Abstract
Social insect colonies are like fortresses, well protected and rich in shared stored resources. This makes them ideal targets for exploitation by predators, parasites and competitors. Colonies of Myrmica rubra ants are sometimes exploited by the parasitic butterfly Maculinea alcon. Maculinea alcon gains access to the ants' nests by mimicking their cuticular hydrocarbon recognition cues, which allows the parasites to blend in with their host ants. Myrmica rubra may be particularly susceptible to exploitation in this fashion as it has large, polydomous colonies with many queens and a very viscous population structure. We studied the mutual aggressive behaviour of My. rubra colonies based on predictions for recognition effectiveness. Three hypotheses were tested: first, that aggression increases with distance (geographical, genetic and chemical); second, that the more queens present in a colony and therefore the less-related workers within a colony, the less aggressively they will behave; and that colonies facing parasitism will be more aggressive than colonies experiencing less parasite pressure. Our results confirm all these predictions, supporting flexible aggression behaviour in Myrmica ants depending on context.
Keywords: aggression, defence, social parasite, Maculinea
1. Introduction
The ability to discriminate between relatives and non-relatives should give an advantage to the inclusive fitness of an individual, and is therefore expected to be favoured by kin selection [1,2]. Inclusive fitness can only increase if the benefits of shared labour can be directed towards colony members or close relatives rather than towards foreigners [2]. Social insects, such as ants, have indeed achieved sophisticated nest-mate recognition skills, and high levels of aggression are generally expressed towards non-nest-mates, which potentially pose a cost to the society. Even though social insects have evolved an array of discrimination cues, odours are thought to be the predominant cues for the discrimination of nest-mates and non-nest-mates. Considerable direct and indirect evidence has been collected in favour of cuticular hydrocarbons serving as the main cues for nest-mate recognition in ants [3–5].
Several models of the proximate recognition mechanisms have been put forward to explain how ants in huge societies are able to tell friends from foes. Crozier & Dix [6] proposed the ‘Gestalt’ model, which assumes that colony members mix their odours and recognize a composite odour for their own colony, which allows for discrimination of workers from other colonies that have a different distribution of odour locus alleles. Reeve [7] proposed an additional action component to the model, triggering aggression according to cues perceived by the actor exceeding or falling below a certain threshold. The model aims to adjust the threshold to minimize acceptance errors (acceptance of a non-nest-mate) and rejection errors (rejection of a nest-mate) according to the needs of a colony. The more costly an acceptance or rejection error will be, the more likely it is that the thresholds will shift in order to avoid any costly mistake.
To test the implications of this model, we examined colonies from a population of the ant Myrmica rubra, at a site where it co-occurs with its social parasite Maculinea alcon. Myrmica rubra is a common Palaearctic ant species, with a highly variable colony structure, ranging from monogynous and monodomous to highly polygynous and polydomous populations [8], with queen number within populations being a very variable trait [9,10]. Relatedness among My. rubra nest-mates has been shown to be directly correlated with the number of reproductively active queens in the colony [11]. Myrmica rubra sexuals tend to mate close to the home nest, and new nests are usually started when the mother colony fissions (i.e. the colony splits and workers leave the mother colony together with at least one inseminated queen) [12].
Maculinea alcon is a socially parasitic butterfly whose larvae take advantage of the shelter and resources of ant nests. The butterfly caterpillars feed on their host plant Gentiana pneumonanthe for their first three instars before they leave the plant in their fourth and final instar, and await carriage back to the nest by a Myrmica ant. This is facilitated by mimicking the ant brood's cuticular hydrocarbon cues [13]. Once inside the nest, the ants will treat the caterpillars like their own larvae, feeding them by trophallaxis. Additionally, the caterpillars feed directly on ant brood. Since G. pneumonanthe is patchily distributed relative to the common Myrmica host ants, areas where ants face strong parasitism by My. alcon are typically small ‘islands’ defined by gentian patches within a local Myrmica population. Nevertheless, it has been shown [14] that the viscous population structure of My. rubra has allowed M. alcon to enter into a coevolutionary arms race with its host ants, altering their cuticular hydrocarbon profiles.
