Abstract
Background
To assess diagnostic efficacy of plasma total homocysteine (tHcy) and C-reactive protein (CRP) levels for ocular ischemic syndrome (OIS).
Methods
In all, 87 patients with retinal vein occlusion (RVO), 955 patients with a stenosis of internal carotid artery (ICA) <90% and 159 patients with a stenosis of ICA >90% were included between 2003 and 2009. A total of 43 patients with a stenosis ICA >90% were diagnosed as OIS. Fasting tHcy, CRP, lipid profiles, creatinine were measured, and diagnostic values of hyperhomocysteinemia or elevated CRP for OIS were evaluated.
Result
The mean plasma levels of tHcy (18.8 μmol/l) and CRP (1.1 mmol/l) were the highest in patients with OIS among the groups. The prevalences of hyperhomocysteinemia (72%) and elevated CRP (77%) were the highest in OIS among the groups. In patients with stenosis of ICA, the diagnostic sensitivity/specificity for OIS was 70/79% in hyperhomocysteinemia and 73/73% in elevated CRP. The diagnostic sensitivity and specificity for OIS were 53 and 86% in both hyperhomocysteinemia and elevated CRP. The lipid profiles and creatinine levels were similar among the groups.
Conclusion
Our results suggest that hyperhomocysteinemia and elevated CRP may be associated with the development of OIS. The measurements of tHcy and CRP in blood may help to assist the diagnosis of OIS in a stenosis of ICA.
Keywords: ocular ischemic syndrome, homocysteine, C-reactive protein, carotid artery stenosis
Introduction
Severe stenosis of internal carotid artery (ICA) may cause ocular ischemic syndrome (OIS) because of the reduction in ipsilateral perfusion pressure of the eye.1 Chronic progressive ocular ischemia may lead to permanent blindness secondary to neovascular glaucoma and optic atrophy.1, 2 OIS still poses difficulty in the diagnosis and has poor vision prognosis despite the various treatment modalities.1, 2, 3
Elevated total homocysteine (tHcy) in blood have been considered a risk factor for future development of stroke in preexisting diseases of carotid artery.4 A meta-analysis reported that retinal vascular occlusion including arterial and vein occlusion was associated with elevated plasma tHcy concentrations.5 However, recent studies have suggested that the plasma tHcy level in retinal vein occlusion (RVO) was not a risk factor of RVO but was a marker of atherosclerosis.6, 7 Inflammation is now widely accepted as central to every aspect of the atherosclerotic process, from its initiation to progression. C-reactive protein (CRP) is another biological predictor of atherosclerotic disease.8 There have been little evidences to the relationships between plasma levels of tHcy or CRP and OIS.
To address this issue, a retrospective review of patients who were tested for plasma tHcy levels, CRP levels, lipid profiles, and other risk factors of atherosclerosis over 7 years were conducted in single tertiary referral hospital. Patients with RVO or stenosis of ICA were selected as controls to compare the plasma levels and the frequencies of hyperhomocyteinemia and elevated CRP with patients with OIS. The author assessed the diagnostic efficacy of hyperhomocyteinemia and elevated CRP for OIS.
