Abstract
Background
Constraint-induced movement therapy (CIMT) has received considerable attention as an intervention to enhance motor recovery and cortical reorganization after stroke.
Objective
The present study represents the first multicenter effort to measure cortical reorganization induced by CIMT in subjects who are in the subacute stage of recovery.
Methods
A total of 30 stroke subjects in the subacute phase (>3 and <9 months poststroke) were recruited and randomized into experimental (receiving CIMT immediately after baseline evaluation) and control (receiving CIMT after 4 months) groups. Each subject was evaluated using transcranial magnetic stimulation (TMS) at baseline, 2 weeks after baseline, and at 4-month follow-up (ie, after CIMT in the experimental groups and before CIMT in the control groups). The primary clinical outcome measure was the Wolf Motor Function Test.
Results
Both experimental and control groups demonstrated improved hand motor function 2 weeks after baseline. The experimental group showed significantly greater improvement in grip force after the intervention and at follow-up (P = .049). After adjusting for the baseline measures, the experimental group had an increase in the TMS motor map area compared with the control group over a 4-month period; this increase was of borderline significance (P = .053).
Conclusions
Among subjects who had a stroke within the previous 3 to 9 months, CIMT produced statistically significant and clinically relevant improvements in arm motor function that persisted for at least 4 months. The corresponding enlargement of TMS motor maps, similar to that found in earlier studies of chronic stroke subjects, appears to play an important role in CIMT-dependent plasticity.
Keywords: Plasticity, Recovery, Transcranial magnetic stimulation, Upper extremity
Stroke is the leading cause of long-term disability among adults in the United States. Approximately 70% to 88% of persons with ischemic stroke have some degree of motor impairment.1,2 Although variable degrees of recovery may occur after stroke, only recently have consistent efforts been made to understand the mechanisms underlying motor recovery.3-9
Constraint-induced movement therapy (CIMT) has produced promising results among subjects with limited upper extremity motor function after stroke.10-14 CIMT involves intense, functionally oriented task practice with the paretic upper extremity along with restraint of the less-impaired upper extremity for most waking hours.11 The concept of this treatment strategy was derived from studies in nonhuman primates.10 Unilaterally deafferented monkeys relearned use of the affected limb when the intact limb was restrained. This observation led to several studies in humans, where subjects in the chronic phase poststroke wore a sling on the less-impaired side and were intensely trained on the paretic side for 6 hours daily during 2 consecutive weeks. These studies demonstrated that subjects can improve motor function well after their stroke.10-14 Recent evidence from the EXCITE multicenter trial has shown statistically significant and clinically relevant improvements in paretic arm motor ability and use compared with participants receiving usual and customary care.15
Although CIMT is a promising intervention, there is a need to better understand the neural mechanisms, including cortical changes that might occur. Such insight could help to improve currently available interventions. Our understanding of central nervous system plasticity associated with CIMT is particularly poor in the subacute phase after stroke. Expansion of motor maps derived using transcranial magnetic stimulation (TMS) has been demonstrated in a small number of chronic subjects (>12 months poststroke ) undergoing CIMT.3,7 For instance, Liepert et al16 suggested that an increase in the size of the motor map correlated with functional recovery as demonstrated by significant improvement on the motor activity log (MAL)17 in 13 chronic stroke subjects (6 months to 17 years poststroke injury). Another study using functional magnetic resonance imaging (fMRI) to measure motor physiological changes showed that after 2 weeks of home-based CIMT, 7 subjects (6 to 84 months poststroke) improved hand motor function (ie, mean grip strength ratio); this was associated with enhanced activation of premotor and secondary somatosensory cortex of the affected hemisphere and bilateral cerebellum during motor tasks.18
Whereas several small studies in chronic-phase stroke subjects have provided imaging data following repetitive task practice,3,6,7 the present study represents the first multicenter effort using TMS to evaluate cortical reorganization longitudinally among subjects 3 to 9 months poststroke in response to CIMT. Because this subacute period may be one of physiological changes unrelated to type of therapy, it was important to include a control group that did not receive CIMT.
We therefore tested the hypothesis that subacute stroke patients receiving CIMT would have larger motor output maps in the ipsilesional primary motor cortex compared to a control group receiving usual and customary care. The apparent map expansion would occur without changes in motor thresholds and might be associated with increased slope of the TMS stimulus response curve, resulting in less localized responses to the same pattern of stimulation. If the map expansion occurred without any change in cortical excitability, this would suggest an increased area of motor representation. We expected that map expansion would be correlated with improvements in motor recovery and that these changes would remain stable over time.