In the present study, we compared how aggressively workers from colonies of My. rubra behave towards conspecific intruders from areas of the same population where the social parasite M. alcon is present with areas where it is absent. We collected data on the genetic structure and the chemical profiles of colonies to be able to test several predictions arising from Reeve's [7] optimal acceptance threshold model:
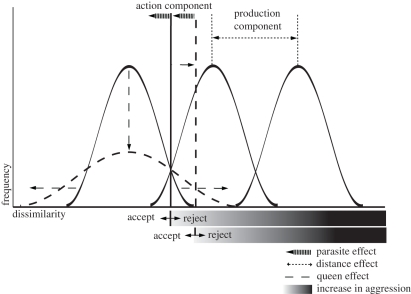
— If neighbouring colonies are more closely related to each other than to colonies further away, recognition cue similarity should decline with geographical distance. Without a change in acceptance threshold, aggression is therefore expected to rise with distance (production component; figure 1).
— With more queens in a colony, genetic diversity will rise. As a consequence, unless there is perfect mixing of the gestalt odour within colonies, the recognition cue distribution will broaden. We thus expect the acceptance threshold to right-shift (ants get less aggressive) in order to avoid too many costly rejection errors (action component; figure 1).
— In populations that suffer from social parasitism, the acceptance threshold is expected to shift to the left, since acceptance errors are more costly. Colonies from areas where Maculinea is present are therefore expected to act more aggressively compared with colonies that live in areas where the Maculinea parasite is absent (action component; figure 1).
Figure 1.
Frequency distribution of cue dissimilarities between the actor template (left of acceptance threshold) and recipients (non-nest-mates) phenotype (right of the acceptance threshold). Queen effect (dashed lines) causes the cue dissimilarity distribution (CDD) to broaden and as a result to shift the acceptance threshold to the right in order to avoid rejection errors (rejection of nest-mates); at the same time, with the right shift the risk of acceptance errors (acceptance of non-nest-mates) increases. Social parasites can take advantage of this ‘open door’. In order to avoid social parasites, the threshold needs to be set to more restrictive values (action component; dashed arrow). With increasing distance (physically, chemically or genetically; dotted lines) overlaps between template CDD and recipient phenotype CDD are lower, since the CDDs diverge (production component) and it becomes safer to unleash higher aggression towards an encountered recipient.
2. Material and methods
(a). Ant collection and housing
We collected a total of eight My. rubra colonies (hereafter ‘test colonies’) in June 2006 from the Nordmarken area of the Danish island of Læsø. Myrmica rubra colonies were collected along two parallel transects, 50 m apart. One of the transects was laid out within a 1.1 hectare patch of G. pneumonanthe plants, where the social parasite Maculinea alcon is common and My. rubra faces severe parasitism [15]. The second transect ran through an area separated from the flying areas of M. alcon by low shrubs, and too dry for G. pneumonanthe to grow. Along the second transect, Maculinea parasitism has not been observed since we started monitoring in 1998 and is highly unlikely since My. rubra do not forage more than a few metres from their nests [16]. Our collections took place at two sampling points 100 m apart along each transect. At each sampling point, we completely excavated two neighbouring Myrmica rubra nests (resulting in four colonies per transect).
Colony social structure was examined based on the number of excavated queens and using three highly variable microsatellite loci (for more detailed information see the electronic supplementary material).
In natural colonies, the number of queens observed in a nest and the genetic diversity among the workers in the nest (which is expected to influence cue diversity), while correlated, often diverge from each other owing to reproductive skew, variation in mating frequency among queens and queen turnover. To try to tease apart the effects of genetic relatedness and queen number per se, we collected an additional nine My. rubra colonies from the area without parasitism just after hibernation in March 2008, and controlled queen number (matrilines) within the nests (hereafter ‘matriline colonies’) by removing queens and allowing a new cohort of workers to develop. This allowed us to decouple the possible effect of innate aggressiveness on genetic diversity that could arise if less aggressive colonies are more likely to adopt new queens (for further information see the electronic supplementary material).
(b). Aggression assays
Directed aggression tests in a controlled, neutral arena were performed with the eight test colonies using a full factorial design, with each colony acting as either defenders (five workers per encounter) or intruders (one worker per encounter) in interactions with every other colony. Three replicates were conducted for each of the 64 possible pairs of colonies. For the matriline colonies, we were not able to test the colonies in a full factorial design since too few workers were produced in the small laboratory colonies, but instead each colony was tested against every other as either defender or intruder. For each test, an aggression index was calculated for how aggressively the defenders responded to the intruder (for further information see the electronic supplementary material).
