL, Jones K, Hansen S 1988. Tetrahydroisoquinoline lacks dopaminergic nigrostriatal neurotoxicity in mice. Neurosci Lett 85: 101–104 [DOI] [PubMed] [Google Scholar]
- Porter CC, Totaro JA, Stone CA 1963. Effect of 6-hydroxydopamine and some other compounds on the concentration of norepinephrine in the hearts of mice. J Pharmacol Exp Ther 140: 308–316 [PubMed] [Google Scholar]
- Porter CC, Totaro JA, Burcin A 1965. The relationship between radioactivity and norepinephrine concentrations in the brains and hearts of mice following administration of labeled methyldopa or 6-hydroxydopamine. J Pharmacol Exp Ther 150: 17–22 [PubMed] [Google Scholar]
- Przedborski S 2007. Neuroinflammation and Parkinson’s disease. Handb Clin Neurol 83: 535–551 [DOI] [PubMed] [Google Scholar]
- Przedborski S, Tieu K 2006. Toxic animal models. In Neurodegenerative diseases (ed. Beal MF, et al. ), pp. 196–221 Cambridge University Press, Cambridge [Google Scholar]
- Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM 1995. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience 67: 631–647 [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini A, Jakowec M, Petzinger G, Miller R, Akram M 2001. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): A technical review of its utility and safety. J Neurochem 76: 1265–1274 [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT 2007. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55: 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold PM, Tieu K 2010. Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics 7: 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Sabol KE, Seiden LS 1994. Functional consequences of neurotoxic amphetamine exposure. In Amphetamine and its analogs: Psychopharmacology, toxicology, and abuse (ed. Cho AK, Segal DS), pp. 297–313 Academic, New York [Google Scholar]
- Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW 2005. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci 88: 193–201 [DOI] [PubMed] [Google Scholar]
- Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J 2009. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect 117: 964–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Simola N, Kermath BA, Kane JR, Schallert T, Gonzalez-Lima F 2009. Striatal neuroprotection with methylene blue. Neuroscience 163: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelspacher H, Susilo R 1985. Tetrahydroisoquinolines and beta-carbolines: Putative natural substances in plants and mammals. Prog Drug Res 29: 415–459 [DOI] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA 2006. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 12: 1259–1268 [DOI] [PubMed] [Google Scholar]
- Rozas G, López-Martín E, Guerra MJ, Labandeira-García JL 1998. The overall rod performance test in the MPTP-treated-mouse model of parkinsonism. J Neurosci Meth 83: 165–175 [DOI] [PubMed] [Google Scholar]
- Saint-Pierre M, Tremblay ME, Sik A, Gross RE, Cicchetti F 2006. Temporal effects of paraquat/maneb on microglial activation and dopamine neuronal loss in older rats. J Neurochem 98: 760–772 [DOI] [PubMed] [Google Scholar]
- Saner A, Thoenen H 1971. Model experiments on the molecular mechanism of action of 6-hydroxydopamine. Mol Pharmacol 7: 147–154 [PubMed] [Google Scholar]
- Sarre S, Yuan H, Jonkers N, Van HA, Ebinger G, Michotte Y 2004. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J Neurochem 90: 29–39 [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH 1994. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59: 401–415 [DOI] [PubMed] [Google Scholar]
- Scally MC, Ulus IH, Wurtman RJ, Pettibone DJ 1978. Regional distribution of neurotransmitter-synthesizing enzymes and substance P within the rat corpus striatum. Brain Res 143: 556–560 [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD 1989. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1: 1269. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW 1985. Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther 233: 539–544 [PubMed] [Google Scholar]
- Scorza MC, Carrau C, Silveira R, Zapata-Torres G, Cassels BK, Reyes-Parada M 1997. Monoamine oxidase inhibitory properties of some methoxylated and alkylthio amphetamine derivatives: Structure-activity relationships. Biochem Pharmacol 54: 1361–1369 [DOI] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RK, Huston JP 2001. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav Brain Res 125: 109–125 [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R 1994. Behavioral pharmacology of amphetamine. In Amphetamine and its analogs: Psychopharmacology, toxicology, and abuse (ed. Cho AK, Segal DS), pp. 115–150 Academic, New York [Google Scholar]
- Senoh S, Witkop B 1959. Nonenzymatic conversions of dopamine to norepinephrine and trihydroxyphenethylamine. J Am Chem Soc 81: 6222–6231 [Google Scholar]
- Senoh S, Creveling CR, Udenfriend S, Witkop B 1959. Chemical, enzymatic and metabolic studies on the mechanism of oxidation of dopamine. J Am Chem Soc 81: 6236–6240 [Google Scholar]
- Shimizu K, Ohtaki K, Matsubara K, Aoyama K, Uezono T, Saito O, Suno M, Ogawa K, Hayase N, Kimura K, et al. 2001. Carrier-mediated processes in blood–brain barrier penetration and neural uptake of paraquat. Brain Res 906: 135–142 [DOI] [PubMed] [Google Scholar]
- Smith P, Heath D 1976. Paraquat. CRC Crit Rev Toxicol 4: 411–445 [DOI] [PubMed] [Google Scholar]
- Smith Y, Kieval JZ 2000. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci 23: S28–S33 [DOI] [PubMed] [Google Scholar]
- Snyder SH, D’Amato RJ 1985. Predicting Parkinson’s disease. Nature 317: 198–199 [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Jochnowitz ND, Zeevalk GD, Oostveen JA, Hall ED 1996. Treatment of mice with methamphetamine produces cell loss in the substantia nigra. Brain Res 738: 172–175 [DOI] [PubMed] [Google Scholar]
