Abstract
Background
The beneficial effect of intravitreal ranibizumab in the treatment of neovascular age-related macula degeneration (nAMD) is well known. Outcome data for eyes presenting with visual acuity better than 6/12 is limited.
Aims
To assess the effect of baseline vision on outcome in ranibizumab-treated nAMD eyes, including a subgroup with baseline vision ≥6/12 (<0.30 logmar).
Design
Prospective, consecutive and interventional case series.
Methods
A consecutive cohort of patients treated with intravitreal ranibizumab for nAMD with 52-week follow-up were studied. Patients who had received previous treatment for nAMD were excluded. Eyes were stratified according to baseline logmar visual acuity into four groups: <0.30 (>6/12), 0.30–0.59 (6/12–6/24), 0.60–0.99 (6/24–6/60) and 1.00–1.20 (6/60–6/96). Intravitreal ranibizumab (0.5 mg in 0.05 ml) was administered in three loading monthly doses followed by PRN dosing according to optical coherence tomography (OCT) findings.
Results
A total of 615 eyes were studied including 88 eyes with baseline vision <0.30. The mean change in logmar letters at 52 weeks was +5.5 (entire study group), −0.5 (<0.30 subgroup), +2.2 (0.30–0.59 subgroup), +6.5 (0.60–0.99 subgroup) and +15.3 (1.00–1.20 subgroup). In the <0.30 subgroup, 60 of 88 eyes (68%) had best-corrected visual acuity (BCVA) equal to or better than baseline and 82 of 88 eyes (93%) lost <15 letters at 52 weeks. Within this subgroup 56 of 67 eyes (84%) maintained UK driving standard BCVA visual acuity over the study period.
Conclusions
This study provides evidence that intravitreal ranibizumab treatment stabilises good vision in nAMD presenting with vision better than 6/12 over 52 weeks follow-up.
Keywords: macular degeneration, ranibizumab, lucentis, neovascularisation, visual acuity, treatment
Introduction
Age-related macular degeneration (AMD) is the leading cause of permanent visual impairment in the developed world, accounting for over half of blind and partial sight registrations in those over 50 years in the United Kingdom.1 Neovascular AMD (nAMD) rapidly progresses, if untreated, leading to irreversible central vision loss within 3 months and significant economic consequences.2
Intravitreal ranibizumab (Lucentis, Novartis Pharma AG, Basel, Switzerland; Genetech Inc., San Francisco, CA, USA) is a recombinant, humanised, monoclonal Fab antibody fragment, which inhibits all VEGF-A isoforms and is currently the standard treatment for nAMD in the United Kingdom. It has been found to improve visual acuity by at least 15 letters in one-third of patients and prevent visual loss of at least 15 letters in 90% of cases, after 2 years of monthly intravitreal injections.3, 4
Outcome data for ranibizumab in nAMD has until recently been limited to baseline best-corrected visual acuities (BCVAs) between 6/12 and 6/96 due to the inclusion criteria of the major clinical trials.3, 4, 5, 6 As baseline vision was not a limiting factor for outcome in these studies and all baseline vision subgroups benefited from ranibizumab,7, 8 a favourable response could also be expected for eyes with baseline BCVA better than 6/12. This subgroup has the potential to maintain vision for driving and reading.
The CATT study9 is the first large-scale multicentre trial to include eyes with baseline BCVA better than 6/12 (inclusion criteria 20/25–20/320 (6/7.5–6/96)). However, outcome for this specific group of eyes with the best baseline BCVA was not specifically addressed.
Published data for eyes with better that 6/12 vision is limited to a small retrospective case series of 14 eyes by Raja et al,10 which found an improvement in mean logmar vision from 0.18 to 0.13 after a 12-month follow-up with a mean 7.5 injections. All but one maintained vision over the same period.
The Royal College of Ophthalmologists AMD guidelines11 recommend intravitreal ranibizumab treatment for active nAMD, if baseline BCVA is equal to or better than 6/96. However, clinical practice varies throughout the United Kingdom. In England, NHS funding is typically based on the National Institute for Health and Clinical Excellence (NICE) 2008 guidelines,12 which uses the 6/12 to 6/96 baseline vision range used in clinical trials. In Wales, Welsh Assembly Government funding is possible if a Consultant Retinal Ophthalmologist recommends treatment.13 This clinical decision is based on the Royal College of Ophthalmologists guidelines and thus includes eyes with baseline vision above 6/12.
We report the effect of baseline BCVA on the outcome in ranibizumab treatment of nAMD in the South East Wales with particular focus on eyes with baseline BCVA above 6/12.
Materials and methods
A consecutive series of patients diagnosed with nAMD and deemed eligible for treatment with intravitreal ranibizumab since February 2007 were prospectively studied.
