Abstract
Many insects use the polarization pattern of the sky for obtaining compass information during orientation or navigation. E-vector information is collected by a specialized area in the dorsal-most part of the compound eye, the dorsal rim area (DRA). We tested honeybees' capability of learning certain e-vector orientations by using a classical conditioning paradigm with the proboscis extension reflex. When one e-vector orientation (CS+) was associated with sugar water, while another orientation (CS−) was not rewarded, the honeybees could discriminate CS+ from CS−. Bees whose DRA was inactivated by painting did not learn CS+. When ultraviolet (UV) polarized light (350 nm) was used for CS, the bees discriminated CS+ from CS−, but no discrimination was observed in blue (442 nm) or green light (546 nm). Our data indicate that honeybees can learn and discriminate between different e-vector orientations, sensed by the UV receptors of the DRA, suggesting that bees can determine their flight direction from polarized UV skylight during foraging. Fixing the bees' heads during the experiments did not prevent learning, indicating that they use an ‘instantaneous’ algorithm of e-vector detection; that is, the bees do not need to actively scan the sky with their DRAs (‘sequential’ method) to determine e-vector orientation.
Keywords: polarization vision, insect, dorsal rim area, navigation, proboscis extension reflex
1. Introduction
Many animals show striking capabilities in spatial orientation and navigation over short-range and long-range distances. For example, sea turtles, birds and butterflies travel thousands of kilometres for seasonal habitats or a nest [1–3]. Insects such as honeybees [4] and desert ants [5] are capable of navigating up to several kilometres in straight lines back to their nest after complicated feeding journeys. To solve navigational tasks, animals are forced to choose the appropriate travelling direction [6]. Spatial orientation should be encoded as internal representations of the spatial relationship between the animal's body and its surrounding space. In rats and other mammals, place cells and head-direction cells are well-studied examples of neurons encoding spatial properties in the brain [7–9]. However, the brain mechanisms of mammals for spatial orientation and calculating distances are not fully understood. The smaller brain of an insect allows investigation of basic brain mechanisms for spatial navigation at a single-neuron level [10].
It is well known that insects use a skylight compass to obtain directional information during navigation by path integration (i.e. they continually monitor their net distance and direction from the starting point [11–15]). A major source of skylight compass information is the polarization pattern of blue sky. As a result of sunlight scattering, light from blue sky is partially polarized with the celestial e-vectors arranged along concentric circles around the Sun [16]. Behavioural studies have shown that desert ants use celestial e-vector patterns during travel for finding the correct direction to the nest [12,15]. The finding that honeybees failed to transfer the direction to the food source by a waggle dance under an unpolarized light condition [11,17] suggests the use of e-vectors of polarized light for deducing the direction.
The e-vector detection in insects is mediated by a group of specialized ommatidia located in the most dorsal part of the compound eye, the dorsal rim area (DRA). The sensory function of DRA ommatidia as polarization sensors has been studied broadly across species anatomically, electrophysiologically and behaviourally (for reviews, see [13,18]). In honeybees, the DRA consists of four to five rows of ommatidia at the dorsal margin of the compound eye [19,20]. Electron microscopic studies have shown that in each DRA ommatidium there are two sets of the photoreceptors with the microvilli oriented at 90° to each other (i.e. they are tuned to mutually orthogonal e-vector orientations [19,21]). These two receptor types have been suggested to connect antagonistically to second-order neurons, effectively enhancing e-vector contrast [22]. Electrophysiological and behavioural studies indicate that UV-sensitive photoreceptors in the DRA ommatidia with high polarization sensitivities and wide visual fields are the primary information carriers for polarization vision in honeybees [23,24]. Wavelength dependence for e-vector-guided waggle dances was previously found to be strictly UV-sensitive [25].
In contrast to the peripheral sensory system, neural mechanisms of polarization vision in the brain have been studied in only few species, mainly in crickets and locusts. In crickets, e-vector information from the DRA is processed by a group of polarization-opponent interneurons in the optic lobe (POL1-neurons [26–28]). There are only three types of POL1-neurons, which are tuned to different e-vectors oriented at 10°, 60° and 130° to the body axis, suggesting that e-vector orientation is coded as signals of three differently tuned information channels by the so-called instantaneous method of e-vector detection [13]. In the higher centre of the brain, the central complex has been suggested to be the location of the internal compass [29]. Electrophysiological studies have revealed a number of different types of polarization-sensitive neurons in the central complex [29–32]. In the locust, zenithal e-vector orientations are topographically represented in columnar organization in the protocerebral bridge of the central complex [29]. In the cricket, Sakura et al. [32] proposed that polarization information is represented by e-vector orientation-selective neurons (the so-called ‘compass neuron’) in the central body, as a result of integration of combinatory activations by the three types of POL1-neurons. The compass neurons have been discussed as analogues of head-direction cells in mammals [13,32,33].
Since no electrophysiological data are available for honeybees beyond the retinal level, it is unknown whether the neural basis of e-vector detection in honeybees is similar to that of crickets and locusts. In principle, every organism has two ways of measuring celestial e-vector orientation: the instantaneous method described above, and the so-called sequential method [13]. With the instantaneous method, e-vector orientation can be recognized at a glance, since each e-vector is unambiguously defined by a certain neural activity pattern in the brain. In contrast, with the sequential method, an animal has to make rotatory movements around its vertical body axis, thereby scanning the sky with their DRA. Two previous studies with honeybees came to opposite conclusions. Based on the idea that the insects perceive polarization not as a separate modality of light but as a modulation of light intensity when the bees made rotational movements, Rossel & Wehner [11,22,34] were able to elicit directed waggle dances when the light intensity of an unpolarized dorsal stimulus was modulated as a function of body orientation. Edrich & von Helversen [35], on the other hand, were unable to disturb the oriented dances of bees when the degree of polarization of a dorsal polarized stimulus was temporally modulated at different rates. This suggested instantaneous processing because the animal should have been severely confused if it had made sequential comparisons. These hypotheses for polarized light perception have been neither proven nor disproven, because of a paucity of direct evidence.