METHODS
Subjects
A total of 30 subjects (3 to 9 months poststroke) with partial recovery of a weak upper extremity following a single stroke were included in this study. Inclusion criteria were identical to those of the EXCITE trial,15 and some subjects were studied during their participation in EXCITE. The remainder participated after recruitment for EXCITE was concluded but were subject to identical inclusion criteria and training. Active movement in the paretic arm had to include at least 20° of wrist extension and 10° of extension at the thumb and 2 other digits.19 Subjects were randomized into an experimental group (n = 17, age ± standard error of the mean [SEM]: 54.4 ± 3.8), who received CIMT immediately after baseline testing (TMS and motor assessments), and a control group (n = 13, age ± SEM: 58.5 ± 3.5) who received CIMT after all the assessment sessions were performed. Each individual participant gave informed consent. The protocol was approved by the Institutional Review Boards at each participating site (Wake Forest University, Emory University, and The Ohio State University).
Study Design
Experimental group
Each subject in the experimental group participated in 10 consecutive weekdays of intensive upper extremity therapy during which time he or she donned a padded mitt covering the less affected hand. This mitt worn for at least 90% of waking hours over a 2-week period of therapy, including 2 weekends, for a total of 14 days.15 Each subject was requested to complete a diary to record home activities undertaken while the mitt was worn. Treatment focused on unimanual skill acquisition and functional retraining and was based on the principles of behavioral training20,21 that can also be described in terms of motor learning derived from adaptive or part-task practice.22,23 Tasks emphasized grasp as well as manipulation and release of objects. Subjects also performed general activities related to daily living, coordination, and balance. Tasks were chosen based on subject preference and movement limitations. Task difficulty was progressively increased by using a training strategy in which targets for motor ability goals were kept just beyond the level of performance already achieved. The specific tasks chosen and the time of training for each task were recorded. Rest intervals were provided, lasting no longer than the practice segments, and the duration of both types of period was documented.
Control group
The control group continued with their usual and customary care. Because this care might affect functional gains among participants, an attempt was made to track care received through participant reports collected during monthly phone calls by project staff and during the scheduled testing sessions. Usual and customary care ranged from no treatment to the application of mechanical interventions (orthotics) or various occupational and physical therapy approaches in the home, day treatment programs, or outpatient hospital visits. Participants in the control condition were offered the same CIMT regimen as those in the experimental group, after the 4-month evaluation session.
Outcome Measures
Wolf Motor Function Test (WMFT)
The WMFT was chosen as the primary clinical outcome measure15 and was performed by blinded evaluators at all sites. This test has established reliability, and validity and has also been used extensively to evaluate upper extremity motor function in the EXCITE trial.15 It encompasses a battery of 15 time-based tasks and 2 force-based tasks (items 7 lift weight and 14 grip weight).
Neurophysiological Assessment—TMS
Comparability and standardization of TMS data collection techniques across all 3 sites was ensured prior to enrollment of subjects. After completion of intensive training for TMS data acquisition, data of at least 2 normal volunteers and 2 subjects with chronic stroke were acquired at each site. Additionally, 1 healthy volunteer was consistently evaluated across all sites to ensure reproducibility of the data. The TMS data were centrally analyzed at Wake Forest University.
All testing was conducted on 3 occasions (baseline, after 2 weeks, and at 4-month follow-up) by trained staff blinded to group assignment. Subjects were seated in a comfortable chair with elbows slightly flexed and supported to facilitate a relaxed posture. Adhesive monitoring electrodes (H59P, Kendall soft-E, Chicopee, MA) were placed over the belly of the extensor digitorum communis (EDC) muscle bilaterally with the reference electrode placed proximally. To ensure reproducibility of electrode placements at different time points, a template was obtained for each subject at baseline using a plastic film. The EDC muscle was selected because this muscle is the primary effector of finger extension, its activity represents part of the minimal motor criteria for inclusion for the CIMT, and the inevitable volume-conduction of motor evoked potentials (MEPs) from neighboring muscles would most likely capture additional functionally related wrist extensor activity.24 The electromyographic (EMG) signals were amplified and filtered (band-pass 30 Hz to 1 kHz) using an isolated bioelectric amplifier (James Long Co., Caroga Lake, NY), and fed into a laboratory computer for off-line analysis. Auditory feedback of EMG was used to ensure quiescence in target muscle activity prior to stimulation.