Eligibility for treatment was based on the Royal College of Ophthalmologists AMD guidelines at the time of presentation.11 Patients were managed at two centres in South East Wales (University Hospital of Wales (UHW), Cardiff and Royal Gwent Hospital (RGH), Newport) using the same management protocol. Eyes that had completed 52-week follow-up were included in the study.
Exclusion criteria were CNV secondary to causes other than nAMD and previous treatment for nAMD in the affected eye (argon laser photocoagulation, photodynamic therapy or previous anti-VEGFs).
Study eyes were subdivided into four subgroups based on baseline logmar BCVA (Snellen equivalent): <0.30 (better than 6/12), 0.30–0.59 (6/12–6/24), 0.60–0.99 (6/24–6/60) and 1.00–1.20 (6/60–6/96).
Baseline examination
A complete ophthalmological examination was completed for each patient including BCVA, intraocular pressure measurement, dilated fundus biomicroscopy, optical coherence tomography (OCT) and fluorescein angiography.
Treatment regime
Three loading doses of intravitreal ranibizumab (0.5 mg in 0.05 ml) were administered at monthly intervals followed by PRN treatment 4–6 weekly based on OCT assessment (persistent or recurrent intraretinal and/or subretinal fluid) or slit lamp examination (new subretinal or retinal haemorrhage). Time domain OCT was in use for the first 18 months of the study (Stratus OCT, Carl Zeiss, Welwyn Garden City, UK), but later it was replaced by spectral domain 3D OCT (Cirrus HD-OCT, Carl Zeiss; Topcon 3D OCT 1000 and 2000, Topcon, Newbury, UK).
Outcome measures
The main outcome measure was the mean change in logmar letters at 52 weeks for each baseline vision subgroup.
Results
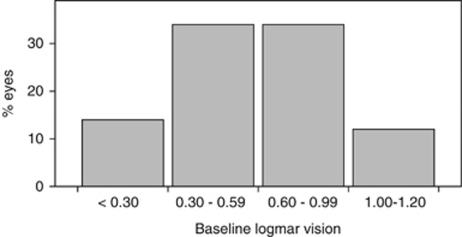
A total of 615 eyes (306 UHW; 309 RGH) were included in this study. Baseline demographic details of the baseline BCVA subgroups are shown in Table 1 and Figure 1. In all, 527 of 615 eyes (86%) had baseline BCVA between 0.30 and 1.20 and the remaining 88 eyes (14%) had baseline BCVA of <0.30.
Table 1. Baseline characteristics.
| <0.30 | 0.30–0.59 | 0.60–0.99 | 1.00–1.20 | All eyes | |
|---|---|---|---|---|---|
| No eyes | 88 | 210 | 211 | 106 | 615 |
| Mean age (years) | 77.5 | 79.9 | 78.9 | 81.3 | 79.3 |
| Mean BCVA | 0.19 | 0.42 | 0.73 | 1.06 | 0.60 |
Abbreviation: BCVA, best-corrected visual acuity.
Figure 1.
Baseline visual acuity groups.
The mean follow-up interval between the first three visits (loading phase) was 35 days, and the mean follow-up interval between visits during the prn phase was 45 days.
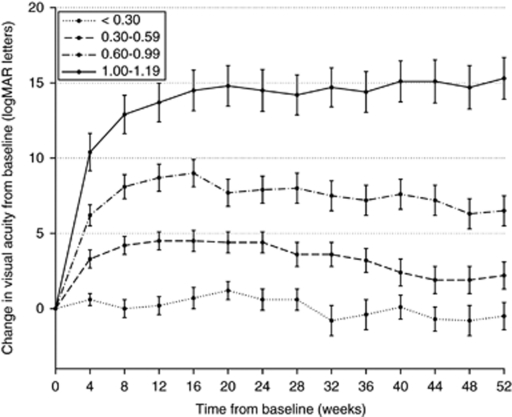
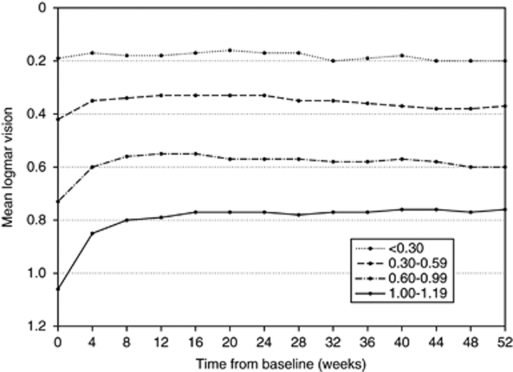
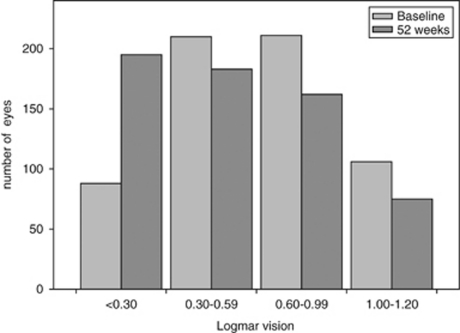
Results at 52 weeks are summarised in Table 2. There was no significant difference in the number of treatments over the study period between the baseline subgroups. The mean change in logmar letters at 52 weeks was +5.5 letters (all patients) and −0.5 (<0.30 subgroup), +2.2 (0.30–0.59 subgroup), +6.5 (0.60–0.99 subgroup) and +15.3 (1.00–1.20 subgroup). The mean change in logmar letters and mean logmar vision in each subgroup over the 52-week follow-up is shown in Figures 2 and 3, respectively. The greatest gain in logmar letters occurred between week 0 and 4 in all baseline vision groups with the exception of the <0.30 group. BCVA at baseline for the entire study group compared with 52 weeks is shown in Figure 4.