To study the cognitive functions of polarization vision, monitoring of behavioural outputs and the establishment of a reliably reproducible experimental paradigm are required. In honeybees, the Pavlovian classical conditioning with the proboscis extension reflex (PER) has been widely used as a tool for determining cognitive capabilities. A honeybee will extend its proboscis when sugar water, an unconditioned stimulus (US), is applied to its antenna, proboscis or leg [36]. When sugar solution is applied to the bee shortly after presentation of a conditioned sensory stimulus (CS), which is not originally related to the PER, the bee will show PER when CS alone is presented without sugar water. By using this associative learning paradigm, the capabilities of bees for sensory discrimination and cognition have been investigated [37–39].
In the present study, we establish a new behavioural paradigm for studying polarization vision in honeybees using PER, allowing us to examine two controversial mechanisms for polarization recognition, because the head of the bee can be easily fixed in place under this condition. We demonstrate (i) that honeybees can learn e-vector orientations by using the classical conditioning paradigm with PER, and (ii) that bees discriminate certain e-vector orientations under these conditions. We show (iii) that the DRA is crucial for the discrimination of e-vector orientations, and (iv) that polarization vision is restricted to the UV range of light. Most importantly, our data indicate (v) that honeybees can use an instantaneous mechanism to code e-vector orientation in the brain.
2. Material and methods
(a). Experimental animals
The honeybees, Apis mellifera ligustica, used in this study were reared in a normal 10-frame hive at the campus of Tokushima Bunri University. All experimental bees were collected from the same colony. Forager honeybees with pollen loads were collected at the hive entrance in the evening (mostly between 16.00 and 18.00 h), at least 1 day before the experiment. The bees were anaesthetized on ice and mounted in a plastic tube as described previously [36,40]. Contrary to the previous conditioning experiments using a visual stimulus [37,38], the antennae of the bees were kept intact throughout the experiments. The bees were fed four or five drops of 30 per cent sucrose solution and kept at room temperature until the next day in a dark environment. In the morning, each bee was fed with one drop of the sucrose solution. Although it was generally difficult for the bees to move their heads under these conditions, the heads were further immobilized at the neck with wax in some experiments.
(b). Experimental set-up
The experiments were performed using a custom-made experimental box (electronic supplementary material, figure S1a) in a dark room. The box consisted of two parts divided by a horizontal partition with a circular opening containing a polarizer; the upper part was for generating unpolarized light while the lower part was for manipulating an experimental animal. The inside of the box was covered with black non-glossy paper to avoid reflection of the light. Only the frontal plane of the box was open for manipulating. Light from a xenon lamp (LC8, Hamamatsu Photonics, Hamamatsu, Japan) was applied from the top of the upper part by a light guide. In addition to white light, we used UV, blue and green monochromatic light to examine the wavelength dependency of polarization vision. For these monochromatic stimuli, interference filters with λmax of 350, 441.6 or 546 nm and half-widths of 10 nm (VPF, Sigma-Koki, Tokyo, Japan) were mounted at the end of the light guide. Within the box, the light passed through a hollow tube, the wall of which was the diffusing paper for removing any specific polarized light components. After passing through the diffusing paper, unpolarized light passed through the opening in the centre of the partition between the upper and the lower parts of the box. For the polarized light stimulus, a slider with a linear polarizer (HN42HE, Polaroid Company, Cambridge, MA, USA) was inserted manually into a slit below the diffusing paper. The e-vector orientation of the stimulus could be changed by changing the inserting direction of the slider. Two bees were placed on the black platform in the centre of the lower part so that the DRA would face the light stimulus. The intensity of the polarized white light at the animal level was 8700 lx (measured by LM-331, AS ONE, Osaka, Japan), and the powers of the polarized UV, blue and green lights at the animal level were 5.0, 17.1 and 13.4 µW cm−2, respectively (measured by PM100, ThorLabs, Newton, NJ, USA for blue and green light, and by YK-34UV, Lutron Electronic Enterprise, Taipei, Taiwan for the UV light). The size of the stimulus was 62° as seen by the bees.
(c). Visual stimulation and conditioning
The procedures of the conditioning paradigm were adapted from the colour-conditioning paradigm [37]. Differential conditioning was applied with two polarized light stimuli with different e-vector orientations (electronic supplementary material, figure S1b). The angular difference between the e-vector orientations presented during conditioning was 90°. Here we refer to the e-vector parallel to the body axis of the bee as 0° and that at a right angle to the body axis as 90°, for convenience. At least 5 min before conditioning began, the bees were placed in the centre of the lower part below the unpolarized light stimulus to become familiar with the environment. Unpolarized light illumination was kept on throughout the experiments and the polarizer was inserted manually only during polarized light stimulation. One e-vector orientation was selected as the conditioned stimulus (CS+) and was applied for 4 s, followed by manual application of 30 per cent sucrose solution (US) to the right antenna or to the proboscis for 3 s together with the CS+. US application was performed by the standard procedure [36,40]. The other e-vector orientation was presented for 7 s without sucrose (CS−). CS+ and CS− were balanced among animals (i.e. one of the bees in the box was conditioned with 0° of the e-vector as CS+, while the other was with 90° at the same time). The interval between CS+ and CS− was 2 min. This differential conditioning trial was repeated 15 times (inter-trial interval = 4 min). In the test phase, the animal was exposed to polarized light once without sucrose 4 min after the last conditioning trial. Since bees could freely extend their probosces, and as the CS+ light during the conditioning phase preceded the US by 4 s, the response to the CS+ could be monitored visually and expressed as an acquisition function. For the experiments under UV light, a small spotlight of dim red light was focused on the bee's head to observe the bee's behaviour.