Transcranial magnetic stimulation was delivered using a Magstim 200 stimulator with a figure-8 coil (Magstim, Whitland, Dyfed, UK). The coil handle pointed posteriorly yielding approximately posterior–anterior current flow across the central sulcus.25,26 The coil was placed on the frontoparietal region contralateral to the target muscle and moved until the “hot-spot” for the EDC muscle was found.
Resting motor thresholds
The resting motor threshold (rMT) was defined as the minimum TMS intensity (measured to the nearest 1% of the maximum output of the magnetic stimulator) required to elicit at least 5 out of 10 MEPs ≥25 μV on consecutive trials, a modification of the standard27 because of the smaller potentials recorded on bipolar versus belly-tendon montages.
Active motor thresholds
The active motor threshold (aMT) was defined as the lowest TMS intensity resulting in MEPs of about 200 μV on 50% of trials during isometric contraction of the EDC muscle.27
Mapping of motor cortical area
Stimulus sites were located using a latitude/longitude based coordinate system.28 Subjects wore a tight-fitting, flexible cap premarked with a 1-cm grid referenced to the vertex (Cz). The cap was positioned using cranial landmarks (nasion, inion) and the preauricular crease as references. Latitude was defined as the mediolateral distance from Cz, and longitude as the distance from Cz along a line of constant latitude from the interaural line. Stimulation intensity was set at 110% of rMT and 10 stimuli were delivered to each scalp site at a rate of approximately 1 stimulus every 5 seconds. Stimulation was continued on each site of the grid, until a border position without a response of at least 25 μV peak to peak in 5 successive or less than 50% of 10 stimulations was encountered. The average response of every series of 10 stimuli was calculated offline.
Map area
The normalized map area (nMV) is a simple measure of the spread of the motor representation over multiple scalp sites. It is calculated as the sum of the normalized MEP (nMEP – the mean MEP at each location, divided by the largest mean MEP) over all locations. The nMV ranges from 1, for a map with only 1 active location, to a value that is equal to the number of active locations, if all locations gave equal responses.
Center of gravity mapping
The center of gravity (COG) is an estimate of the center of the motor map, and is an average of all active location vectors, each weighted by the MEP amplitude at that location.28 If there are N locations, the COG is calculated by for the x-coordinate (COGx) and similarly for the y-coordinate (COGy).7
Recruitment curves
To study changes in cortical excitability in a range relevant to map acquisition, limited recruitment (stimulus–response) curve measurements were performed.29 The coil was kept at the optimal position for stimulation of the EDC muscle (hot spot). The stimulus intensity was increased in 10% steps between 90% and 120% of rMT, and 10 MEPs were recorded at each stimulus intensity.
Silent period
This is a measure of cortical inhibition that can be measured using a single stimulator.27,30 Stimulation was delivered at 150% of aMT and 5 recordings of at least 500 milliseconds were performed.
Data Analyses
All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
Statistical analyses were performed on performance assessment (WMFT) outcomes (for both paretic and nonparetic sides) and TMS measures (motor map area, rMT, aMT, silent period, COGx, and COGy for both hemispheres). We compared baseline measures for the 2 groups (experimental vs control). The normality of each measure was checked using the Kolmogorov–Smirnov test. Because of the skewed distribution of the WMFT, a logarithmic transformation was performed on the 15 time-based WMFT measures.31 t tests were used to compare normally distributed measures (either before or after transformations). Transformations did not correct nonnormality of aMT, silent period, and COGy of the less-affected side, therefore the nonparametric Wilcoxon rank–sums test was used for these 3 measures to make comparisons between groups. An analysis of variance (ANOVA) model with repeated measures was fitted to each variable to evaluate group (experimental vs control) and visit (time points 2 and 3) main effects, adjusting for baseline values by including the baseline as a covariate in the ANOVA model. The interaction effect between group and visit was also included in the initial model. If the interaction was not significant at the level of .05, then it was removed from the final model.
