Abstract
The metacopines represent one of the oldest and most important extinct groups of ostracods, with a fossil record from the Mid-Ordovician to the Early Jurassic. Herein, we report the discovery of a representative of the group with three-dimensionally preserved soft parts. The specimen—a male of Cytherellina submagna—was found in the Early Devonian (416 Ma) of Podolia, Ukraine. A branchial plate (Bp) of the cephalic maxillula (Mx), a pair of thoracic appendages (walking legs), a presumed furca (Fu) and a copulatory organ are preserved. The material also includes phosphatized steinkerns with exceptionally preserved marginal pore canals and muscle scars. The morphology of the preserved limbs and valves of C. submagna suggests its relationship with extant Podocopida, particularly with the superfamilies Darwinuloidea and Sigillioidea, which have many similar characteristic features, including a large Bp on the Mx, the morphology of walking legs, Fu with two terminal claws, internal stop-teeth in the left valve, adductor muscle scar pattern, and a very narrow fused zone along the anterior and posterior margins. More precise determination of affinities will depend on the soft-part morphology of the cephalic segment, which has not been revealed in the present material.
Keywords: soft-part preservation, bacteria-like organisms, Podolia, Metacopina, Ostracoda, Early Devonian
1. Introduction
The ostracods are the best-represented group of arthropods in the fossil record. More than 65 000 living and extinct species of ostracods have been described. However, knowledge of ostracod relationships in the Early Palaeozoic still remains inadequate. The systematics of living ostracods are based principally on the soft-part anatomy, which is almost unknown in the fossil record.
Here, we describe a single metacopine ostracod specimen of Cytherellina submagna [1] from the Lower Devonian (Lochkovian) of Podolia in the Ukraine, with partly preserved soft-part anatomy. The material also contains many specimens of the species with exceptionally preserved muscle scars, marginal structures and phosphatized marginal pore canals. The species belongs to the Podocopida (Metacopina)—one of the three major Palaeozoic ostracode groups. This taxonomic assignment is based on the hard-part characteristics.
The Lower Devonian sediments in Podolia also yield other Palaeocopida and Podocopida ostracod species. Preservation of the fossils in the locality is generally very good. Even the original colour pattern of the valves of some of the brachiopods is preserved [2].
Metacopine ostracods occur in the fossil record from the Mid-Ordovician to the Early Jurassic. They survived the end-Permian extinction, and were diverse and relatively abundant in the Triassic and Early Jurassic. The extinction of the metacopines in the Early Toarcian time coincided with the onset of the Early Toarcian Oceanic Anoxic Event and a global eustatic sea-level rise. However, the metacopines probably lost in competition with more advanced cytheroidean ostracods in unfavourable environments [3].
In the Palaeozoic, examples of soft-part preservation of ostracods are extremely rare. Myodocopid ostracod species with soft parts preserved were described from the Lower Silurian Herefordshire Konservat-Lagerstätte in England [4–6]. A single specimen of a kirkbyacean ostracod (Palaeocopida) with poorly preserved appendages has been described from the Upper Devonian of Italy [7]. Ostracods with soft-part preservation occur very rarely in Mesozoic and Cenozoic deposits (e.g. [8]).
2. Geological setting
The described specimen was found in the uppermost part of the marine Lochkovian succession cropping out in the right escarpment of the River Dniester near the village Ivanye Zlote, Podolia, Ukraine (coordinates: 48°42′21.7″ N, 25°39′12.5″ E). The deposits belong to the upper part of the Ivanye Horizon of the Tyver Series (see map and section, figs 1, 2 and 4 in [9]) and are composed of shallow-marine argillites interbedded with argillaceous carbonates. They contain abundant fish remains, ostracods, brachiopods, some bivalves, conodonts and scolecodonts. Upwards, they pass gradually into the Old Red Sandstone facies. The described specimen occurred in a carbonate layer located about 5 m below the Old Red deposits.
3. Material and methods
The material comprises rare complete carapaces, isolated pyritized valves and numerous phosphatized steinkerns showing well-preserved muscle scars, pore canals and marginal structures. One specimen preserves soft parts in the posterior part of the carapace.