Materials and methods
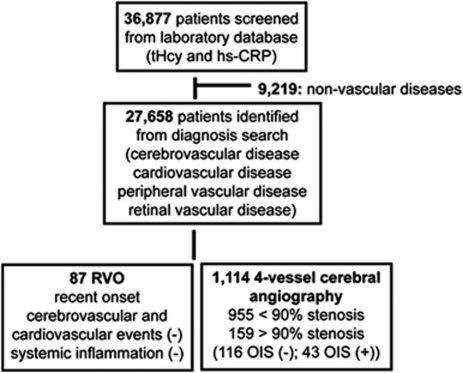
We screened the laboratory database of plasma tHcy, CRP, creatinine and lipid profiles at the Kangdong Sacred Heart Hospital of Hallym University Medical Center and the Chonnam National University Hospital over 7-year-period from January 2003 to December 2009. We identified 36 877 individuals who gave the blood sample at the same time and reviewed the diagnosis in the medical records. We selected 27 568 patients with the diagnosis of cardiovascular disease, cerebrovascular disease, peripheral vascular disease, and retinal vascular disease. Measuring plasma tHcy and CRP are not commonly indicated in normal patient screening, we selected patients with RVO as controls. Inclusion criteria were a recent onset, no history of cerebrovascular events, cardiovascular events, and no evidence of systemic inflammation. Because cerebral vascular imaging procedures were not indicated in RVO patients without neurological symptoms, all patients with RVO did not receive cerebrovascular imaging procedures. We selected 87 patients who were diagnosed with RVO (36 with central RVO and 51 with branch RVO). Among 27 568 patients, we screened patients who had the cerebral vascular imaging of ICA including four vessels cerebral angiography, neck CT angiography, or MR angiography. A total of 1178 patients with neurological symptoms received non-invasive CT or MR angiography. In all, 64 of 1178 patients (5.4%) had no stenosis of ICA in CT or MR angiography. Because four-vessel cerebral angiography is an invasive procedure, it is usually indicated in patients who had a stenosis in CT or MR angiography. Four-vessel cerebral angiography shows a stenosis of ICA in all patients. A total of 1114 patients had a stenosis of ICA in four-vessel cerebral angiography. In all, 159 (14.2%) of 1114 patients had severe stenosis of ICA that was defined as obstruction of arterial lumen ≥90%, and the 955 patients had a stenosis of <90% and obstruction of arterial lumen ≥30%. Routine ocular evaluations were not indicated in 1071 patients without ocular symptoms who had received four-vessels cerebral angiography. In all, 43 (3.9%) of 1114 patients were diagnosed as OIS by single ophthalmologist (JKA). Selection of patients was illustrated in Figure 1.
Figure 1.
Flow chart of selection of patients. tHcy, total homocysteine; hs-CRP, high sensitivity C-reactive protein; ICA, internal carotid artery; RVO, retinal vein occlusion; OIS, ocular ischemic syndrome.
Diagnostic criteria of OIS were as follows: symptomatic onset including visual loss, visual field defect, amaurosis fugax, and/or ocular pain; ophthalmological examinations including new vessels at iris, retina or optic disc, narrowing of retinal arteries, dilatation of retinal veins, and/or retinal hemorrhages; fluorescein angiography examinations including delayed patchy choroidal filling and increased retinal arteriovenous transit time; the presence of ICA stenosis of >90% using four vessels cerebral angiography.
Fasting (>8 h) venous blood samples were drawn from participants into vacuum tubes containing EDTA. Plasma was separated immediately from blood cells by centrifugation at 3000 g at 25°C for 10 min and stored at −20°C until analysis. Total plasma homocysteine was measured by an ELISA kit (Bio-Rad Laboratories. Inc., Hercules, CA, USA). Hyperhomocysteinemia was defined as plasma concentrations >14.0 μmol/l. CRP levels were measured by a highly sensitive nephelometric assay using a monoclonal antibody to high sensitivity CRP coated on polystyrene beads (Dade Behring, Marburg, Germany).9 The elevation of high sensitivity CRP has been considered emerging risk factor for cardiovascular events.10 Elevated CRP was defined as plasma concentrations >0.5 mmol/l. Creatinine levels and lipid profiles (plasma total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides) were also measured. Institutional Review Board/Ethics Committee approval was obtained from the Chonnam National University Clinical Research Institute. The study protocol was in accordance with the provisions of the Declaration of Helsinki.
Statistical analyses were performed with SPSS for Windows version 15.0 software (SPSS, Chicago, IL, USA). ANOVA test was used to compare the mean plasma levels of tHcy, CRP, creatinine, and cholesterols among the groups. χ2 was used to compare prevalences of hyperhomocysteinemia, hyperlipidemia, and elevated CRP among the groups. Cross tabulation analysis was used to measure diagnostic sensitivity, specificity, positive predictive value and negative predictive value of hyperhomocysteinemia, and elevated CRP in patients with stenosis of ICA. A P-value of <0.05 was considered to have statistical significance.