RESULTS
Wolf Motor Function Test
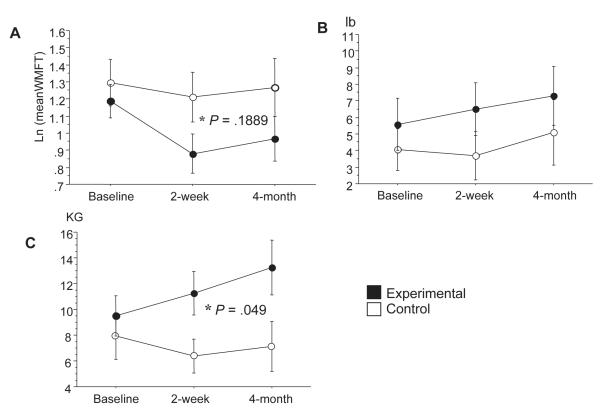
At baseline, there was no difference on time-based and force-based measures for the paretic arm between experimental and control groups (P = .893, .725, and .894, respectively for 15 time-based evaluations, lift, and grip strength). Both groups improved on time-based and force-based measures (Figure 1). The experimental group showed a nonsignificant trend (P = .189) to greater improvement in time-based measures when compared with the control group over a 4-month period (Figure 1A). The weight that could be lifted was not significantly different in experimental subjects compared with control subjects (P = .311, Figure 1B). But grip strength was significantly higher in experimental subjects than in control subjects at postbaseline visits (P = .049, Figure 1C). Time-based and force-based measures remained stable on the nonparetic arm following the 2-week CIMT intervention (Table 1) indicating specificity of the intervention to the paretic arm.
Figure 1.
Effect of constraint-induced movement therapy on Wolf Motor Function Test (WMFT) collected at baseline, after 2 weeks, and at 4-month follow-up. A, Mean of time-based evaluations. B, Mean of force-based measure. C, Mean of force-based measure (grip strength, kg).
Note that the experimental group (black dots) showed improvement in all performance measures after 2 weeks and at 4-month follow-up compared with the control group (white dots). Grip force showed significant improvement compared with the control group (P = .049).
Table 1.
Functional Measures of the Less-Affected Handa
| Experimental | Control | |
|---|---|---|
| Ln mean WFMT (1) | 0.36 ± 0.04 | 0.48 ± 0.05 |
| Ln mean WFMT (2) | 0.32 ± 0.43 | 0.43 ± 0.04 |
| Ln mean WFMT (3) | 0.42 ± 0.07 | 0.45 ± 0.05 |
| Lift (1) lb | 17.21 ± 1.01 | 16.87 ± 1.08 |
| Lift (2) | 17.67 ± 0.74 | 17.08 ± 1.10 |
| Lift (3) | 17.53 ± 0.90 | 16.46 ± 1.78 |
| Grip (1) kg | 30.41 ± 2.81 | 30.08 ± 3.26 |
| Grip (2) | 32.70 ± 2.56 | 33.25 ± 3.20 |
| Grip (3) | 31.99 ± 3.95 | 28.99 ± 3.89 |
Natural log (Ln) mean of 15 time-based measures, force-based subtests 7 (lift) and 14 (grip) collected at baseline (1), after 2 weeks (2), and at 4-month follow-up (3). Data are expressed as mean ± standard error.
Neurophysiological Assessment—TMS
There were no baseline differences for TMS measures, including rMT, aMT, motor map area, COGx, COGy, recruitment curves, and silent periods, comparing experimental and control groups. There was no significant change over time in resting (Table 2). There was a nearly significant increase in TMS motor map area in the experimental group compared with the control (P = .053, Figure 2). Longitudinal changes in TMS motor map area of 2 representative subjects are shown in Figure 3. There was no significant change in COGs over time (Table 2).
Table 2.
Transcranial Magnetic Stimulation Measures of the Contralesional Side in the 2 Treatment Groupsa
| Experimental | Control | |
|---|---|---|
| rMT 1 | 52.06 ± 3.16 | 47.38 ± 3.48 |
| rMT 2 | 51.31 ± 2.69 | 47.23 ± 3.41 |
| rMT 3 | 52.44 ± 2.52 | 49.10 ± 4.46 |
| aMT 1 | 45.28 ± 3.72 | 36.69 ± 3.23 |
| aMT 2 | 42.00 ± 2.90 | 37.69 ± 3.20 |
| aMT 3 | 45.69 ± 3.02 | 36.11 ± 3.04 |
| Motor map area 1 (cm2) | 4.87 ± 0.42 | 4.39 ± 0.43 |
| Motor map area 2 (cm2) | 5.42 ± 0.54 | 5.49 ± 0.71 |
| Motor map area 3 (cm2) | 4.62 ± 0.63 | 4.83 ± 0.97 |
| COGx 1 | 4.70 ± 0.27 | 4.37 ± 0.22 |
| COGx 2 | 4.58 ± 0.24 | 4.16 ± 0.31 |
| COGx 3 | 4.65 ± 0.28 | 3.87 ± 0.34 |
| COGy 1 | 2.76 ± 0.57 | 3.43 ± 0.46 |
| COGy 2 | 2.95 ± 0.57 | 3.44 ± 0.40 |
| COGy 3 | 2.62 ± 0.54 | 3.37 ± 0.64 |
Resting motor threshold (rMT), active motor threshold (aMT), transcranial magnetic stimulation (TMS) motor map area, and centers of gravity for the x-coordinate (COGx) and y-coordinate (COGy) collected in the extensor digitorum communis muscle of the less-affected forearm at baseline (1), after 2 weeks (2), and at 4-month follow-up (3). Data are expressed as mean ± standard error.