(c). Surface chemistry
The cuticular hydrocarbons of ten of the genetically analysed workers were extracted and analysed on a gas chromatograph with mass spectrometer (GC-MS) detector, using standard methods (for further information see the electronic supplementary material).
(d). Statistical analysis
Correlations between geographical, genetic and chemical distances between colonies were assessed using Mantel tests, as implemented in GenAlEx v. 6.2 [17].
To test the various hypotheses generated by the adjustable threshold model, we assessed the relationship between the measured index of aggression and transect (i.e. presence or absence of M. alcon), three different measures of distance between colonies (geographical, chemical and genetic) and three different measures of colony social structure (the number of queens excavated with the colony, and the number of queens and the within-colony relatedness estimated from microsatellite markers). The aggression index was loge-transformed for the analysis, to normalize its distribution, and nine separate models were run for each of the combinations of different distance and social structure measurements.
For the matriline colonies, the effect of number of matrilines and geographical distance on loge aggression index was assessed with a nested ANCOVA including number of matrilines, geographical distance and their interaction. Since we assigned colonies to have a particular number of matrilines, matriline number (1, 3, 5 or 9) was treated as a categorical variable, and colony identity was nested within matriline number. Analyses were conducted using the software package JMP v. 7.02 (SAS Institute).
Analysis of cuticular hydrocarbon profiles was based on the areas of 26 chromatogram peaks, which represented single or co-eluting cuticular hydrocarbons. Prior to multivariate analysis, variable number was reduced and multicollinearity was eliminated by using principal components analysis (PCA), which yielded two principal components (PCs) explaining significantly more of the variance than expected under a broken stick model [18]. Discriminant analysis based on those two PCs was performed to distinguish between our predefined groupings, the colonies, the sampling points and the transects. Chemical distance between colonies was calculated as the Euclidean distance between the samples from pairs of colonies in PC space.
3. Results
Two of the four colonies collected within the parasitized transect had one and four M. alcon caterpillars present, respectively. The number of queens collected with the test colonies ranged from 1 to 17 (mean 8.6). However, based on three microsatellite markers, mean number of queens per colony was estimated as 11.25, ranging from 7 to 15. The correlation between excavated number of queens and estimated number of mother queens was +0.558 (n = 8, p = 0.125). Within-colony relatedness estimates ranged from 0.020 to 0.314 (mean 0.139), and were significantly negatively correlated with both the excavated number of queens (r = −0.731, p = 0.040) and the number of queens estimated from microsatellite markers (r = −0.806, p = 0.016).
For the analysis of cuticular hydrocarbon profiles, the first PC explained 59.0 per cent of the total variance and the second PC explained an additional 13.9 per cent. A discriminant analysis based on the two PCs and grouping by colony, sampling point or transect found significant diversification, with colonies, sampling points and transects being significantly different from each other (colonies: Wilks' λ = 0.247, F14,146 = 10.54, p < 0.0001; sampling points: Wilks' λ = 0.412, F6,154 = 14.32, p < 0.0001; transects: Wilks' λ = 0.845, F2,79 = 7.24, p = 0.0013).
As predicted, based on the previously reported genetic viscosity of M. rubra populations, genetic, chemical and geographical distances between the test colonies were significantly correlated (Mantel tests: geographical versus genetic distances: r = +0.373, p = 0.001; geographical versus chemical distances: r = +0.388, p = 0.036; genetic versus chemical distances: r = +0.417, p = 0.032).