The material was extracted from limestone by processing the samples with acetic acid using a method commonly applied for the search of conodonts. As a result, most of the specimens are preserved as phosphatic coatings of the originally calcitic valves, presumably because any remaining calcite was dissolved by the acetic acid. The internal phosphate layer is usually preserved, and therefore the internal morphological features of the carapace are preserved in the phosphate layer as negative reliefs. The pore canals within the valves have been cast in phosphate. The material is housed in the Institute of Paleobiology, Polish Academy of Sciences, Warsaw (ZPAL O.60).
4. Systematic palaeontology
Phylum: Arthropoda, Subphylum: Crustacea, Class: Ostracoda, Subclass: Podocopa, Order: Podocopida, Suborder: Metacopina, Superfamily: Healdioidea.
Family: Healdiidae [10].
Genus: Cytherellina [11].
Type species: Beyrichia siliqua Jones [12], p. 90, pl. 5, fig. 22; by original designation of Jones & Holl [11]; Late Silurian, late Ludlow Series, England.
Remarks: The genus Cytherellina is currently known from more than 45 species [13]. The specific differentiation in many descriptions of the Cytherellina species may be incorrect, which is caused by the lack of distinctive external morphological characters or insufficient knowledge on the interior valve morphology. The most distinctive feature of Cytherellina is the presence of an adductorial recess on the interior surface of the valves, reflected as an inflation bordered by two depressions (‘undulated contours’ of Jones & Holl [11]) on steinkerns of the type species Cytherellina siliqua from the Ludlow Series of England and a Scandinavian erratic boulder from the Wrocław area, Poland (Jones & Holl [11], pl. 14: 6a–e). Two specimens illustrated by Jones & Holl ([11], pl. 14: 2, 5) as C. siliqua have been referred to the new species Cytherellina ruperti [14]. The adductorial recess has been documented only in Cytherellina decliva [15] from the Early Devonian of Moldavia, Cytherellina oleskoiensis [16] from the Early Devonian of Podolia [17,18] and Cytherellina crepiduloides [19] from the Late Silurian of central New York, USA.
Stratigraphic and geographical range of the species known from internal morphology: Late Silurian–Early Devonian of Europe and USA.
Cytherellina submagna [1] (figures 1–3c).
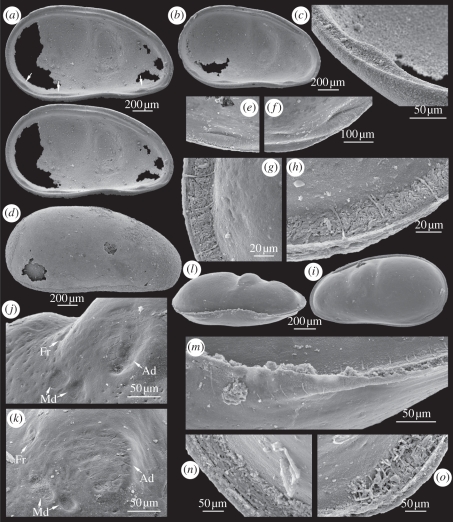
Figure 1.
Cytherellina submagna: (a) LV, internal view (stereo pair), internal teeth (arrows), ZPAL O.60/60; (b,c) LV, internal view and close up of caudo-ventral tooth, ZPAL O.60/62; (d) RV, lateral external view, ZPAL O.60/76; (e,f) antero-ventral, postero-ventral and caudo-ventral teeth visible as depressions on external surface of steinkern, ZPAL O.60/81; (g–i) steinkern, left lateral view, (g,h) marginal pore canals in anterior and posterior parts, (i) negative relief of adductorial recess and stop-teeth, ZPAL O.60/002; (j,k) central muscle field, anterior to left, ZPAL O.60/003, ZPAL O.60/043; (l,m) ventral view on steinkern and close up of marginal pore canals in antero-ventral and ventral parts, ZPAL O.60/038; (n,o) steinkern with bacterial-like phosphatized organisms in anterior and posterior part of fused zone, ZPAL O.60/033.
Figure 3.