Figure 2.
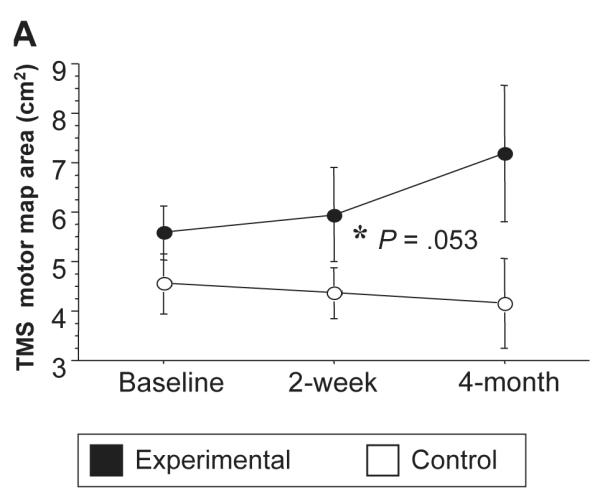
Longitudinal changes in transcranial magnetic stimulation (TMS) motor map area (3A) on the ipsilesional hemisphere. Note that there was a trend for an increased map area when comparing experimental (black dots) and control groups (P = .053) over a 4-month period.
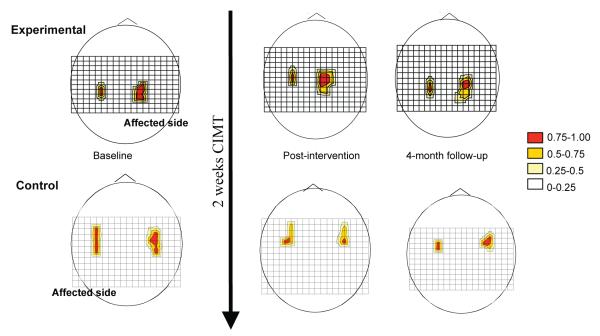
Figure 3.
Longitudinal changes in transcranial magnetic stimulation (TMS) motor map area of 2 representative subjects. The grid size is 1 cm and (0,0) is Cz in the 10-20 EEG system. Motor responses at each scalp position are color-coded by motor evoked potential size (relative to the maximal response). Increased TMS motor map area of ipsilesional hemisphere was observed in a subject assigned to the experimental group over a 4-month period (top diagrams) whereas the map area of the subject assigned to the control group tended to decrease (bottom diagrams).
As mentioned above, thresholds did not change over time in either group. Stimulation used to acquire maps was set to 110% rMT. The stimulus response (recruitment) curve, in the range of stimulus strength used to acquire the maps, did not change over time. A simple measure of inhibition, the silent period, actually increased over time in the experimental group, although not significantly (Figure 4) suggesting a resurgence of inhibition related to recovery, rather than disinhibition that might have explained the increased map size. However, no correlation was found between the silent period change and functional changes (time-based evaluations, lift, and grip strength).
Figure 4.
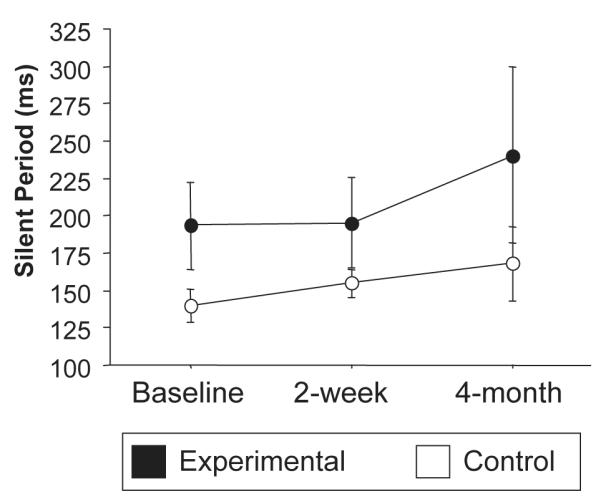
Longitudinal changes in silent periods in the ipsilesional hemisphere. No significant changes were observed when comparing experimental (black) and control (white) groups.