(d). Selective painting of the compound eye
The DRA of the compound eye is visually identifiable because its cornea appears slightly grey and cloudy [19]. Either both DRAs or the whole of both compound eyes except for the DRAs (exDRA) were painted with black acrylic emulsion paint (Herbol, Cologne, Germany) under a dissecting microscope at least 1 h before experiments. Because it was technically not possible to cover the DRA alone, which consists of only four to five horizontal rows of ommatidia (see [19,24]), a small area of the unspecialized dorsal part next to the DRA was covered together with the DRA. Vice versa, a small area of the dorsal eye part near the DRA was not covered when the DRA was left open because it was impossible to leave only the DRA open. We estimated that a threefold area of the DRA was covered in DRA-painted animals and a less than twofold area of the DRA was not covered in exDRA animals. After the experiments, the paint cover was checked in all animals under the dissecting microscope. Data for cases in which even a small area of paint was missing were excluded from further analysis. The three ocelli, which are not involved in polarization vision [41], were not painted in the experiments.
(e). Analysis and statistics
Animals that extended their probosces during the 4 s polarized light stimulation period prior to US application were counted as showing PER. Data for animals that failed to respond to US were excluded from analysis.
Comparisons of the learning effects across the experimental parameters were performed using generalized linear models (GLMs) calculated by R statistical software (v. 2.11.0, R Development Core Team, http://www.R-project.org). Based on the number of bees that exhibited PER in each trial, we constructed four models for binomial distributions with different parameter sets, trial number, CS, both trial number and CS, and null (i.e. neither trial number nor CS). Akaike information criterion (AIC) was calculated for each model, and the best model was selected as the model providing the smallest AIC value among the four models.
To determine the number of the trials to establish the e-vector orientation learning, we performed change point analysis using GLMs. Sixteen trials were divided into two groups: the former and latter groups. For set 1, the former group included trial 1 and the latter group included trials 2–16. We applied the null model to the former group and the CS model to the latter group. AIC for this set was obtained by summing AICs of both groups. Next, for set 2, trials 1 and 2 were in the former group and trials 3–16 were in the latter group, and we then calculated AIC for set 2. We repeated this procedure until set 15, with trials 1–15 for the former group and trial 16 for the latter group. Finally, the number of trials for learning establishment was determined by the set that provided the smallest AIC value among 15 sets.
3. Results
(a). General features of polarized light learning
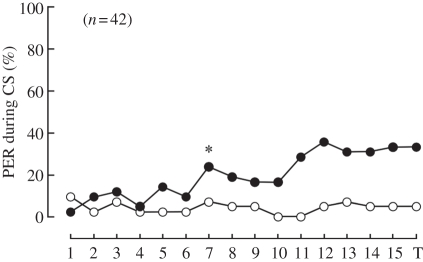
First, we examined the capability of a honeybee to discriminate between different e-vector orientations. The differential conditioning paradigm with two mutually orthogonal e-vector orientations revealed that honeybees could discriminate those e-vectors (figure 1). Model selection using AIC values of GLMs indicated that the best model was the model including both the trial and CS as the parameters (table 1). In the first acquisition trial, only one (2.4%) and four (9.5%) of 42 bees exhibited PER to CS+ and CS−, respectively. After 15 acquisition trials, 14 (33.3%) and two (4.8%) of the 42 bees exhibited PER to CS+ and CS−, respectively. The change point analysis indicated that seven trials were needed to discriminate CS+ and CS− (figure 1). At about the seventh trial, the number of bees showing PER to CS+ became large and then slightly decreased until the 10th trial. The number of bees showing PER to CS+ increased again at about 12th trial. In the test trial (16th trial from the beginning), the number of animals showing PER was slightly smaller than that in the 12th trial. In most cases, the proportion of animals showing PER did not exceed 50 per cent. On the other hand, the number of animals showing PER to CS− remained small (less than 10%) throughout the experiment. When 15 acquisition trials were divided into two parts (first part: 1–8; second part: 9–15) and the number of PERs to CS+ in each part was counted, there were only three bees among 42 bees in which the number of PER in the first part was higher than that in the second part. This indicates that the most bees learned CS+ although the learning score was only about 35 per cent. The learning properties described above were generally observed in all experiments in this study.
Figure 1.
Associative learning of e-vector orientation. Proboscis extension response probabilities in each trial are shown. Bees exhibited learning performance after the seventh conditioning trial. The asterisk indicates the point when the effect of conditioning was found by the change point analysis. 1–15, number of each conditioning trial; T, test trial. Black circles, CS+; white circles, CS−.
Table 1.
AICs of GLMs with different parameter sets in each experimental group. Asterisks indicate selected model with minimum AIC.
| parameters | normal (figure 1) | DRA (figure 2a) | exDRA (figure 2b) | UV (figure 3a) | blue (figure 3b) | green (figure 3c) | neck-fixed (figure 4) |
|---|---|---|---|---|---|---|---|
| null | 243.66 | 116.21* | 240.84 | 210.21 | 86.84* | 84.88* | 201.92 |
| only trial | 216.90 | 138.13 | 207.63 | 205.33 | 90.44 | 96.37 | 214.72 |
| only CS | 161.80 | 118.17 | 168.83 | 152.86 | 88.33 | 85.36 | 139.86* |
| trial + CS | 133.22* | 140.08 | 129.69* | 145.75* | 91.14 | 96.81 | 151.51 |
We further analysed the behavioural responses to the two e-vector orientations (0° and 90°) when each e-vector was used as CS+ separately using GLMs. No bias was found for either of the two e-vectors as CS+ (GLM including CS as a parameter was not selected as the best model, meaning that 0° and 90° for CS+ was not different). Also, no clear interactive effects were observed in terms of PER responses between two simultaneously conditioned bees (only six cases were potentially interactive cases; data not shown). Therefore, we pooled the data for these two e-vectors according to their functions.