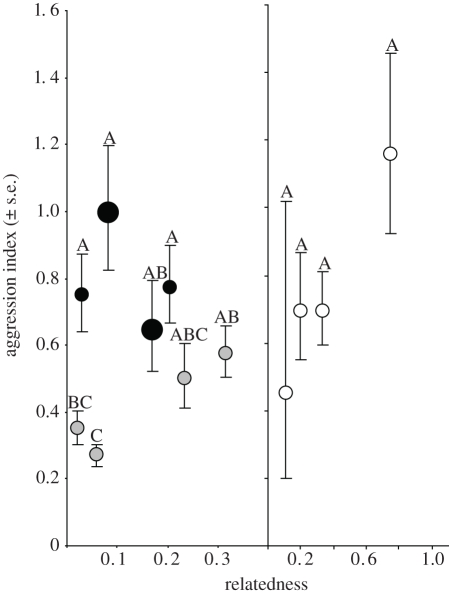
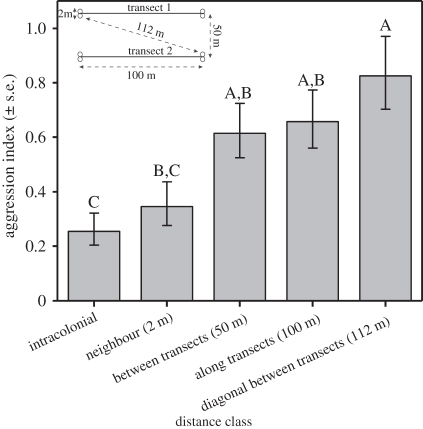
Overall, test colonies showed significant differences in aggression levels (figure 2; ANOVA, F7,184 = 6.91, p < 0.0001). Colonies collected from the transect where M. alcon was present were significantly more aggressive than those from the transect where M. alcon was absent, and the difference between transects explained 65 per cent of the variance in aggression that could be attributed to differences between colonies (nested ANOVA: between transects, F1,6 = 11.3, p = 0.015; colonies nested within transects, F6,184 = 2.79, p = 0.013). When we constructed ANCOVA models using measures of distance between defender-colony and intruder-colony, defender-colony social structure, and transect from which the defender-colony was collected, some general patterns emerged for all estimates of distance and social structure (table 1). Once again, transect was highly significant in all analyses, with aggression being higher on the M. alcon transect. In six of the nine analyses, there was also a significant interaction between transect and social structure of the defender colony, and this effect was close to significance in the other three models. This arose because there was no relationship between social structure and aggression on the transect with M. alcon present (where aggression was uniformly high), but on the transect with M. alcon absent, aggression decreased as the number of queens increased and within-colony relatedness decreased (figure 2). Geographical distance between home and intruder colonies also explained a significant portion of the variance in aggression index in the analysis in which it was included, but there was no effect of the other distance measures (i.e. genetic and chemical distances; table 1). The effect of distance was primarily because aggression was significantly lower between colonies collected at the same location (and not significantly different from aggression towards nest-mates) than between colonies collected further apart (figure 3).
Figure 2.
Relationship between within-colony relatedness and mean aggression levels per colony (±s.e.). White circles are colonies with artificially constructed matrilines, grey circles are colonies collected outside M. alcon areas, while black circles are colonies collected within a M. alcon area. Larger circles indicate the presence of Maculinea caterpillars when collected. Levels not connected by the same letter are significantly different (Tukey–Kramer HSD).
Table 1.
Results of ANCOVA examining the effect of distance between home and intruder colonies, social structure of home colonies and area of origin (transect) of home colonies on loge of the aggression index for the test colonies. Significant effects (p < 0.05) are highlighted in bold.