Correlation of map changes with functional changes
Map size correlated with lifting force (r = 0.39, P = −.03) and grip strength (r = 0.44, P = .01) in the experimental group, with no such correlation in the control group. There was a significant positive correlation of map size increase with lifting between the first and third visit (r = 0.75, P = .03) in the experimental group, and a similar, although nonsignificant, correlation in the control group (r = 0.89, P = .11).
Changes in the unaffected hemisphere
TMS measures (thresholds, map size, location, recruitment curve, and silent period) did not differ in the ipsilesional hemisphere. These measures did not change over time or differ significantly between groups (Table 3).
Table 3.
Transcranial Magnetic Stimulation Measures of the Ipsilesional Side in the 2 Treatment Groupsa
| Experimental | Control | |
|---|---|---|
| rMT 1 | 64.54 ± 5.62 | 60.78 ± 7.72 |
| rMT 2 | 62.54 ± 4.45 | 59.33 ± 7.07 |
| rMT 3 | 62.00 ± 4.82 | 66.20 ± 7.72 |
| aMT 1 | 58.08 ± 5.94 | 52.78 ± 6.92 |
| aMT 2 | 52.73 ± 5.01 | 48.11 ± 5.42 |
| aMT 3 | 52.27 ± 4.40 | 51.50 ± 5.78 |
| COGx 1 | 4.36 ± 0.36 | 4.07 ± 0.32 |
| COGx 2 | 5.53 ± 0.32 | 4.06 ± 0.30 |
| COGx 3 | 4.53 ± 0.31 | 3.95 ± 0.65 |
| COGy 1 | 2.63 ± 0.54 | 3.82 ± 0.44 |
| COGy 2 | 2.82 ± 0.41 | 3.87 ± 0.56 |
| COGy 3 | 2.18 ± 0.43 | 3.74 ± 0.27 |
Resting motor threshold (rMT), active motor threshold (aMT), and centers of gravity for the x-coordinate (COGx) and y-coordinate (COGy) collected in the extensor digitorum communis muscle of the more-affected forearm at baseline (1), after 2 weeks (2), and at 4-month follow-up (3). Data are expressed as mean ± standard error.
DISCUSSION
Constraint-induced movement therapy improved hand function after stroke, as assessed by the grip strength, measure of the WMFT, relative to the control group. Our results suggest that CIMT in subjects 3 to 9 months poststroke is associated with an increase in the TMS motor map area of the EDC muscle in the ipsilesional hemisphere, whereas map area remains constant on the contralesional side. There are no significant changes in other TMS parameters that would explain away the map increase as an epiphenomenon resulting from changes in excitability. This observation was most evident for the experimental group, and the map area increased further at 4-month follow-up.
Several studies have provided evidence that CIMT improves the motor recovery of subjects in the chronic poststroke period.10-14 Recently, a large multicenter study enrolling subacute subjects (the EXCITE Trial) showed that CIMT produced superior and more enduring effects when compared with customary care in subjects 3 to 9 months poststroke.15 Imaging studies performed in the chronic poststroke phase have demonstrated associated cortical reorganization.3,6,7,18 However, the underlying mechanism(s) associated with CIMT-induced motor improvements remain(s) unclear, particularly in the subacute phase, which served as the focus of this exploratory study.
Complex patterns of cortical reorganization have been described after recovery from brain lesions such as those resulting from strokes.3,8,9,32,33 Evidence of functional reorganization of areas near the infarcted area, increased activation of the undamaged hemisphere and activation of collateral pathways in the same hemisphere have been suggested as possible mechanisms underlying recovery.33 Carey et al34 reported that subjects in the chronic phase poststroke who were assigned to 20 sessions of training employing finger tracking exhibited improvement on tracking tests, which were correlated with increased activation in primary motor cortex, primary sensory cortex, and premotor cortex of the affected hemisphere.