From the comparison of bees' learning performance, we found that the effect of conditioning depended on the season, although the bees were from the same colony. The maximum ratios of animals responding to CS+ were 50.0 and 27.8 per cent in spring and autumn, respectively. On the other hand, the maximum ratios of animals responding to CS− were 8.3 and 5.6 per cent in spring and autumn, respectively (with PER probability in the first trial excluded). These results suggest that spring bees learned e-vector orientation better than autumn bees, but we cannot exclude other possibilities such as ageing, experience, etc. Although we do not know any causal factors, we used only spring bees for further experiments because of this difference in the learning score.
(b). Selective stimulation of eye regions
To confirm the sensory input area for e-vector learning in the eye, we covered part of each compound eye restricting the area receiving light stimulation to the DRA or exDRA (electronic supplementary material, figure S2).
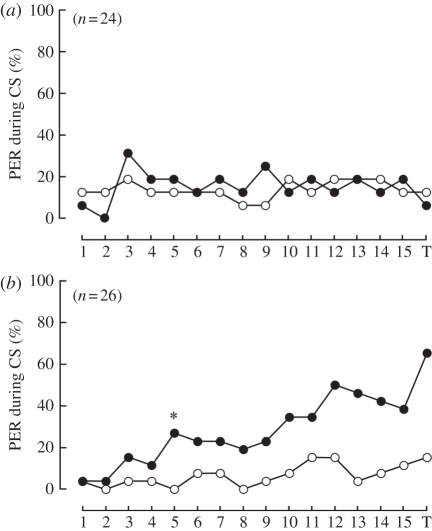
We found that the exDRA-painted animals exhibited the PER to CS+ but not to CS−, whereas the DRA-painted animals could not learn CS+ (figure 2 and table 1). The number of PER animals increased gradually with increase in acquisition trials. In the 5th trial, CS+/CS− discrimination was established (figure 2b). In the test trial, 17 (65.4%) of 26 exDRA-painted animals showed PER for CS+. The best model, ‘trial and CS’, indicates that bees learned CS+ along the acquisition trials (table 1). In general, the probability of DRA-painted animals showing PER to CS− was slightly higher than that of exDRA-painted animals throughout the experiment (mean values of 14.6 and 6.7% for DRA-painted and exDRA-painted animals, respectively).
Figure 2.
Learning curves of (a) DRA-painted and (b) exDRA-painted bees. (a) The DRA-painted bees did not learn CS+ throughout the trials. (b) The exDRA-painted bees exhibited leaning performance after the fifth conditioning trial. The asterisk indicates the point when the effect of conditioning was found by the change point analysis. 1–15, number of each conditioning trial; T, test trial. Black circles, CS+; white circles, CS−.
(c). Wavelength dependence of e-vector discrimination
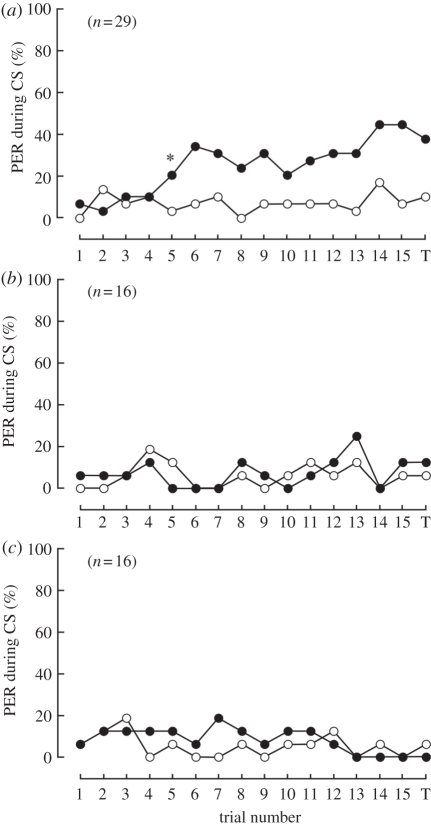
The compound eye of the honeybee contains three types of photoreceptors with different spectral sensitivities, peaking in UV, blue and green light [42]. Here, we attempted to determine the wavelength selectivity for e-vector discrimination learning. The bees exhibited e-vector discrimination by conditioning with UV polarized light only (table 1 and figure 3a). The maximum ratios of animals that responded were 44.8 and 17.2 per cent for CS+ and CS −, respectively. In the case of blue or green polarized light, the bees did not learn CS+ because the best model was ‘null’ (table 1 and figure 3b,c). The probabilities for PER during CS+ or CS− were both in the same low range. These results indicate that a honeybee perceives polarized light information from UV photoreceptors.
Figure 3.
Learning curves for (a) UV, (b) blue and (c) green polarized light. (a) The UV-trained bees exhibited learning performance after the fifth conditioning trial. The asterisk indicates the point when the effect of conditioning was found by the change point analysis. (b,c) The blue- and green-trained bees did not learn CS+ throughout the trials. 1–15, number of each conditioning trial; T, test trial. Black circles, CS+; white circles, CS−.
(d). Instantaneous or sequential system for e-vector detection?
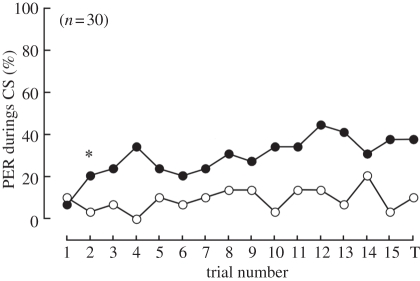
Next, we fixed the bees' heads with wax, preventing any scanning movements necessary for a sequential approach, and trained them with UV polarized light. If the bees depended on spatial scanning, they would not be able to discriminate CS+ from CS− under this condition. On the other hand, if the bees could discriminate the two e-vectors, this suggested that e-vector discrimination ‘at a glance’ was possible by the instantaneous mechanism. Fixation of the head revealed the honeybees' ability for significant discrimination between CS+ and CS− because the ‘CS’ model was selected (table 1 and figure 3b,c) same as the result for animals without fixation. The PER probability reached a plateau with a maximum value at 43.3 per cent. As in the case of DRA-painted animals, head-fixed animals showed slightly higher PER responses to CS− in general than the animals shown in figure 1 (10.0% and 8.3% of the bees showed PER for CS− in the test phase in head-fixed and normal animals, respectively).