Table 2. Results at 52 weeks.
| <0.30 | 0.30–0.59 | 0.60–0.99 | 1.00–1.20 | All eyes | |
|---|---|---|---|---|---|
| Mean no. of Rx (including three loading doses) | 5.4 | 5.6 | 5.8 | 5.2 | 5.6 |
| Mean letter gain | −0.5 | 2.2 | 6.5 | 15.3 | 5.5 |
| Mean BCVA | 0.20 | 0.37 | 0.60 | 0.76 | 0.49 |
| <15 letter loss | 0.93 | 0.88 | 0.92 | 1.00 | 0.92 |
| >15 letter gain | 0.01 | 0.16 | 0.33 | 0.46 | 0.24 |
Abbreviation: BCVA, best-corrected visual acuity; Rx, ranibizumab intravitreal injections.
Figure 2.
Mean change in logmar letters over the 52-week follow-up.
Figure 3.
Mean visual acuity over the 52-week follow-up.
Figure 4.
Visual acuity distribution at baseline and 52 weeks.
<0.30 baseline group results
Eighty-eight eyes of 88 patients had baseline BCVA <0.30. The mean change in logmar letter was −0.5 letters at 52 weeks (range −36 letters to +18 letters). Sixty of these 88 eyes (68%) had BCVA equal to or better than baseline at 52 weeks. Eighty-two of 88 eyes (93%) lost fewer than 15 letters at 52 weeks.
Within this baseline vision group, 67 eyes had baseline BCVA of 0.22 or better (Snellen 6/10) equivalent to UK driving standard BCVA. At 52 weeks, 56 of these eyes maintained this driving standard vision (84%).
Conclusions
The benefits of ranibizumab treatment for nAMD has been demonstrated by several large-scale randomised controlled trials.3, 4, 5, 6 Due to the inclusion criteria of such trials, there is little information in the literature regarding the outcome of treatment in eyes with vision better than 6/12 (<0.30 logmar). The aim of this study was to evaluate the effect of baseline vision on outcome in ranibizumab-treated nAMD eyes, including eyes with baseline vision <0.30 logmar.
Our study demonstrated favourable results for the group as a whole with a mean gain of 5.5 letters at 52 weeks with a mean 5.6 injections. All baseline vision groups, except the <0.30 group, had a mean letter gain at 52 weeks with mean letter gain of −0.5, +2.2, +6.5 and +15.3 letters for the <0.30, 0.30–0.59, 0.60–0.99 and 1.00–1.20 subgroups, respectively. The letter gain was generally inversely proportional to the baseline logmar vision with greatest letter gains occurring in eyes with worse baseline vision.
Similar findings were reported in retrospective subgroup analyses of MARINA and ANCHOR first year results,8, 14 which found baseline vision to be the most important predictor of response to treatment. ‘Ceiling and floor effects' were suggested to explain this effect, that is better baseline vision eyes were less likely to improve and worse baseline vision eyes less likely to lose further vision. Similarly, a small retrospective review of 87 eyes by Shona et al15 found poor baseline vision to be a predictor of maximal gain in visual acuity but better outcome in those presenting with better vision.
Our study group included 88 eyes with baseline vision better than 0.30 logmar, constituting 14% of the total group. These eyes would not be eligible for treatment under current NICE guidelines. The mean visual acuity in this subgroup was unchanged over the 52-week follow-up (0.19 at baseline and 0.20 at 52 weeks) with a mean of 5.4 injections. Raja et al10 reported a similar stabilisation of vision in a small retrospective case series of 14 eyes with baseline vision <0.30 with a mean 7.5 injections over 52 weeks. Eighty-four percent of the eyes with driving standard vision at baseline maintained this level of vision at 52 weeks.