Expansion of TMS motor maps has been reported in earlier studies applying intensive motor training. Pascual-Leone et al35 undertook topographic motor mapping to investigate motor skill acquisition during piano-playing in healthy subjects, and demonstrated an enlargement of the motor map after 5 days of training.35 Bastings et al36 evaluated 12 subjects with stroke and demonstrated that participants with good recovery had larger TMS cortical maps in the affected hemisphere compared with age-matched healthy volunteers.36 Similarly, Wittenberg et al3 reported expansion of ipsilesional and decrease of contralesional TMS motor maps after CIMT in chronic stroke. Results from fMRI studies have shown greater activation of both ipsilesional nonprimary motor cortices and contralesional motor cortex.4,18
Hamzei et al6 reported that stroke subjects with preserved motor cortical hand areas had decreased ipsilesional activation measured by fMRI, combined with an increase in intracortical excitability measured by TMS. More recently, a reduction in perilesional M1 activation magnitude which tended to be associated with post-CIMT behavior gains (percent decrease in WMFT) has been observed.37 The authors suggest this reflects strengthened synaptic efficacy among the M1 motor neuron pools spared by the lesion, representing a training-related functional consequence of CIMT.37
In our study, the TMS motor map area expansion occurred in the absence of changes in rMT, aMT, recruitment curves and silent period. Therefore, non-specific corticomotor excitability is not likely to confound the interpretation of map area changes in participants undergoing CIMT and meeting inclusion criteria of the EXCITE trial criteria in our study. Additionally, expansion of the TMS motor map area occurred in the absence of shifts of the COG in our study. It has been demonstrated that TMS motor map area as well as the COG after stroke are highly variable, and changes in these parameters do not correlate with clinical outcomes.36 On the other hand, our results differ from those of Liepert et al,7 who demonstrated that enlargement of the TMS cortical motor map area was associated with a mediolateral shift of the COG after CIMT in chronic stroke. These apparent discrepancies in measures of cortical excitability and COG observed in our study compared with TMS studies performed in chronic stroke might also be attributable to differing placement of electrodes,3,7,18 the muscles from which recordings were made24 or the way in which the COG was calculated.38
Expansion of TMS motor map area in the experimental group did not correlate with overall increased performance measured by WMFT, a finding that might be attributable to our limited sample size, compared with the EXCITE trial.15 However, map size did correlate with the force-based measures (lifting force and grip strength), with a significant positive correlation of map size increase with lifting force between the first and third visit, in the experimental but not control group. Both force-based measures probe activity that is relevant to EDC activation: The lifting task recruits both proximal and distal muscles of the upper extremity, as the task requires shoulder flexion, extension of the elbow and wrist, as well as activation of the EDC muscle. The grip force task requires that the subjects are able to activate the EDC muscle prior to activation of the finger flexors. It is possible that the difference in the number of subjects in the experimental and control groups could have led to a type I error in our study. However, in light of the strong effects meassured in the EXCITE trial it is unlikely. In fact, the EXCITE trial enrolled 222 subjects and found significant differences in the time-based evaluations after 2 weeks of intervention. In the current study, we found only non-significant trends in time-based evaluations between the experimental and control groups. It is conceivable that a much larger number of subjects may be required in order to conclusively unveil patterns of brain reorganization after CIMT. But, intriguingly, the ipsilesional TMS motor maps tended to decrease in area among the control subjects across time, suggesting that the concept of learned nonuse may apply during the subacute period. We are currently analyzing data on chronic stroke subjects undergoing the same therapy; it is possible that subjects in this group may have smaller than normal maps, with more potential for expansion due to therapy.
Mechanism of map area expansion
Expansion of TMS motor map area was not associated with specific measurable differences in motor cortical excitability, such as recruitment curves, changes in motor thresholds, or inhibition, represented by silent periods. This appears to eliminate the possibility of bias of map size by changes in excitability. This suggests that CIMT induces brain reorganization that results in a more distributed motor representation for upper-extremity muscles. This may extend more laterally, along the precentral sulcus, or more sagitally, with recruitment of neurons in anterior (Brodmann area 6) or posterior (areas 1-3) regions. The stimulation method is not particularly sensitive to distinguish between these.
In conclusion, this study provides evidence that subjects undergoing CIMT in the subacute phase after stroke may have functional cortical reorganization as demonstrated by enlargement of TMS motor maps. Although this study represents the first multicenter effort to measure the cortical reorganization induced by CIMT in subjects who are in the subacute phase of recovery, it remains to be determined which specific cortical areas are engaged in the recovery of motor function.
ACKNOWLEDGMENTS
This study was sponsored by NICHD 1 RO1 HD-40984-01A1. We gratefully thank the therapists, nurses, and research assistants from all sites for invaluable work during data collection.