4. Discussion
(a). General properties of e-vector learning
We have established a new appetitive-learning paradigm in which an e-vector orientation was associated with sugar solution and demonstrated that honeybees can discriminate between two different e-vectors. In this study, we used 0° and 90° e-vector orientations for CS+, and could not find any differences in learning performances between these two e-vectors. If we used e-vector orientations for CS that are coded by POL-neurons in the medulla (e.g. 10°, 60° and 130° in the crickets), bees might show better discrimination ability. Nevertheless, we first have to find orientations coded by polarization-sensitive neurons because neither selective orientations nor such brain neurons have been studied in the honeybee yet. Learning of e-vector orientation was slower than olfactory learning: the bees required seven acquisition trials for polarized light learning, whereas only one trial was sufficient for olfactory learning [36,43]. Furthermore, PER probability after 15 trials was much lower than in olfactory learning (about 80% in the third trial) [36]. These differences are probably based on the different sensory modality for learning. PER probability of harnessed honeybees was 40 per cent after 20 trials in colour learning [37]. This is comparable with our results, although colour learning required more acquisition trials (14 trials for colour learning and seven trials for polarized light learning). Rather than colour learning in honeybees, the properties of polarized light learning might be similar to those of motion learning. In motion learning [38], harnessed honeybees were presented with black–white gratings moving from front to back or vice versa, mimicking optical flow during foraging, and the bees were conditioned to associate the movement direction. In these experiments, eight acquisition trials were required to reach a plateau of learning performance at approximately 40 per cent (i.e. the same as in the present study). Since information on optical flow and e-vector orientation are both closely related to foraging behaviour (i.e. navigation and orientation [15,44]), and since the learning performance for these two sensory cues are similar, this information may have similar biological meaning for bees.
The comparatively low performance of polarized light learning was not improved by either a longer (20 min) inter-trial interval (ITI) or a larger number of trials (data not shown). In olfactory learning, spaced training (ITI > 3 min) induces higher learning performance than massed training (ITI = 30 s), and the memory established by spaced training is consolidated better than that by massed training [45]. It would be interesting to examine how long polarized light memory is maintained under natural conditions. This memory is probably short-term memory, like working memory in mammals, because it is unlikely that a foraging bee needs to retain e-vector memory for path integration during flight. Our preliminary experiments in which bees were tested three times (4, 8 and 12 min after the last conditioning trial) by CS+ without any rewards suggested that the memory extinction curve for e-vector orientation was comparable to that of olfactory learning with the short ITI (data not shown [46]). The 4 min ITI in our experiments might be long, considering their normal flight speed of 210–350 m min−1 [47]. Assuming that polarized light learning is related to navigation, we might have obtained a better performance score if we had selected a massed training paradigm (ITI < 4 min), because each type of learning paradigm has a specifically optimal ITI in general [45,48].
We found a seasonal difference in the polarized light learning score. Honeybees are well known to have an annual life cycle in their colony [49]. Both olfactory and tactile learning performances by using PER were affected by the foraging season [50]. Our finding that spring bees showed better performance in polarized light learning than autumn bees, together with the seasonality previously found in three different sensory modalities (i.e. olfactory, tactile and visual), suggests that the seasonal difference in learning ability is a general feature for honeybees. We still need additional experiments to reveal seasonal difference in learning capability for polarized light.
(b). Polarized light information processing mechanism in the sensory system
Bees learned e-vector orientation only when UV light was used for a CS, but no significant learning performance was observed for blue or green stimuli, even though the intensity of UV light was about a third of other wavelength intensities (figure 3). This finding supports a previous study in which the direction of waggle dances could be controlled by e-vector orientation provided that the stimulus contained UV [25]. These data indicate that honeybees rely on polarized UV light of skylight to detect e-vector information. In addition, our covering experiments demonstrate that the DRA is the eye region responsible for e-vector learning (figure 2). This result is in agreement with a previous study showing that blocking visual inputs to the DRA abolished polarized light-guided orientation in the honeybee [24]. Their data perfectly agree with the high polarization sensitivity in the UV receptors of the DRA found in an electrophysiological study [23]. Thus, all available data strongly indicate that the UV receptors of the DRA are the input elements for polarized light perception in honeybees.
(c). Instantaneous system of polarization vision
In the sequential method, successive readings of an insect's POL-neurons are compared while the insect scans a dorsal polarized stimulus (e.g. the sky) by rotating about its vertical axis. Whenever one of the POL-neurons exhibits maximal activity, this indicates that the e-vector of the stimulus is aligned with the e-vector tuning axis of the POL-neuron. With (for instance) three tuning types of POL-neuron, just three body orientations can be determined directly by the polarization vision system. Based on these reference orientations, all other orientations must be determined either by proprioreception or by evaluating the optic displacement of the retinal image of the world perceived on making a turn. In the instantaneous method, the brain compares the simultaneously available signals of differently tuned POL-neurons. Theory shows that the signals of three independent polarization-sensitive channels suffice to encode e-vector orientation unambiguously [51–53]. In other words, each body orientation is defined by a unique combination of activity levels in the three POL-neurons. Using the instantaneous approach, an insect can perceive e-vector orientation (or body orientation relative to it) at a glance without performing any movements.