Results
Demographic features of patients included in this study are summarized in Table 1. The demographic characteristics of OIS were similar to those of severe stenosis of ICA. Male preponderance and old age were noted in severe stenosis of ICA and OIS. The prevalences of hypertension and diabetes were significantly higher in severe stenosis of ICA and OIS compared with RVO (P<0.001).
Table 1. Demographic features of study subjects.
| Variables | RVO |
Disease controls (ICA stenosis) |
OIS | |
|---|---|---|---|---|
| <90% Stenosis | >90% Stenosis | |||
| No. of patients | 87 | 955 | 116 | 43 |
| Age, years (range) | 60 (34–81) | 68 (48–93) | 70 (45–93) | 71 (53–85) |
| Gender (female/male) | 53/34 | 315/640 | 26/90 | 9/34 |
| Smoking (%) | 22 (25) | 287 (30) | 36 (31) | 14 (33) |
| Hypertension (%) | 35 (40) | 401 (42) | 76 (65) | 25 (63) |
| Diabetes (%) | 13 (15) | 191 (20) | 38 (36) | 18 (42) |
Abbreviations: ICA, internal carotid artery; OIS, ocular ischemic syndrome; RVO, retinal vein occlusion.
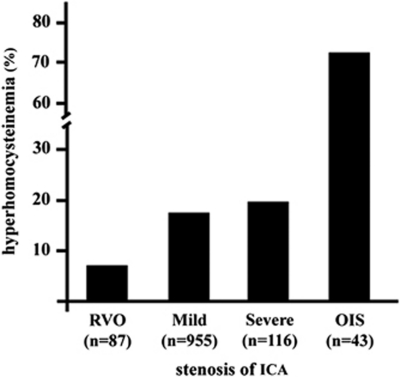
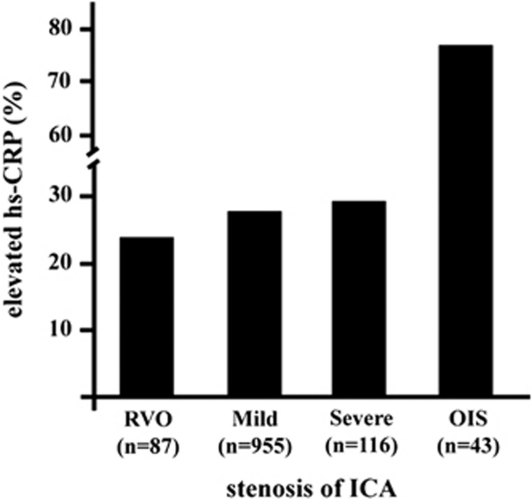
The mean plasma levels of tHcy were significantly the highest in patients with OIS (18.8±7.9 μmol/l, ANOVA, P<0.001) among the groups. The prevalence of hyperhomocysteinemia (tHcy>14.0 μmol/l) was 72% in OIS (Figure 2). This value was significantly the highest among the groups. The mean CRP levels were the highest in the OIS group (1.07±1.4 mmol/l, ANOVA, P<0.001). The prevalence of elevated CRP levels (>0.5 mmol/l) was 77% in the OIS group (Figure 3). This value was the highest among the groups. The plasma levels of total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and creatinine were similar among the groups.
Figure 2.
Frequencies of hyperhomocysteinemia (>14.0 μmol/l).
Figure 3.
Frequencies of elevated high sensitivity CRP (>0.5 mmol/l).
The sensitivity and specificity of hyperhomocysteinemia and elevated CRP levels for diagnosing OIS in patients with stenosis of ICA were summarized in Table 2. The diagnostic sensitivity and specificity were 70 and 79% in hyperhomocysteinemia and 73 and 73% in elevated CRP levels. The diagnostic sensitivity and specificity were 53 and 86% in both hyperhomocysteinemia and elevated CRP levels. Positive predictive value was 56% in hyperhomocysteinemia, 50% in elevated CRP, and 58% in hyperhomocysteinemia and elevated CRP. The negative predictive value was 80% in hyperhomocysteinemia, 73% in increased CRP, and 83% in hyperhomocysteinemia and elevated CRP.