| distance measure |
||||||
|---|---|---|---|---|---|---|
| geographical |
genetic |
chemical |
||||
| source | F1,160 | p | F1,160 | p | F1,160 | p |
| social structure as number of queens counted during excavation | ||||||
| distance (D) | 19.1 | <0.001 | 1.08 | 0.301 | 2.10 | 0.149 |
| no. queens (Q) | 2.72 | 0.101 | 0.849 | 0.358 | 1.10 | 0.295 |
| transect (T) | 38.9 | <0.001 | 24.3 | <0.001 | 26.0 | <0.001 |
| D × Q | 0.429 | 0.514 | 0.465 | 0.497 | 0.022 | 0.882 |
| D × T | 1.41 | 0.238 | 0.450 | 0.503 | 1.83 | 0.178 |
| Q × T | 4.62 | 0.033 | 3.79 | 0.053 | 4.85 | 0.029 |
| D × Q × T | 0.224 | 0.637 | 0.068 | 0.795 | 0.427 | 0.515 |
| r2 | 0.332 | 0.252 | 0.263 | |||
| social structure as number of queens estimated from microsatellites | ||||||
| distance (D) | 11.3 | <0.001 | 0.129 | 0.720 | 3.07 | 0.081 |
| no. queens (Q) | 0.001 | 0.986 | 0.005 | 0.942 | 0.013 | 0.909 |
| transect (T) | 36.9 | <0.001 | 23.5 | <0.001 | 22.9 | <0.001 |
| D × Q | 0.808 | 0.370 | 0.860 | 0.355 | 0.299 | 0.585 |
| D × T | 0.676 | 0.412 | 1.25 | 0.265 | 0.732 | 0.393 |
| Q × T | 7.47 | 0.007 | 5.29 | 0.023 | 5.37 | 0.022 |
| D × Q × T | 2.00 | 0.160 | 1.22 | 0.272 | 0.013 | 0.908 |
| r2 | 0.347 | 0.266 | 0.277 | |||
| social structure as within-colony relatedness | ||||||
| distance (D) | 16.3 | <0.001 | 1.02 | 0.314 | 2.26 | 0.134 |
| relatedness (R) | 0.996 | 0.320 | 1.33 | 0.251 | 0.780 | 0.378 |
| transect (T) | 44.4 | <0.001 | 29.0 | <0.001 | 28.7 | <0.001 |
| D × R | 0.003 | 0.955 | 0.349 | 0.556 | 0.133 | 0.716 |
| D × T | 1.71 | 0.192 | 0.486 | 0.487 | 1.64 | 0.203 |
| R × T | 5.30 | 0.023 | 1.94 | 0.165 | 3.65 | 0.058 |
| D × R × T | 1.26 | 0.263 | 0.831 | 0.364 | 0.006 | 0.939 |
| r2 | 0.327 | 0.247 | 0.252 | |||
Figure 3.
The relationship between distance and aggression index for the test colonies. Levels not connected by the same letter are significantly different (Tukey–Kramer HSD). The schematic in the top left corner shows relationship between transects and distances.
For the matriline colonies, there was a significant effect of number of matrilines on loge aggression index (nested ANCOVA: F3,9.37 = 5.21, p = 0.022), but no effect of geographical distance (F1,23 = 0.235, p = 0.633) nor the interaction between matriline number and distance (F1,23 = 0.284, p = 0.836), and there was no difference in aggression index of colonies within each matriline treatment (F4,23 = 0.549, p = 0.701). Aggression decreased with increasing number of matrilines, with monogynous colonies being more aggressive than those with three or five queens (figure 2).
4. Discussion
We demonstrated that aggressive behaviour of Myrmica rubra workers is a variable trait that depends on both external and internal factors. The three hypotheses that we tested were all largely verified. In accordance with Reeve's [7] optimal acceptance threshold model, we found that acceptance thresholds became more restrictive as fitness costs for the colony rise.
In our aggression assays, five ‘defender’ ants encountered one intruder in a neutral area. The five ants should have a higher eagerness to fight since the presence of nest-mates is very likely to indicate the proximity of the home colony, and aggressive defence behaviour should therefore be more beneficial to the ‘defender’ ants. The intruder should benefit from retreat, and is less likely to act aggressively [19]. Buczkowski & Silverman [20] showed that acceptance thresholds shift according to the presence of nest-mates or nest material indicating colony proximity in Linepithema humile. We performed our assays under standardized conditions in a neutral arena to keep the influence of the surrounding conditions to a minimum. Differences in aggression are therefore expected to be caused by something other than the presence of nest-mates or nest material.
We confirmed our first hypothesis: that aggression will increase with increasing distance. The rise in geographical distance is correlated with a rise in genetic distance as well as chemical distance. These differences in cue distribution represent the production component in Reeve's [7] optimal acceptance threshold model. Colonies further apart have more dissimilar recognition cue distributions, the overlap in cue dissimilarity distribution (CDD) is reduced, and rejection and acceptance errors are less likely to occur. The aggression level rises gradually and is not an ‘all or nothing’ response (figure 1), as has been shown previously in other studies (e.g. [21,22]). Selection should generally favour narrow CDDs with high frequencies of desirable recipients, since this allows for restrictive thresholds and at the same time minimizes rejection errors [7]. Genetically diverse ant societies achieve this by mixing their recognition cues throughout the colony, to form a ‘gestalt’ odour [6,23], narrowing down the CDD. This mechanism is less efficient in more diverse and larger colonies. Monogamous monogyny is thought to be the ancestral state in social insects [24], and also the condition under which CDDs can be expected to be narrowest. Polygyny has evolved later [24,25] to improve colony efficiency [26], and perhaps reduce parasite pressure [27]. Our source Myrmica rubra population was highly polygynous, as is typical for this species [28,29]. We collected only one apparently monogynous colony, which contained multiple matrilines after estimating queen number using genetic markers. Overall, our queen numbers estimated using genetic markers were higher than the numbers of queens found during excavation, particularly when one considers that estimates based on genetic markers are minimum estimates. This pattern could emerge if workers in polydomous colonies were to move and mix freely between the nests [30] or if queen turnover was so high that we collected workers from queens that were no longer alive [30,31]. We can also not rule out that some queens escaped our collection routine.
