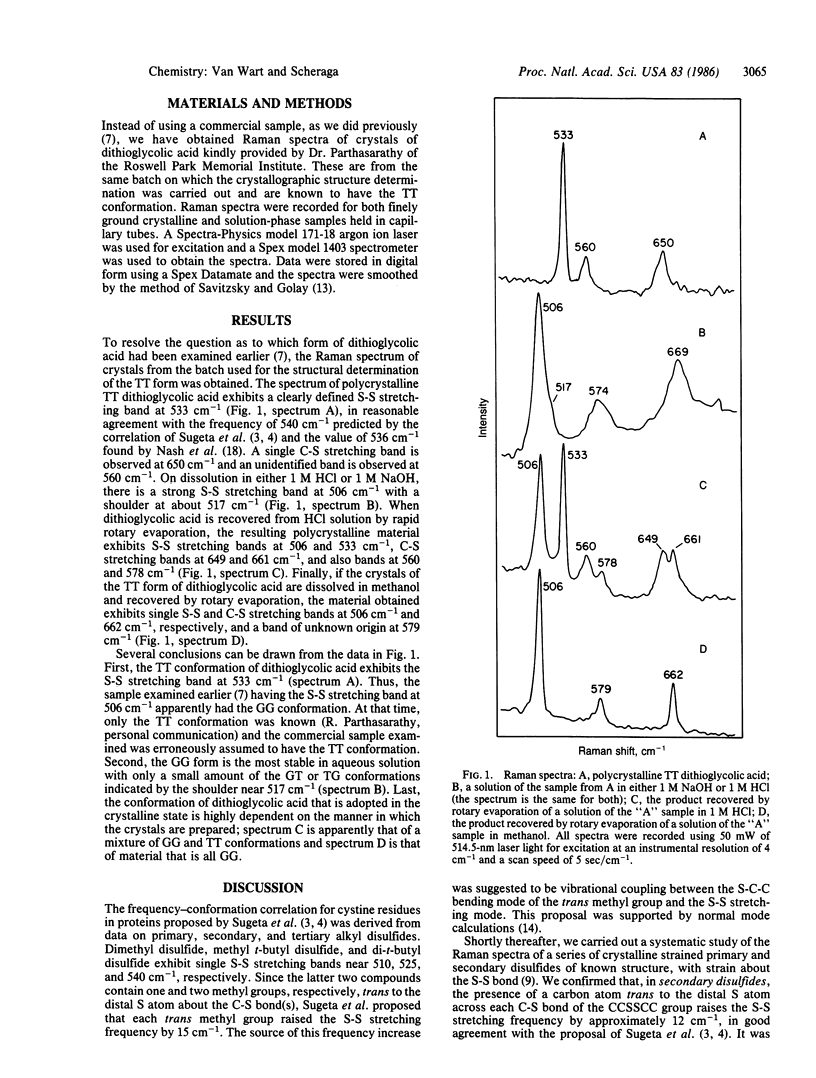
Abstract
Two hypotheses have been advanced to explain the conformational dependence of the disulfide stretching frequency of the CCSSCC moiety in unstrained disulfides. Such compounds can adopt conformations that exhibit disulfide stretching bands near 510, 525, and 540 cm-1. Sugeta et al. [Sugeta, H., Go, A. & Miyazawa, T. (1973) Bull. Chem. Soc. Jpn. 46, 3407-3411] have attributed these three bands to conformations having none, one, and two trans conformations, respectively, about the C-S bonds. In apparent contradiction to this correlation, Van Wart and Scheraga [Van Wart, H. E. & Scheraga, H. A. (1976) J. Phys. Chem. 80, 1812-1823] found that dithioglycolic acid crystals, believed at the time to exist only in the trans conformation about both C-S bonds, exhibited the S-S stretching band near 510 cm-1. On the basis of this observation, it was suggested instead that the 510, 525, and 540 cm-1 bands arose from CCSSCC moieties having none, one, and two A conformations (i.e., those with CC-SS dihedral angles close to 30 degrees), respectively, about the C-S bonds. It has recently been shown by Nash et al. [Nash, C. P., Olmstead, M. M., Weiss-Lopez, B., Musker, W. K., Ramasubbu, M. & Parthasarathy, R. (1985) J. Am. Chem. Soc. 107, 7194-7195] that dithioglycolic acid can crystallize in two different forms, one with trans and the other with gauche conformations about both C-S bonds, and that these have S-S stretching bands at 536 and 510 cm-1, respectively. These results are confirmed here and it is shown that our earlier data were collected on the all-gauche (rather than the all-trans) form. Thus, the correlation proposed by Sugeta et al. is correct.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Lord R. C., Yu N. T. Laser-excited Raman spectroscopy of biomolecules. I. Native lysozyme and its constituent amino acids. J Mol Biol. 1970 Jun 14;50(2):509–524. doi: 10.1016/0022-2836(70)90208-1. [DOI] [PubMed] [Google Scholar]
- Lord R. C., Yu N. T. Laser-excited Raman spectroscopy of biomolecules. II. Native ribonuclease and alpha-chymotrypsin. J Mol Biol. 1970 Jul 28;51(2):203–213. doi: 10.1016/0022-2836(70)90137-3. [DOI] [PubMed] [Google Scholar]
- Van Wart H. E., Lewis A., Scheraga H. A., Saeva F. D. Disulfide bond dihedral angles from Raman spectroscopy. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2619–2623. doi: 10.1073/pnas.70.9.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart H. E., Scheraga H. A. Stable conformations of aliphatic disulfides: influence of 1,4 interactions involving sulfur atoms. Proc Natl Acad Sci U S A. 1977 Jan;74(1):13–17. doi: 10.1073/pnas.74.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]