REFERENCES
- 1.Ottenbacher K. Cerebral vascular accident: some characteristics of occupational therapy evaluation forms. Am J Occup Ther. 1980;34:268–271. doi: 10.5014/ajot.34.4.268. [DOI] [PubMed] [Google Scholar]
- 2.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 3.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 4.Schaechter JD, Kraft E, Hilliard TS, et al. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 5.Hamada I, DeLong MR. Excitotoxic acid lesions of the primate subthalamic nucleus result in reduced pallidal neuronal activity during active holding. J Neurophysiol. 1992;68:1859–1866. doi: 10.1152/jn.1992.68.5.1859. [DOI] [PubMed] [Google Scholar]
- 6.Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. Neuroimage. 2006;31:710–720. doi: 10.1016/j.neuroimage.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Liepert J, Miltner WH, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 8.Rossini PM, Caltagirone C, Castriota-Scanderbeg A, et al. Hand motor cortical area reorganization in stroke: a study with fMRI, MEG and TCS maps. Neuroreport. 1998;9:2141–2146. doi: 10.1097/00001756-199806220-00043. [DOI] [PubMed] [Google Scholar]
- 9.Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14(1 Suppl):S57–S76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 10.Taub E, Uswatte G. Constraint-induced movement therapy: bridging from the primate laboratory to the stroke rehabilitation laboratory. J Rehabil Med. 2003;(41 Suppl):34–40. doi: 10.1080/16501960310010124. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 12.Ostendorf CG, Wolf SL. Effect of forced use of the upper extremity of a hemiplegic patient on changes in function. A single-case design. Phys Ther. 1981;61:1022–1028. doi: 10.1093/ptj/61.7.1022. [DOI] [PubMed] [Google Scholar]
- 13.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Devillé WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 14.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 15.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 16.Liepert J, Uhde I, Gräf S, Leidner O, Weiller C. Motor cortex plasticity during forced-use therapy in stroke patients: a preliminary study. J Neurol. 2001;248:315–321. doi: 10.1007/s004150170207. [DOI] [PubMed] [Google Scholar]
- 17.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 18.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 19.Wolf SL, Binder-MacLeod SA. Electromyographic biofeedback applications to the hemiplegic patient. Changes in upper extremity neuromuscular and functional status. Phys Ther. 1983;63:1393–1403. doi: 10.1093/ptj/63.9.1393. [DOI] [PubMed] [Google Scholar]
- 20.Taub E, Crago JE, Burgio LD, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61:281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panyan M. How to Use Shaping. H & H Enterprises; Lawrence, KS: 1980. [Google Scholar]
- 22.Winstein C. Designing practice for motor learning: clinical impressions. In: Lister M, editor. Contemporary Management of Motor Control Problems: Proceedings of the II Step Conference; Alexandria, VA. Foundation for Physical Therapy; 1991. [Google Scholar]
- 23.Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. Human Kinetics; Champaign, IL: 1999. [Google Scholar]
- 24.Wolf SL, Butler AJ, Campana GI, et al. Intra-subject reliability of parameters contributing to maps generated by transcranial magnetic stimulation in able-bodied adults. Clin Neurophysiol. 2004;115:1740–1747. doi: 10.1016/j.clinph.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol. 1992;85:9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 27.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 28.Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- 29.Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell JC. Physiological studies of electric and magnetic stimulation of the human brain. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:29–35. [PubMed] [Google Scholar]
- 31.Wolf SL, Thompson PA, Morris DM, et al. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 32.Chollet F, Weiller C. Imaging recovery of function following brain injury. Curr Opin Neurobiol. 1994;4:226–230. doi: 10.1016/0959-4388(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 33.Chollet F, Loubinoux I, Carel C, Marque P, Albucher JF, Guiraud-Chaumeil Mechanisms of motor recovery after cerebrovascular accident [in French] Rev Neurol (Paris) 1999;155:718–724. [PubMed] [Google Scholar]
- 34.Carey JR, Kimberley TJ, Lewis SM, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 35.Pascual-Leone A, Grafmann J, Hallet M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- 36.Bastings EP, Greenberg JP, Good DC. Hand motor recovery after stroke: a transcranial magnetic stimulation mapping study of motor output areas and their relation to functional status. Neurorehabil Neural Repair. 2002;16:275–282. doi: 10.1177/154596802401105207. [DOI] [PubMed] [Google Scholar]
- 37.Dong Y, Winstein CJ, Albistegui-Dubois R, Dobkin BH. Evolution of FMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21:412–428. doi: 10.1177/1545968306298598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler AJ, Wolf SL. Transcranial magnetic stimulation to assess cortical plasticity: a critical perspective for stroke rehabilitation. J Rehabil Med. 2003;(41 Suppl):20–26. doi: 10.1080/16501960310010106. [DOI] [PubMed] [Google Scholar]