In accordance with our second hypothesis, aggression level decreased with increasing genetic diversity within colonies, but only in those areas where the social parasite Maculinea alcon was absent (see below). We see the same pattern in the matriline colonies even though the results do not reach significance, probably owing to the low number of colonies we had available for testing. To make sure that increased genetic diversity (in terms of queen number) was in itself not an effect of low aggressiveness, we reared colonies from different combinations of laboratory queens, performed the same aggression assays and obtained the same results: aggression level dropped with increasing numbers of queens. We are therefore confident that genetic diversity determines aggressiveness and not vice versa.
Ants in areas where the social parasite M. alcon is present were far more aggressive than ants collected in habitat where this parasite is absent. However, the pattern of decreasing aggression with increasing colony genetic diversity was not apparent in these areas (figure 2). This is initially surprising, since Thomas Als (results reported in [5]) was able to show that within-nest relatedness of parasitized My. rubra colonies was lower than that of unparasitized colonies collected on the island of Læsø, implying that increased genetic diversity facilitates exploitation. From these results, we would thus expect to find lower aggression levels in colonies with high queen numbers facilitating the acceptance of the parasite. The lack of this pattern in the parasite area might be caused by colonies having reached the upper limit of their aggression level, rendering any more aggression too costly for a colony. Another explanation would be our somewhat low sample size not picking up the pattern.
As hypothesized, we found strong context-dependent aggression in Myrmica ants, with large differences in aggression within only a few metres between the two transects. Chemical analysis showed a significant diversification between ants from parasite areas and ants outside parasite areas, which is in accordance with the findings of Nash et al. [14], who could confirm a coevolutionary arms race between the low-dispersal My. rubra ants and M. alcon. While we believe this to be a likely consequence of parasite pressure by Maculinea butterflies, we cannot rule out that habitat differences also play a role. While the transect with the parasite present was rather boggy, the transect without the parasite was relatively dry; whether this has any effect on the aggression is unknown, although it may have an influence on the chemical profile [20]. Our whole-model approach showed that geographical distance and transect explain aggression best, while genetic and chemical distances do not explain aggression. Our study makes an interesting comparison with that of Lorenzi [32] on the wasp Polistes biglumis and its social parasite Polistes atrimandibularis. Wasps learn new compounds added by the parasite to the colony as being from their own colony, and therefore widen their template. Lorenzi found colonies with parasites to be more permissive towards non-nest-mates, but also less permissive to nest-mates, compared with unparasitized colonies. While this apparently illogical result was explained in terms of impaired template learning abilities of the parasitized wasp colonies, it can also be readily interpreted in terms of Reeve's [7] adjustable threshold model. As the template gets wider, the host workers need to shift towards a more permissive overall template, and more acceptance errors will occur when compared with unparasitized colonies. At the same time, this threshold should shift, relative to the unparasitized colonies, towards a less permissive acceptance level caused by the parasite pressure, explaining the relative higher number of nest-mate rejections.
In summary, the variable aggression shown by My. rubra colonies can be largely explained by Reeve's [7] adjustable threshold model. The additional costs of failure to recognize intruders imposed by social parasites appear from our results to have a major influence on acceptance or rejection thresholds, which are adjusted in an adaptive manner.
Acknowledgements
We thank T. A. Hansen and E. Stafflinger for assistance in the field, J. J. Boomsma for helpful comments on the manuscript, and the Centre for Social Evolution for providing such a stimulating work environment. The project was founded via a Danish National Research Foundation grant to the Centre for Social Evolution.