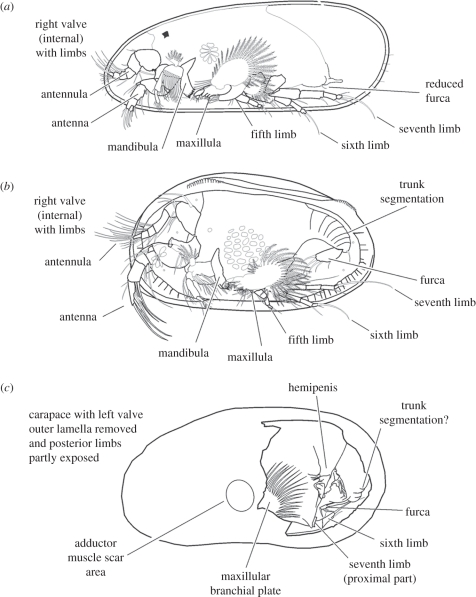
Diagrams showing comparative anatomy of (a) a darwinuloidean, (b) a sigillioidean and (c) Cytherellina submagna, with only one of each pair of limbs shown for clarity; (a) and (b) are ‘general arrangement’ diagrams that are not intended to represent single species and are based on illustrations published by (a) Rossetti & Martens [24] and Smith et al. [25], and (b) McKenzie [26], Maddocks [27], Schornikov & Gramm [28] and Wouters [29].
1963 Healdianella submagna sp. nov. [1], p. 99 (part), pl. 10, figs 1, 2, 4 (not pl. 9, fig. 10).
1971 Cytherellina? submagna; [17], p. 113, pl. 37, figs 13–16.
Holotype: Institute of Geology and Geochemistry, NAS Ukraine, Kyiv, no. 32/10 [1].
Type locality and stratigraphy: Kasperovcy village, left border of the river Seret, Podolia, Ukraine; Chortkov Horizon, Early Devonian.
Material: Several tens of steinkerns, of which one preserves soft parts, and some isolated valves.
Description: Carapace subovate in lateral view, moderately inflated posteriorly; maximum length 1.3 mm; greatest length below mid-height; greatest height at posterior cardinal angle. Dorsal margin asymmetrically arched with highest point in posterior part; anterior part of dorsal margin inclined anteriorly. Anterior margin narrowly and sharply rounded; posterior margin broadly rounded; ventral margin weakly concave. Left valve (LV) overlaps right valve (RV). LV with narrow ventral lip (i.e. ‘bow-shaped projection’ of Adamczak [20,21]). External surface smooth, lacking any lobal/sulcal structures. Sexual dimorphism not observed.
Internally, valves with deep adductorial recess in antero-median part, anteriorly and posteriorly bordered by two transverse ridges, posterior ridge best developed. Ridges distinguishable as depressions on steinkerns.
Adductor muscle scar (Ad) located in ventral part of adductorial recess, developed as closely packed cluster with up to 40 polygonal scars. Cluster outline transversely extended, asymmetric, with slightly concave anterior margin and curved posterior margin. Two elongated mandibular scars (Md) located antero-ventrally to Ad. Frontal muscle scar (Fr) transversely elongated, located antero-dorsally to Ad.
Hinge of LV consists of groove (crenulated?) and lists. LV with contact groove interrupted in the middle part of the ventral margin. Three inner stop-teeth (antero-ventral, postero-ventral and caudo-ventral) developed in LV by thickening of inner list. Antero-ventral and postero-ventral teeth located in front of ventral lip and behind it. Caudo-ventral tooth developed in posterior part of inner list, below mid-length and is best developed. Stop-teeth preserved as elongate depressions on surface of steinkerns.
Fused zone very narrow in the anterior and posterior parts of the carapace.
Marginal pore canals straight, comparatively short along anterior and posterior margins; canals crossing ventral lip simple, long. Adult specimens with 15–20 marginal pore canals along anterior margin, less canals along posterior margin.
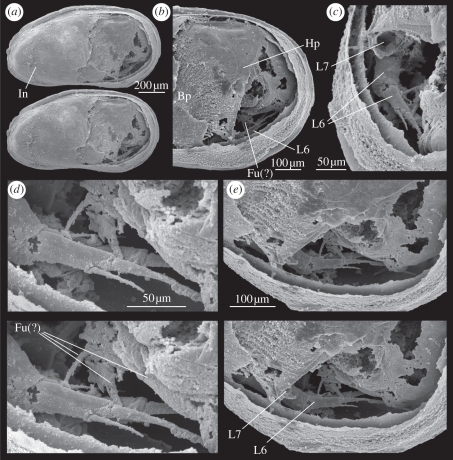
Carapace with soft parts preserved with valves closed. Branchial plate (Bp) of left maxillula (Mx), three-dimensionally preserved pair of walking legs, presumed furca (Fu) and hemipenis (Hp) preserved in posterior part of carapace.