Which of these alternative algorithms is implemented in honeybees? This question has been addressed by behavioural experiments. Rossel & Wehner [11,22] supported the idea of the sequential mechanism by directing waggle-dance experiments with an unpolarized dorsal stimulus. Edrich & von Helversen [35], on the other hand, have shown that bees kept the oriented dances even when the degree of polarization of a dorsal polarized stimulus was temporarily modulated, suggesting the instantaneous mechanism. In the present study, fixing the bees' heads to prevent scanning movements during the conditioning experiments did not prevent the insects from discriminating between two sequentially presented orthogonal e-vector orientations (figure 4). This finding can only be explained by an instantaneous algorithm of e-vector detection. Tethered flying locusts and walking crickets responded spontaneously to the e-vector of a dorsally presented polarized stimulus even with their heads fixed, suggesting instantaneous e-vector detection in orthopteran insects ([54]; T. Labhart 2000, unpublished data).
Figure 4.
Learning curves of head-fixed bees for UV polarized light. The head-fixed bees exhibited learning performance after the second conditioning trial. The asterisk indicates the point when the effect of conditioning was found by the change point analysis. 1–15, number of each conditioning trial; T, test trial. Black circles, CS+; white circles, CS−.
Recent electrophysiological studies in the central complex of orthopteran insect brains have provided data strongly consistent with instantaneous processing. The central complex has been suggested to be involved in spatial orientation in Drosophila [55], and many types of polarization-sensitive neurons have been found electrophysiologically in the central complex of crickets and locusts [29–32]. A group of neurons in the central complex of crickets have been suggested to code e-vector orientation [32], supporting the idea of an internal compass. In the locust, the protocerebral bridge of the central complex consists of 16 columns, with each column containing polarization-sensitive neurons that respond to specific e-vector orientation [29]. Thus, information on e-vector orientation seems to be represented in a topographical manner in the protocerebral bridge of the central complex. These findings imply that actual e-vector orientation is finely encoded in the orthopteran brain. On the other hand, because the polarized light stimuli were applied by a slowly rotating polarizer in all these studies, there is still a possibility that the animals are able to analyse the e-vector orientation ‘sequentially’ even under the fixation of the heads.
Our present conclusion that honeybees use the instantaneous mechanism for polarization vision does not exclude the possibility that the sequential mechanism is also used, as proposed by the analysis of dance behaviour under unpolarized light [11,22]. Another learning paradigm using PER, in which bees can scan polarized stimuli, will allow us to examine whether or not they use sequential mechanisms. These two essentially different mechanisms are possibly not exclusive but may function complementarily depending on the circumstantial conditions the animal is facing (e.g. light intensity, degree of polarization and visible size of the blue sky). If so, it would be interesting to reveal situations in which one mechanism dominates. It remains unknown what angular view of the sky and what intensity of polarized light are needed for honeybees to discriminate two e-vectors. Elucidation of these factors will facilitate an understanding of the manner of selection of those two mechanisms. Since directional discrimination capability is necessary for all navigating animals [6], similar neural mechanisms probably underlie navigation in both invertebrates and vertebrates.
Acknowledgements
The authors are deeply grateful to Dr T. Labhart for fruitful discussions and critical comments about this work. We thank Dr H. Takeuchi for technical advice on experiments, Dr K. Kagaya for statistical analysis, and Drs G. Nishimura and M. Mizunami for measuring light intensity. We also thank to Dr E. Ito for his offers of finance, experimental space and so on. This work was partly supported by grants-in-aid for Scientific Research from JSPS to M.S. (22770064) and from MEXT to H.A. (17075001).
References
- 1.Lohmann K. J., Lohmann C. M. F. 1996. Orientation and open-sea navigation in sea turtles. J. Exp. Biol. 199, 73–81 [DOI] [PubMed] [Google Scholar]
- 2.Walcott C. 1996. Pigeon homing: observations, experiments and confusions. J. Exp. Biol. 199, 21–27 [DOI] [PubMed] [Google Scholar]
- 3.Reppert S. M., Gegear R. J., Merlin C. 2010. Navigational mechanism of migrating monarch butterflies. Trends Neurosci. 33, 399–406 10.1016/j.tins.2010.04.004 (doi:10.1016/j.tins.2010.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menzel R., De Marco R., Gregger U. 2006. Spatial memory, navigation and dance behaviour in Apis mellifera. J. Comp. Physiol. A 192, 889–903 10.1007/s00359-006-0136-3 (doi:10.1007/s00359-006-0136-3) [DOI] [PubMed] [Google Scholar]
- 5.Wehner R., Wehner S. 1990. Insect navigation: use of maps or Ariadne's thread? Ethol. Ecol. Evol. 2, 27–48 10.1080/08927014.1990.9525492 (doi:10.1080/08927014.1990.9525492) [DOI] [Google Scholar]