- Spectors S, Sjoerdsma A, Udenfriend S 1965. Blockade of endogenous norepinephrine synthesis by alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J Pharmacol Exp Ther 147: 86–95 [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, Rayport S 1993. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem 60: 527–535 [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen T-K, Lau YY, Kristensen H, Rayport S, Ewing A 1995. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci 15: 4102–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadaiesky MT, Dombrowski PA, Figueiredo CP, Cargnin-Ferreira E, Da CC, Takahashi RN 2008. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience 156: 830–840 [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, et al. 2011. Rotenone, paraquat and Parkinson’s disease. Environ Health Perspect 119: 866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin JP, Cheramy A, Blanc G, Thierry AM, Glowinski J 1976. Topographical distribution of dopaminergic innervation and of dopaminergic receptors in the rat striatum. I. Mictoestimation of [3H] dopamine uptake and dopamine content in microdiscs. Brain Res 107: 291–301 [DOI] [PubMed] [Google Scholar]
- Taylor TN, Greene JG, Miller GW 2010. Behavioral phenotyping of mouse models of Parkinson’s disease. Behav Brain Res 211: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA 2000a. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: Environmental risk factors for Parkinson’s disease? Brain Res 873: 225–234 [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA 2000b. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: Implications for Parkinson’s disease. J Neurosci 20: 9207–9214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA 2003. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur J Neurosci 18: 589–600 [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM 2008. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem 105: 605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumen A, Behnecke A, Qadri F, Bauml E, Thumen A, Behnecke CA, Qadri F, Bauml E, Moser A 2002. N-Methyl-norsalsolinol, a putative dopaminergic neurotoxin, passes through the blood–brain barrier in vivo. Neuroreport 13: 25–28 [DOI] [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, et al. 2003. d-β-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson’s disease. J Clin Invest 112: 892–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranzer JP, Thoenen H 1968. An electron microscopic study of selective, acute degeneration of sympathetic nerve terminals after administration of 6-hydroxydopamine. Experientia 24: 155–156 [DOI] [PubMed] [Google Scholar]
- Tranzer JP, Thoenen H 1973. Selective destruction of adrenergic nerve terminals by chemical analogues of 6-hydroxydopamine. Experientia 29: 314–315 [DOI] [PubMed] [Google Scholar]
- Trulson ME, Cannon MS, Faegg TS, Raese JD 1985. Effects of chronic methamphetamine on the nigral-striatal dopamine system in rat brain: Tyrosine hydroxylase immunochemistry and quantitative light microscopic studies. Brain Res Bull 15: 569–577 [DOI] [PubMed] [Google Scholar]
- Ungerstedt U 1968. 6-Hydroxydopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 5: 107–110 [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott G 1970. Quantitative recording of rotational behaviour in rats after 6-hydroxydopamine lesions of the nigrostriatal dopamine system. Brain Res 24: 485–493 [DOI] [PubMed] [Google Scholar]
- Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S 2000. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem 74: 721–729 [DOI] [PubMed] [Google Scholar]
- West MJ 1993. New stereological methods for counting neurons. Neurobiol Aging 14: 275–285 [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ 1991. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231: 482–497 [DOI] [PubMed] [Google Scholar]
- West MJ, Ostergaard K, Andreassen OA, Finsen B 1996. Estimation of the number of somatostatin neurons in the striatum: An in situ hybridization study using the optical fractionator method. J Comp Neurol 370: 11–22 [DOI] [PubMed] [Google Scholar]
- Widmann R, Sperk G 1986. Topographical distribution of amines and major amine metabolites in the rat striatum. Brain Res 367: 244–249 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Niwa T, Nagatsu T 1990. Parkinsonism in monkeys produced by chronic administration of an endogenous substance of the brain, tetrahydroisoquinoline: The behavioral and biochemical changes. Neurosci Lett 119: 109–113 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Ogawa M, Suzuki K, Nagatsu T 1993. Parkinsonism produced by tetrahydroisoquinoline (TIQ) or the analogues. Adv Neurol 60: 207–211 [PubMed] [Google Scholar]
- Yuan J, Callahan BT, McCann UD, Ricaurte GA 2001. Evidence against an essential role of endogenous brain dopamine in methamphetamine-induced dopaminergic neurotoxicity. J Neurochem 77: 1338–1347 [DOI] [PubMed] [Google Scholar]
- Yuan J, Cord BJ, McCann UD, Callahan BT, Ricaurte GA 2002. Effect of depleting vesicular and cytoplasmic dopamine on methylenedioxymethamphetamine neurotoxicity. J Neurochem 80: 960–969 [DOI] [PubMed] [Google Scholar]
- Yuan J, Darvas M, Sotak B, Hatzidimitriou G, McCann UD, Palmiter RD, Ricaurte GA 2010. Dopamine is not essential for the development of methamphetamine-induced neurotoxicity. J Neurochem 114: 1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Vadasz C 2001. The midbrain dopaminergic system: Anatomy and genetic variation in dopamine neuron number of inbred mouse strains. Behav Genet 31: 47–59 [DOI] [PubMed] [Google Scholar]
- Zaczek R, Culp S, De Souza EB 1991a. Interactions of [3H]amphetamine with rat brain synaptosomes. II. Active transport. J Pharmacol Exp Ther 257: 830–835 [PubMed] [Google Scholar]
- Zaczek R, Culp S, Goldberg H, Mccann DJ, De Souza EB 1991b. Interactions of [3H]amphetamine with rat brain synaptosomes. I. Saturable sequestration. J Pharmacol Exp Ther 257: 820–829 [PubMed] [Google Scholar]
- Zhu C, Vourc’h P, Fernagut PO, Fleming SM, Lacan S, DiCarlo CD, Seaman RL, Chesselet MF 2004. Variable effects of chronic subcutaneous administration of rotenone on striatal histology. J Comp Neurol 478: 418–426 [DOI] [PubMed] [Google Scholar]