Bp large, with up to 24 feathered rays, of which 19 definitely belong to the Mx Bp because in most cases their proximal point of attachment can be seen, and the rest are evident only by their distal ends protruding from under the inner lamella (In). These last five could belong to an L5 or sixth limb (L6) Bp.
Pair of presumed L6 is preserved in the postero-ventral part of the carapace. Endopodite of left limb with two complete visible segments: first visible segment (proximal) with a dorsal apical seta. Second visible segment elongate, with a ventral sub-apical seta and a very long, slender, slightly curved terminal claw. Right limb poorly visible behind the left, showing distal segment and terminal claw. The distal end of a third (more proximal) segment of the left L6 is visible in some views.
Presumed seventh limb (L7) represented by a proximal endopodite segment on the left side, hidden behind the triangular ventral tip of the Hp, with an oval cross-section (visible in figure 2c); distal segments and setae missing, presumed broken off.
Figure 2.
(a–e) Cytherellina submagna: (a) left lateral view of steinkern (stereo pair) with soft anatomy in posterior part, ZPAL O.60/001; (b) close-up view of soft parts showing Bp of Mx, walking legs, Hp and presumed Fu; (c) posterior oblique view of walking leg (L6) and the remnant of a basal podomere or proximal endopodite podomere of L7; (d) close-up of walking legs and presumed Fu (stereo pair), right appendage hidden behind the left one; (e) close-up (stereo pair) of soft parts in ventral part.
Hp large, triangular-ovate in outline, occupying a large part of the posterior part of carapace, with a rounded triangular ventral tip overhanging the L6 and L7 on the left side, and a complex structure protruding about halfway along the posterior side.
Fu a pair of elongate, lamellar rami extending anteriorly from the posterior body extremity; the distal (anterior) end of the left one is broken off, but that of the right one (much of which is hidden behind the left) bears distally two large terminal claws/setae with a slender seta in between, and the broken stub of a fourth seta posterior to these, all directed ventrally.
Posterior body showing possible traces of abdominal segmentation and bearing a small, posteriorly directed seta at the distal extremity.
Abundant phosphatized bacteria-like elongate organisms, each 3–4 µm in diameter and 10–15 µm in length, preserved in anterior and posterior marginal part of one specimen (figure 1n,o).
Remarks: C. submagna is closely related to C. oleskoiensis [16] from the Early Devonian of Podolia (Borshchiv and Lower Chortkiv horizons), from which it can be distinguished by a more elongate and slightly angular anterior margin, and by weaker LV–RV overlap along the ventral margin. The outline and negative relief of the adductorial recess in steinkerns of C. submagna are very similar to those in the specimen of C. siliqua from the erratic border from Poland illustrated by Jones & Holl [11].
Stratigraphic and geographical range: Early Devonian, Lochkovian, Chortkiv–Ivanye horizons, Podolia, Ukraine.
5. Phylogenetic implications
The evolution of the soft-part features was very slow in the ostracode lineages; in some cases, an evolutionary stasis has been recorded for more than 400 Myr (e.g. [4]). Therefore, the preservation of soft parts in fossil specimens is extremely important for the reconstruction of the phyletic continuity between Recent and fossil fauna.
As in Recent podocopids, the large Bp of the Mx of C. submagna was probably associated with respiration, being used to produce an oxygenated water current through the internal space of the carapace, and is interpreted as a modified exopod [22,23]. The morphology of the legs preserved in C. submagna, similar to the trunk walking legs in Recent podocopine ostracods, suggests that these appendages were used mainly for locomotion.