- 6.Frost B. J., Mouritsen H. 2006. The neural mechanisms of long distance animal navigation. Curr. Opin. Neurobiol. 16, 481–488 10.1016/j.conb.2006.06.005 (doi:10.1016/j.conb.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 7.Taube J. S. 1998. Head direction cells and the neurophysiological basis for a sense of direction. Prog. Neurobiol. 55, 225–256 10.1016/S0301-0082(98)00004-5 (doi:10.1016/S0301-0082(98)00004-5) [DOI] [PubMed] [Google Scholar]
- 8.Sharp P. E., Blair H. T., Cho J. 2001. The anatomical and computational basis of the rat head direction cell signal. Trends Neurosci. 24, 289–294 10.1016/S0166-2236(00)01797-5 (doi:10.1016/S0166-2236(00)01797-5) [DOI] [PubMed] [Google Scholar]
- 9.Harvey C. D., Collman F., Dombeck K., Tank D. W. 2009. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–949 10.1038/nature08499 (doi:10.1038/nature08499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chittka L., Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008 10.1016/j.cub.2009.08.023 (doi:10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 11.Rossel S., Wehner R. 1987. The bee's e-vector compass. In Neurobiology and behavior of honeybees (eds Menzel R., Mercer A.), pp. 76–93 Berlin, Germany: Springer [Google Scholar]
- 12.Wehner R. 1994. The polarization-vision project: championing organismic biology. In Neural basis of behavioural adaptation (eds Schildberger K., Elsner N.), pp. 103–143 Stuttgart, Germany: Fischer-Verlag [Google Scholar]
- 13.Wehner R., Labhart T. 2006. Polarisation vision. In Invertebrate vision (eds Warrant E., Nilsson D. E.), pp. 291–348 Cambridge, UK: Cambridge University Press [Google Scholar]
- 14.Collett T. S., Collett M. 2000. Path integration in insects. Curr. Opin. Neurobiol. 10, 757–762 10.1016/S0959-4388(00)00150-1 (doi:10.1016/S0959-4388(00)00150-1) [DOI] [PubMed] [Google Scholar]
- 15.Wehner R. 2003. Desert ant navigation: how miniature brains solve complex tasks. J. Comp. Physiol. A 189, 579–588 10.1007/s00359-003-0431-1 (doi:10.1007/s00359-003-0431-1) [DOI] [PubMed] [Google Scholar]
- 16.Strutt J. 1871. On the light from the sky, its polarization and colour. Phil. Mag. 41, 107–120 [Google Scholar]
- 17.Sherman G., Visscher P. K. 2002. Honeybee colonies achieve fitness through dancing. Nature 419, 920–922 10.1038/nature01127 (doi:10.1038/nature01127) [DOI] [PubMed] [Google Scholar]
- 18.Labhart T., Meyer E. P. 1999. Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc. Res. Tech. 47, 368–379 (doi:10.1002/(SICI)1097-0029(19991215)47:6<368::AID-JEMT2>3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 19.Meyer E. P., Labhart T. 1981. Pore canals in the cornea of a functionally specialized area of the honey bee's compound eye. Cell Tissue Res. 216, 491–501 10.1007/BF00238646 (doi:10.1007/BF00238646) [DOI] [PubMed] [Google Scholar]
- 20.Schinz R. 1975. Structure specialization in the dorsal retina of the bee, Apis mellifera. Cell Tissue Res. 162, 23–34 10.1007/BF00223259 (doi:10.1007/BF00223259) [DOI] [PubMed] [Google Scholar]
- 21.Wehner R., Bernard G. D., Geiger E. 1975. Twisted and non-twisted rhabdoms and their significance for polarization detection in the bee. J. Comp. Physiol. 104, 225–245 10.1007/BF01379050 (doi:10.1007/BF01379050) [DOI] [Google Scholar]
- 22.Rossel S., Wehner R. 1986. Polarization vision in bees. Nature 323, 128–131 10.1038/323128a0 (doi:10.1038/323128a0) [DOI] [Google Scholar]
- 23.Labhart T. 1980. Specialized photoreceptors at the dorsal rim of the honeybee's compound eye: polarizational and angular sensitivity. J. Comp. Physiol. A 141, 19–30 10.1007/BF00611874 (doi:10.1007/BF00611874) [DOI] [Google Scholar]
- 24.Wehner R., Strasser S. 1985. The POL area of the honey bee's eye: behavioural evidence. Physiol. Entomol. 10, 337–349 10.1111/j.1365-3032.1985.tb00055.x (doi:10.1111/j.1365-3032.1985.tb00055.x) [DOI] [Google Scholar]
- 25.von Helversen O., Edrich W. 1974. Der polarisationsempfänger im Bienenauge: ein Ultraviolettrezeptor. J. Comp. Physiol. 94, 33–47 10.1007/BF00610156 (doi:10.1007/BF00610156) [DOI] [Google Scholar]
- 26.Labhart T. 1988. Polarization-opponent interneurons in the insect visual system. Nature 331, 435–437 10.1038/331435a0 (doi:10.1038/331435a0) [DOI] [Google Scholar]
- 27.Labhart T., Petzold J., Helbling H. 2001. Spatial integration in polarization-sensitive interneurons of crickets: a survey of evidence, mechanisms and benefits. J. Exp. Biol. 204, 2423–2430 [DOI] [PubMed] [Google Scholar]
- 28.Petzold J. 2001. Polarisationsempfindliche Neuronen im Sehsystem der Feldgrill, Gryllus campestris: Elektrophysiologie, Anatomie und Modellrechnungen. PhD thesis, University of Zurich, Zurich, Germany [Google Scholar]
- 29.Heinze S., Homberg U. 2007. Maplike representation of celestial e-vector orientations in the brain of an insect. Science 315, 995–997 10.1126/science.1135531 (doi:10.1126/science.1135531) [DOI] [PubMed] [Google Scholar]
- 30.Heinze S., Gotthardt S., Homberg U. 2009. Transformation of polarized light information in the central complex of the locust. J. Neurosci. 29, 11 783–11 793 10.1523/JNEUROSCI.1870-09.2009 (doi:10.1523/JNEUROSCI.1870-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitzthum H., Müller M., Homberg U. 2002. Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J. Neurosci. 22, 1114–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakura M., Lambrinos D., Labhart T. 2008. Polarized skylight navigation in insects: model and electrophysiology of e-vector coding by neurons in the central complex. J. Neurophysiol. 99, 667–682 10.1152/jn.00784.2007 (doi:10.1152/jn.00784.2007) [DOI] [PubMed] [Google Scholar]