References
- 1.Hamilton W. D. 1964. Genetical evolution of social behaviour I. J. Theor. Biol. 7, 1–16 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Hamilton W. D. 1964. Genetical evolution of social behaviour II. J. Theor. Biol. 7, 17–52 10.1016/0022-5193(64)90039-6 (doi:10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 3.Lahav S., Soroker V., Hefetz A., Vander Meer R. K. 1999. Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86, 246–249 10.1007/s001140050609 (doi:10.1007/s001140050609) [DOI] [Google Scholar]
- 4.Lenoir A., D'Ettorre P., Errard C., Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599 10.1146/annurev.ento.46.1.573 (doi:10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 5.Nash D. R., Boomsma J. J. 2008. Communication between hosts and social parasites. In Sociobiology of communication (eds d'Ettore P., Hughes D. P.), pp. 55–81 Oxford, UK: Oxford University Press; 10.1093/acprof:oso/9780199216840.003.0004 (doi:10.1093/acprof:oso/9780199216840.003.0004) [DOI] [Google Scholar]
- 6.Crozier R. H., Dix M. W. 1979. Analysis of 2 genetic models for the innate components of colony odor in social hymenoptera. Behav. Ecol. Sociobiol. 4, 217–224 10.1007/BF00297645 (doi:10.1007/BF00297645) [DOI] [Google Scholar]
- 7.Reeve H. K. 1989. The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435 10.1086/284926 (doi:10.1086/284926) [DOI] [Google Scholar]
- 8.Pedersen J. S., Boomsma J. J. 1999. Positive association of queen number and queen-mating frequency in Myrmica ants: a challenge to the genetic-variability hypotheses. Behav. Ecol. Sociobiol. 45, 185–193 10.1007/s002650050552 (doi:10.1007/s002650050552) [DOI] [Google Scholar]
- 9.Elmes G. W., Petal J. 1990. Queen number as an adaptable trait—evidence from wild populations of 2 red ant species (Genus Myrmica). J. Anim. Ecol. 59, 675–690 10.2307/4888 (doi:10.2307/4888) [DOI] [Google Scholar]
- 10.Seifert B. 1988. A taxonomic revision of the Myrmica species of Europe, Asia Minor, and Caucasia (Hymenoptera, Formicidae). Abh. Ber. Naturkundemuseums Goerlitz 62, 1–75 [Google Scholar]
- 11.Seppä P., Walin L. 1996. Sociogenetic organization of the red ant Myrmica rubra. Behav. Ecol. Sociobiol. 38, 207–217 10.1007/s002650050234 (doi:10.1007/s002650050234) [DOI] [Google Scholar]
- 12.Seppä P., Pamilo P. 1995. Gene flow and population viscosity in Myrmica ants. Heredity 74, 200–209 10.1038/hdy.1995.28 (doi:10.1038/hdy.1995.28) [DOI] [Google Scholar]
- 13.Akino T., Knapp J. J., Thomas J. A., Elmes G. W. 1999. Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. Lond. B 266, 1419–1426 10.1098/rspb.1999.0796 (doi:10.1098/rspb.1999.0796) [DOI] [Google Scholar]
- 14.Nash D. R., Als T. D., Maile R., Jones G. R., Boomsma J. J. 2008. A mosaic of chemical coevolution in a large blue butterfly. Science 319, 88–90 10.1126/science.1149180 (doi:10.1126/science.1149180) [DOI] [PubMed] [Google Scholar]
- 15.Zeisset I., et al. Submitted Island populations of the parasitic butterfly Maculinea alcon choose their host ant species on the basis of local availability rather than genetic specialization. J. Evol. Biol. [Google Scholar]
- 16.Elmes G. W., Thomas J. A., Wardlaw J. C., Hochberg M. E., Clarke R. T., Simcox D. J. 1998. The ecology of Myrmica ants in relation to the conservation of Maculinea butterflies. J. Insect Conserv. 2, 67–78 10.1023/A:1009696823965 (doi:10.1023/A:1009696823965) [DOI] [Google Scholar]