In general morphology of the preserved soft parts, C. submagna is relatively similar to some extant Podocopida, especially to members of the superfamilies Darwinuloidea and Sigillioidea (figure 3). The walking appendages of C. submagna may be compared with those of the Recent Podocopida. In the L6 and L7 walking legs of the Cytheroidea, Bairdioidea, Darwinuloidea and Sigillioidea, the endopodite is well developed with a terminal claw, but the exopodite is reduced [22]. The thoracic appendages of living platycopid ostracods differ from those of other ostracods [30]. In C. submagna, the Hp is large and with traces of complex structures.
We interpret the visible soft-part morphology as indicating a close affinity with living Sigillioidea (e.g. the genus Saipanetta), although there are also some interesting similarities with living Darwinuloidea. The Ad pattern, L7 long terminal seta, Fu with large distal setae and the possible trunk segmentation are all indicative of sigillioidean affinities. Olempska [31] reported the merodont hingement, numerous Ads and narrow calcified inner lamella in the Palaeozoic Microcheilinella species, which makes them similar to the Mesozoic to Recent sigillioidean ostracods. The metacopine affinities of Microcheilinella have been suggested by some authors (e.g. [32]). The sigillioidean affinities of C. submagna, which on the basis of hard-part morphology is clearly a metacopine healdioid, are particularly interesting because the sigilliodean genus Saipanetta was orginally considered to be a living metacopine ostracod [33], a view challenged by other authors (e.g. [34]). Our new evidence suggests that a reconsideration of Saipanetta as a living metacopine may not be out of the question, but it would be premature to draw conclusions without the evidence of the anterior appendages of C. submagna.
Recent darwinuloideans are ancient asexual ostracods (e.g. [35–37]) that brood their eggs internally; they probably derived, however, from bisexually reproducing ancestors that became extinct. Sexual reproduction in darwinuloidean ostracods probably disappeared after the end-Permian mass extinction and they are considered to have been asexual for over 200 Myr (e.g. [38]). They were diverse in the Late Palaeozoic, but recently darwinuloids are represented by only one family, the Darwinulidae, with five genera and 28 extant species [39]. Since the Carboniferous, the darwinuloids have been non-marine ostracods (e.g. [40]).
Although the preserved soft parts of C. submagna show a general podocopid affinity, the morphology of the shell clearly shows a similarity to the darwinuloids in having the LV–RV overlap (typical of most darwinilid genera except Darwinula), narrow fused zones with few straight and short marginal pore canals in the anterior and posterior part of the carapace, the presence of valve internal stop-teeth and a smooth external surface [39]; however, sulcal/lobe structures are not seen in post-Palaeozoic darwinuloideans.
The importance of the inner marginal structures of the carapace for the systematics of Palaeozoic podocopid ostracods, especially the morphology of the contact groove and the stop-mechanism, was strongly emphasized by Adamczak [20,21,41]. Characteristic features of the metacopines, such as the interrupted contact groove and narrow ventral lip, occur in the early metacopines (Kuresaaria gotlandica) from the Silurian of Gotland [21] and also in the late metacopine ostracod Ogmoconcha ellipsoidea from the Early Jurassic of England [42].
The inner marginal stop-teeth, interpreted as a blockage for the edge of the smaller valve in tightly closed carapaces, are common in Palaeozoic metacopines [20,41]. Internal stop-teeth on the antero- and postero-ventral margin of larger LV are present in Ordovician and Silurian metacopine genera (e.g. [21,43,44]). Similar structures occur also in the early podocopines (e.g. the bairdioidean Bairdiocypris marginata and Bairdiocypris lamellaris) [20]. The stop-mechanism in Early Palaeozoic species is a transitional character between the Podocopina and Metacopina [41].
Some freshwater Carbonitoidea species appear to have stop-teeth that closely resemble those described above. For example, stop-teeth preserved as ovate depressions ([45], figs 2.9, 2.11, 5.3) can be seen in the steinkerns of Carbonita sp. and Gutschickia sp. from the latest Mississippian of Virginia; this structure, however, is not discussed by the author. These ancient carapace features (such as the stop-mechanism) occur in some Recent and fossil darwinulid ostracods (e.g. Vestalenula, Penthesilenula, Microdarwinula) [25,38,39].
The teeth acted as stoppers to prevent the valves from overlapping one another too much ventrally and, therefore, to protect the eggs or juveniles in the brood space against crushing [25,46]. The stop-mechanism is unknown in Recent Cytheroidea. However, the resemblance between the valve stoppers of metacopines and darwinuloideans may also be a case of homeomorphy.