Table 2. Diagnostic values of hyperhomocysteinemia and elevated C-reactive protein (CRP) for ocular ischemic syndrome in patients with a stenosis of internal carotid artery.
| Variables (%) | tHcy >14.0 μmol/l | CRP >0.5 mg/l | tHcy >14.0 μmol/l |
|---|---|---|---|
| +CRP>0.5 mg/l | |||
| Sensitivity | 70 | 73 | 53 |
| Specificity | 79 | 73 | 86 |
| Positive predictive value | 56 | 50 | 58 |
| Negative predictive value | 80 | 73 | 83 |
Abbreviations: CRP, C-reactive protein; tHcy, total homocysteine.
Discussion
In this study, plasma levels of tHcy and CRP are associated with the development of OIS in patients with stenosis of ICA. The mean plasma concentrations of tHcy and CRP are the highest in patients with OIS. In addition, the prevalences of hyperhomocysteinemia and elevated CRP are also the highest in the OIS group. This is the first evidence to document that plasma tHcy and CRP levels can be used as a serologic marker of OIS. If one detects a stenosis of ICA, the measurements of plasma tHcy and CRP are helpful to assist the diagnosis of OIS.
Recent meta-analyses reported that there has been a significant association between the plasma tHcy levels and the risk of vascular diseases in patients with preexisting vascular diseases.11, 12 A prospective study demonstrated that plasma tHcy levels were strong predictors of ischemic stroke among patients with chronic ischemic heart disease.13 In this study, plasma tHcy levels were associated with the development of OIS in patients with preexisting stenosis of ICA. A meta-analysis reported that retinal vascular occlusion including RVO and arterial occlusion was associated with elevated plasma tHcy levels.5 Recent studies reported that the plasma tHcy level in RVO was not a risk factor of RVO but is a marker of atherosclerosis.6, 7 A recent study suggests that Hcy levels could be associated with the development of retinal arteriosclerosis.14 In this study, the plasma levels of tHcy and the prevalence of hyperhomocysteinemia were much higher in OIS than those in RVO. These results further support the idea that retinal arteriosclerosis may be associated with the plasma levels of tHcy, rather than retinal venous occlusive disease.
CRP has received substantial attention as a promising biological predictor of atherosclerotic disease.8, 12 A population-based study reported that CRP was correlated with the caliber of retinal vessel.15 There have been evidences that CRP may have a direct role in the pathogenesis of atherosclerosis from initiation to progression.16 In this study, CRP was associated with the development of OIS in patients with a stenosis of ICA.
In this study, the prevalences of hypertension and diabetes were much higher in the OIS group than those in the RVO. Even though other atherosclerotic risk factors are similarly present, all patients with severe stenosis of ICA do not have OIS, suggesting that another factor must have a role. In this study, homocysteine and/or CRP probably have a role in the development of OIS in patients with a stenosis of ICA. The measurements of plasma tHcy or CRP have the moderate sensitivity and specificity (70–80%) in diagnosing OIS in patients with a stenosis of ICA. Both measurements have the highest specificity (86%) in diagnosing OIS in patients with a stenosis of ICA. Plasma levels of tHcy and CRP may be considered the promising predictors of OIS.
One may argue that there may be a potential bias in selection of controls. Because the patients with RVO did not have cerebral vascular imaging procedures, it is possible to include a patient with ICA stenosis. However, cerebral vascular imaging procedures are not indicated in patients without neurological symptoms. We selected RVO patients as controls for several reasons. First of all, it is well known that measuring plasma tHcy and CRP are not commonly indicated in normal patient screening and their normal ranges are generally known. Furthermore, RVO is an acute veno-occlusive disease, whereas OIS is a chronic artherosclerotic disease. And RVO is a localized vascular disease, whereas OIS is a systemic vascular disease. The patients with ICA stenosis did not have ocular evaluations. Because of the intrinsic deficit of a retrospective nature in this study, it is possible to underestimate or overestimate the diagnostic efficacy of blood tests of tHcy and CRP. It is unclear why OIS develops in a portion of patients with severe stenosis of ICA. Various factors may be related to the development of OIS in patients with severe stenosis, for example, patency of collateral circulations. Our hypothesis is that developments of OIS may be associated with inflammatory exacerbations in the preexisting severe artherosclerotic status because we often observed inflammatory natures in the eye in patients with OIS.