- 17.Peakall R., Smouse P. E. 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295 10.1111/j.1471-8286.2005.01155.x (doi:10.1111/j.1471-8286.2005.01155.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson D. A. 1993. Stopping rules in principal components analysis: a comparison of heuristic and statistical approaches. Ecology 74, 2204–2214 10.2307/1939574 (doi:10.2307/1939574) [DOI] [Google Scholar]
- 19.Starks P. T., Fischer D. J., Watson R. E., Melikian G. L., Nath S. D. 1998. Context-dependent nestmate discrimination in the paper wasp, Polistes dominulus: a critical test of the optimal acceptance threshold model. Anim. Behav. 56, 449–458 10.1006/anbe.1998.0778 (doi:10.1006/anbe.1998.0778) [DOI] [PubMed] [Google Scholar]
- 20.Buczkowski G., Silverman J. 2005. Context-dependent nestmate discrimination and the effect of action thresholds on exogenous cue recognition in the Argentine ant. Anim. Behav. 69, 741–749 10.1016/j.anbehav.2004.06.027 (doi:10.1016/j.anbehav.2004.06.027) [DOI] [Google Scholar]
- 21.Carlin N. F., Hölldobler B. 1983. Nestmate and kin recognition in interspecific mixed colonies of ants. Science 222, 1027–1029 10.1126/science.222.4627.1027 (doi:10.1126/science.222.4627.1027) [DOI] [PubMed] [Google Scholar]
- 22.Errard C., Hefetz A. 1997. Label familiarity and discriminatory ability of ants reared in mixed groups. Insectes Soc. 44, 189–198 10.1007/s000400050040 (doi:10.1007/s000400050040) [DOI] [Google Scholar]
- 23.van Zweden J. S., D'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry and chemical ecology (eds Blomquist G. J., Bagnères A.-G.), pp. 222–243 Cambridge, UK: Cambridge University Press [Google Scholar]
- 24.Boomsma J. J. 2007. Kin selection versus sexual selection: why the ends do not meet. Curr. Biol. 17, R673–R683 10.1016/j.cub.2007.06.033 (doi:10.1016/j.cub.2007.06.033) [DOI] [PubMed] [Google Scholar]
- 25.Hughes W. O. H., Oldroyd B. P., Beekman M., Ratnieks F. L. W. 2008. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320, 1213–1216 10.1126/science.1156108 (doi:10.1126/science.1156108) [DOI] [PubMed] [Google Scholar]
- 26.Cole B. J. 1983. Multiple mating and the evolution of social behavior in the hymenoptera. Behav. Ecol. Sociobiol. 12, 191–201 10.1007/BF00290771 (doi:10.1007/BF00290771) [DOI] [Google Scholar]
- 27.Liersch S., Schmid-Hempel P. 1998. Genetic variation within social insect colonies reduces parasite load. Proc. R. Soc. Lond. B 265, 221–225 10.1098/rspb.1998.0285 (doi:10.1098/rspb.1998.0285) [DOI] [Google Scholar]
- 28.Elmes G. W. 1973. Observations on density of queens in natural colonies of Myrmica rubra L. (Hymenoptera: Formicidae). J. Anim. Ecol. 42, 761–771 10.2307/3136 (doi:10.2307/3136) [DOI] [Google Scholar]
- 29.Seifert B. 2007. Die Ameisen Mittel- und Nordeuropas. Tauer, Germany: Lutra [Google Scholar]
- 30.Seppä P. 1996. Genetic relatedness and colony structure in polygynous Myrmica ants. Ethol. Ecol. Evol. 8, 279–290 10.1080/08927014.1996.9522918 (doi:10.1080/08927014.1996.9522918) [DOI] [Google Scholar]
- 31.Pedersen J. S., Boomsma J. J. 1999. Effect of habitat saturation on the number and turnover of queens in the polygynous ant, Myrmica sulcinodis. J. Evol. Biol. 12, 903–917 10.1046/j.1420-9101.1999.00109.x (doi:10.1046/j.1420-9101.1999.00109.x) [DOI] [Google Scholar]
- 32.Lorenzi M. C. 2003. Social wasp parasites affect the nestmate recognition abilities of their hosts (Polistes atrimandibularis and P. biglumis, Hymenoptera, Vespidae). Insectes Soc. 50, 82–87 10.1007/s000400300013 (doi:10.1007/s000400300013) [DOI] [Google Scholar]