In Recent ostracods, the marginal pore canals bear sensilla that have a sensory function. They are likely to have played a similar role in Palaeozoic ostracods. The well-developed system of marginal pores in the Palaeocopida dates from at earliest the Early Palaeozoic times [47].
The very narrow fused zone with few straight and short marginal pore canals in the anterior and the posterior part of the carapace distinguished C. submagna from the Cytheroidea, but is more similar to those in the Darwinuloidea [39].
The Ad pattern of C. submagna, composed of many scars, differs from that of most Recent Podocopida, which shows fewer and more discrete scars, developed for example as a row of four scars in the Cytheroidea and arranged in a rosette composed of 9 to 11 elongate scars in the Darwinuloidea. On the other hand, the pattern is very similar to that of the podocopid Sigillioidea. However, the other aspects of the valve morphology of C. submagna, especially the hinge, are quite different from those of Sigillioidea. The possible darwinuloidean and sigillioidean affinities of C. submagna lend credence to the suggestion of Horne [40] that marine Sigillioidea could possibly be ancestral to the non-marine Darwinuloidea.
Acknowledgements
The authors thank two anonymous reviewers for comments made on the manuscript. This work was supported by a grant (no. N N307 057834) from the Ministry of Science and Higher Education to Hubert Szaniawski.
References
- 1.Krandijevsky V. S. 1963. The ostracod fauna of Silurian localities of Podolia. Akad. Nauk Ukr. RSR, Inst. Geol. Nauk, Kiev, 1–176 [in Ukrainian] [Google Scholar]
- 2.Baliński A. 2010. First colour-patterned strophomenide brachiopod from the earliest Devonian of Podolia, Ukraine. Acta Palaeont. Pol. 55, 695–700 10.4202/app.2010.0066 (doi:10.4202/app.2010.0066) [DOI] [Google Scholar]
- 3.Boomer I., Lord A., Crasquin S. 2008. The extinction of the Metacopina (Ostracoda). Senckenb. Leth. 88, 47–53 10.1007/BF03043977 (doi:10.1007/BF03043977) [DOI] [Google Scholar]
- 4.Siveter D. J., Sutton M. D., Briggs D. E. G., Siveter D. J. 2003. An ostracode crustacean with soft parts from the Lower Silurian. Science 302, 1749–1751 10.1126/science.1091376 (doi:10.1126/science.1091376) [DOI] [PubMed] [Google Scholar]
- 5.Siveter D. J., Briggs D. E. G., Siveter D. J., Sutton M. D. 2010. An exceptionally preserved myodocopid ostracod from the Silurian of Herefordshire, UK. Proc. R. Soc. B 277, 1539–1544 10.1098/rspb.2009.2122 (doi:10.1098/rspb.2009.2122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siveter D. J., Siveter D. J., Sutton M. D., Briggs E. G. 2007. Brood care in a Silurian ostracod. Proc. R. Soc. B 274, 465–469 10.1098/rspb.2006.3756 (doi:10.1098/rspb.2006.3756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller K. J. 1979. Body appendages of Paleozoic ostracodes. In Proc. 7th Int. Symp. on Ostracodes, Belgrade (ed. Krstić N.), pp. 5–8 Belgrade, Yugoslavia: Serbian Geological Society [Google Scholar]
- 8.Smith R. J. 2000. Morphology and ontogeny of Cretaceous ostracods with preserved appendages from Brazil. Palaeontology 43, 63–98 10.1111/1475-4983.00119 (doi:10.1111/1475-4983.00119) [DOI] [Google Scholar]
- 9.Małkowski K., Racki G., Drygant D., Szaniawski H. 2009. Carbon isotope stratigraphy across the Silurian–Devonian transition in Podolia, Ukraine: evidence for a global biogeochemical perturbation. Geol. Mag. 146, 652–674 10.1017/S0016756809006451 (doi:10.1017/S0016756809006451) [DOI] [Google Scholar]