In conclusion, homocysteine and CRP are associated with the development of OIS. They maybe considered predictors of OIS in patients with a stenosis of internal carotid artery. The measurements of plasma tHcy and CRP in blood may help to assist the diagnosis of OIS in a stenosis of ICA.
The authors declare no conflict of interest.
References
- Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104:859–864. doi: 10.1016/s0161-6420(97)30221-8. [DOI] [PubMed] [Google Scholar]
- Young LH, Appen RE. Ischemic oculopathy. A manifestation of carotid artery disease. Arch Neurol. 1981;38:358–366. doi: 10.1001/archneur.1981.00510060060009. [DOI] [PubMed] [Google Scholar]
- Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome. III. Visual prognosis and the effect of treatment. Int Ophthalmol. 1991;15:15–20. doi: 10.1007/BF00150974. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Chung CS, Kang SS. Relation of plasma homocysteine to cerebral infarction and cerebral atherosclerosis. Stroke. 1998;29:2478–2483. doi: 10.1161/01.str.29.12.2478. [DOI] [PubMed] [Google Scholar]
- Cahill MT, Stinnett SS, Fekrat S. Meta-analysis of plasma homocysteine, serum folate, serum vitamin B12, and thermolabile MTHFR genotype as risk factors for retinal vascular disease. Am J Ophthalmol. 2003;136:1136–1150. doi: 10.1016/s0002-9394(03)00571-3. [DOI] [PubMed] [Google Scholar]
- Pinna A, Carru C, Zinellu A, Dore S, Deiana L, Carta F. Plasma homocysteine and cysteine levels in retinal vein occlusion. Invest Ophthalmol Vis Sci. 2006;47:4067–4071. doi: 10.1167/iovs.06-0290. [DOI] [PubMed] [Google Scholar]
- Di Crecchio L, Parodi MB, Sanguinetti G, Iacono P, Ravalico G. Hyperhomocysteinemia and the methylenetetrachydrofolate reductase 677C-T mutation in patients under 50 years of age affected by central retinal vein occlusion. Opthalmology. 2004;111:940–945. doi: 10.1016/j.ophtha.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Ledule TB, Weiner DL, Sipe JD, Poulin SE, Collins MF, Rifai N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A, and mannose-binding protein in human serum. Ann Clin Biochem. 1998;35:745–753. doi: 10.1177/000456329803500607. [DOI] [PubMed] [Google Scholar]
- Corrado E, Rizzo M, Coppola G, Fattouch K, Novo G, Marturana I, et al. An update on the role of markers of inflammation in atherosclerosis. J Artheroscler Thromb. 2010;17:1–11. doi: 10.5551/jat.2600. [DOI] [PubMed] [Google Scholar]
- The Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke. A meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease. A critical review of the evidence. JAMA. 2003;290:932–940. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- Tanne D, Haim M, Goldbourt U, Boyko V, Doolman R, Adler Y, et al. Prospective study of serum homocysteine and risk of ischemic stroke among patients with preexisting coronary heart disease. Stroke. 2003;34:632–636. doi: 10.1161/01.STR.0000060203.58958.35. [DOI] [PubMed] [Google Scholar]
- Ghorbanihaghjo A, Javadzadeh A, Argani H, Nezami N, Rashtchizadeh N, Rafeey M, et al. Lipoprotein(a), homocysteine, and retinal arteriorsclerosis. Mol Vis. 2008;14:1692–1697. [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: The Multi-Ethnic Study of Artherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]