- 33.Labhart T., Meyer E. P. 2002. Neural mechanisms in insect navigation: polarization compass and odometer. Curr. Opin. Neurobiol. 12, 707–714 10.1016/S0959-4388(02)00384-7 (doi:10.1016/S0959-4388(02)00384-7) [DOI] [PubMed] [Google Scholar]
- 34.Rossel S. 1993. Navigation by bees using polarized skylight. Comp. Biochem. Physiol. 104A, 695–708 10.1016/0300-9629(93)90146-U (doi:10.1016/0300-9629(93)90146-U) [DOI] [Google Scholar]
- 35.Edrich W., von Helversen O. 1987. Polarized light orientation in honey bees: is time a component in sampling? Biol. Cybern. 56, 89–96 10.1007/BF00317983 (doi:10.1007/BF00317983) [DOI] [Google Scholar]
- 36.Bitterman M. E., Menzel R., Fietz A., Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119 10.1037/0735-7036.97.2.107 (doi:10.1037/0735-7036.97.2.107) [DOI] [PubMed] [Google Scholar]
- 37.Hori S., Takeuchi H., Arikawa K., Kinoshita M., Ichikawa N., Sasaki M., Kubo T. 2006. Associative visual learning, color discrimination, and chromatic adaptation in the harnessed honeybee Apis mellifera L. J. Comp. Physiol. A 192, 691–700 10.1007/s00359-005-0091-4 (doi:10.1007/s00359-005-0091-4) [DOI] [PubMed] [Google Scholar]
- 38.Hori S., Takeuchi H., Kubo T. 2007. Associative learning and discrimination of motion cues in the harnessed honeybee Apis mellifera L. J. Comp. Physiol. A 193, 825–833 10.1007/s00359-007-0234-x (doi:10.1007/s00359-007-0234-x) [DOI] [PubMed] [Google Scholar]
- 39.Menzel R. 2001. Searching for the memory trace in a mini-brain, the honeybee. Learn. Mem. 8, 53–62 10.1101/lm.38801 (doi:10.1101/lm.38801) [DOI] [PubMed] [Google Scholar]
- 40.Okada R., Rybak J., Manz G., Menzel R. 2007. Learning-related plasticity in PE1 and other mushroom body-extrinsic neurons in the honeybee brain. J. Neurosci. 27, 11 736–11 747 10.1523/JNEUROSCI.2216-07.2007 (doi:10.1523/JNEUROSCI.2216-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossel S., Wehner R. 1984. Celestial orientation in bees: the use of spectral cues. J. Comp. Physiol. A 155, 605–613 10.1007/BF00610846 (doi:10.1007/BF00610846) [DOI] [Google Scholar]
- 42.Menzel R., Blakers M. 1976. Colour receptors in the bee eye: morphology and spectral sensitivity. J. Comp. Physiol. 108, 11–33 10.1007/BF00625437 (doi:10.1007/BF00625437) [DOI] [Google Scholar]
- 43.Menzel R., Giurfa M. 2001. Cognitive architecture of a mini-brain: the honeybee. Trends Cogn. Sci. 5, 62–71 10.1016/S1364-6613(00)01601-6 (doi:10.1016/S1364-6613(00)01601-6) [DOI] [PubMed] [Google Scholar]
- 44.Esch H. E., Shang S., Srinivasan M. V., Tautz J. 2001. Honeybee dances communicate distances measured by optic flow. Nature 411, 581–583 10.1038/35079072 (doi:10.1038/35079072) [DOI] [PubMed] [Google Scholar]
- 45.Menzel R., Manz G., Menxel R., Greggers U. 2001. Massed and spaced learning in honeybees: the role of CS, US, the intertribal interval, and the test interval. Learn. Mem. 8, 198–208 10.1101/lm.40001 (doi:10.1101/lm.40001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandoz J. C., Pham-Delègue M. H. 2004. Spontaneous recovery after extinction of the conditioned proboscis extension response in the honeybee. Learn. Mem. 11, 586–597 10.1101/lm.81504 (doi:10.1101/lm.81504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menzel R., et al. 2005. Honey bees navigate according to a map-like spatial memory. Proc. Natl Acad. Sci. USA 102, 3040–3045 10.1073/pnas.0408550102 (doi:10.1073/pnas.0408550102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menzel R. 1999. Memory dynamics in the honeybee. J. Comp. Physiol. A 185, 323–340 10.1007/s003590050392 (doi:10.1007/s003590050392) [DOI] [Google Scholar]
- 49.Gould J. L., Gould C. G. 1988. The honey bee. New York, NY: Scientific American Library [Google Scholar]
- 50.Scheiner R., Barner M., Erber J. 2003. Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie 34, 67–72 10.1051/apido:2002050 (doi:10.1051/apido:2002050) [DOI] [Google Scholar]
- 51.Kirschfeld K. 1972. Die notwendige Anzahl von Rezeptoren zur Bestimmung der Richtung des elektrischen Vektors linear polarisierten Lichtes. Z. Naturforsch. 27, 578–579 [PubMed] [Google Scholar]
- 52.Bernard G. D., Wehner R. 1977. Functional similarities between polarization vision and color vision. Vis. Res. 17, 1019–1028 10.1016/0042-6989(77)90005-0 (doi:10.1016/0042-6989(77)90005-0) [DOI] [PubMed] [Google Scholar]
- 53.Lambrinos D., Möller R., Labhart T., Pfeifer R., Wehner R. 2000. A mobile robot employing insect strategies for navigation. Robot. Auton. Syst. 30, 39–64 10.1016/S0921-8890(99)00064-0 (doi:10.1016/S0921-8890(99)00064-0) [DOI] [Google Scholar]
- 54.Mappes M., Homberg U. 2004. Behavioral analysis of polarization vision in tethered flying locusts. J. Comp. Physiol. A 190, 61–68 10.1007/s00359-003-0473-4 (doi:10.1007/s00359-003-0473-4) [DOI] [PubMed] [Google Scholar]
- 55.Neuser K., Triphan T., Mronz M., Poeck B., Strauss R. 2008. Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244–1248 [DOI] [PubMed] [Google Scholar]