The mean letter gain the in the <0.30 subgroup was less than the other baseline subgroups. This may be due to the ‘ceiling effect' mentioned above. Such eyes have fewer potential letters to gain and pre-existing pigmentary or atrophic macula changes limit acuity gain. This may account for their exclusion from the initial large clinical trials, as their results may have had a negative effect on the letter gain of their study group as a whole. However the maintenance of visual acuity in the <0.30 group over 52 weeks is encouraging and represents the preservation of very good vision for tasks such as reading and driving.
There is limited published data regarding the natural history of nAMD in eyes with good baseline vision. The Verteporfin In Photocoagulation Therapy trial included a placebo subgroup of 24 eyes with subfoveal occult/no-classic CNV, lesion size >4 disc areas and baseline vision ≥20/50.16 At 24 months only 22% maintained vision ≥20/50 without treatment. The MARINA study control arm, which included eyes with minimally classic/occult CNV and baseline vision 6/12–6/96, lost a mean 10 letters over 52 weeks.3 It appears counterintuitive to withhold ranibizumab treatment in <0.30 logmar eyes until acuity falls within NICE treatment levels. The vision lost may not necessarily be regained with detrimental effect on visual function.
Owing to capacity and service constraints of our NHS setting, the mean interval between loading visits was 35 days and not 28 days as planned. Similarly during the PRN period, the mean interval was 45 days and not 4–6 weekly. These prolonged intervals between visits and therefore treatment are likely to have had a detrimental effect on visual outcome.
During the study period there was a change from time domain to spectral domain OCT. Our impression has been that early recurrent leakage was less likely to be missed using the 3D spectral domain OCT. During the entire study period, if intra/subretinal fluid was not detected on OCT assessment, non-contact bio-microscopic fundus examination was routinely performed to identify new intra/subretinal haemorrhages, which may be overlooked on OCT yet indicate the need for treatment.
This prospective study has shown favourable results in the treatment of nAMD with ranibizumab. Letter gain at 52 weeks was inversely proportional to baseline vision. The maintenance of vision at 52 weeks in eyes presenting with good vision <0.30 (better than 6/12) is encouraging. Treatment of this patient subgroup with potential for maintenance of excellent vision should be considered. Further research in this patient group is needed.
The authors declare no conflict of interest.
References
- Owen CG, Fletcher AE, Donoghye M, Rudnicka AR. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom. Br J Ophthalmol. 2003;87:312–317. doi: 10.1136/bjo.87.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MM, Brown GC, Stein JD, Roth Z, Campanella J, Beauchamp GR. Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol. 2005;40:277–287. doi: 10.1016/S0008-4182(05)80070-5. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double-masked, sham controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Boyer DS, Heier JS, Brown DN, Francom SF, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116:1731–1739. doi: 10.1016/j.ophtha.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–252. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–857. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- CATT research group Ranibizumab and bevacizumab for neovascular age related macular degeneration. N Engl J Med. 2011;364 (20:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja MSA, Saldana M, Goldsmith C, Burton BJL.Ranibizumab treatment for neovascular age related macular degeneration in patients with good baseline visual acuity (better than 6/12):12 month outcomes Br J Ophthalmole-pub ahead of print 1 June 2010. [DOI] [PubMed]
- Royal College of Ophthalmologists . Age-Related Macular Degeneration Guidelines For Management. Royal College of Ophthalmologists: London; 2009. [Google Scholar]
- National Institute for Health and Clinical Excellence . Ranibizumab and Pegaptanib for the Treatment for Age Related Macular Deneration. National Institute for Health and Clinical Excellence: London (UK); 2008. [Google Scholar]
- Blyth CP. Delivering Treatment for Age Related Macula Degeneration. The Welsh Retina Group; 2008. [Google Scholar]
- Boyer DS, Antoszyk AN, Awh OC, Bhisitkul RB, Shapiro H, Archarya NR. Subgroup analysis of the MARINA study of ranibizumab in neovascular age related macular degeneration. Ophthalmology. 2007;114:246–252. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Shona O, Gupta B, Vemala R, Sivaprasad S. Visual acuity outcomes in ranibizumab treated neovascular age related macular degeneration stratified by baseline vision. Clin exp ophthalmol. 2011;39:5–8. doi: 10.1111/j.1442-9071.2010.02424.x. [DOI] [PubMed] [Google Scholar]
- Pieramici DJ, Bressler SB, Koester JM, Bressler NM. Occult with no classic subfoveal CNV in age-related macula degeneration. Clinically relevant natural history information in larger lesions with good vision from Verteporfin in Photodynamic Therapy VIP trial: VIP report no 4. Arch Ophthalmol. 2006;124:660–664. doi: 10.1001/archopht.124.5.660. [DOI] [PubMed] [Google Scholar]