- 10.Harlton B. H. 1933. Micropaleontology of the Pennsylvanian Johns Valley shale of the Ouachita Mountains, Oklahoma, and its relationship to the Mississippian Caney shale. J. Paleont. 7, 3–29 [Google Scholar]
- 11.Jones T. R., Holl H. B. 1869. Notes on the Palaeozoic bivalve Entomostraca—9: some Silurian species. Ann. Mag. Nat. Hist. Ser. 4 3, 211–229 [Google Scholar]
- 12.Jones T. R. 1855. Notes on Palaeozoic bivalve Entomostraca—1: some species of Beyrichia from the Upper Silurian Limestones of Scandinavia. Ann. Mag. Nat. Hist. Ser. 2 16, 81–92 [Google Scholar]
- 13.Kempf E. 1986. Index and bibliography of marine Ostracoda. 1. Index A. Geol. Inst. Univ. Köln Sonderveröffentl 50, 1–762 [Google Scholar]
- 14.Petersen L. E., Lundin R. F. 1996. On Cytherellina ruperti sp. nov. Stereo-Atlas Ostracod Shells 23, 49–52 [Google Scholar]
- 15.Abushik A. F., Trandafilova E. E. 1977. New Ostracoda from the Early Devonian of Moldavia. In New species of fossil plants and invertebrates of the SSSR, vol. 4, pp. 75–87 Moscow, Russia: Nauka. [in Russian] [Google Scholar]
- 16.Neckaja A. I. 1958. New species and genera of Ostracoda from the Ordovician and Silurian of the north-western part of the Russian platform. Tr. VNIGRI 115, 349–373 [in Russian] [Google Scholar]
- 17.Abushik A. F. 1971. Ostracoda from the Silurian-Lower Devonian key sections of Podolia. In Paleozoic Ostracoda from key sections in the European part of the USSR (ed. Ivanova V. A.), pp. 7–133 Moscow, Russia: Nauka. [in Russian] [Google Scholar]
- 18.Gramm M. N. 1982. The systematic position of the genus Healdianella Posner, 1951. In Fossil and recent ostracods (eds Bate R. H., Robinson E., Sheppard L.), pp. 193–218 British Micropalaeontology Society Series Chichester, UK: Ellis Horwood Limited [Google Scholar]
- 19.Berdan J. M. 1972. Brachiopoda and Ostracoda of the Cobleskill Limestone (Upper Silurian) of central New York, pp. 1–47 US Geological Survey, Professional Papers 730 Washington, US: US Government Printing Office [Google Scholar]
- 20.Adamczak F. 1976. Middle Devonian Podocopida (Ostracoda) from Poland: their morphology, systematics and occurrence. Senckenb. Leth. 57, 265–467 [Google Scholar]
- 21.Adamczak F. 1967. Morphology of two Silurian metacope ostracodes from Gotland. Geol. Fören. Stockholm Förhandl. 88, 462–475 [Google Scholar]
- 22.Horne D. J., Cohen A., Martens K. 2002. Taxonomy, morphology and biology of Quaternary and living Ostracoda. In The Ostracoda: applications in Quaternary research, vol. 131 (eds Holmes J. A., Chivas A.), pp. 5–36 Geophysical Monograph. Washington, DC: American Geophysical Union [Google Scholar]
- 23.Horne D. J. 2005. Homology and homeomorphy in ostracod limbs. Hydrobiologia 538, 55–80 10.1007/s10750-004-4937-5 (doi:10.1007/s10750-004-4937-5) [DOI] [Google Scholar]
- 24.Rossetti G., Martens K. 1996. Redescription and morphological variability of Darwinula stevensoni (Brady & Robertson, 1870) (Crustacea, Ostracoda). Bull. Inst. R. Sci. Nat. Belgique, Biol. 66, 73–92 [Google Scholar]
- 25.Smith R. J., Kamiya T., Horne D. J. 2006. Living males of the ‘ancient asexual’ Darwinulidae (Ostracoda: Crustacea). Proc. R. Soc. B 273, 1569–1578 10.1098/rspb.2005.3452 (doi:10.1098/rspb.2005.3452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenzie K. G. 1970. The ‘soft parts’ of Saipanetta tumida (Brady, 1890) (Ostracoda, Metacopina), with an expansion of the diagnosis of Saipanettidae. Crustaceana 19, 104–105 10.1163/156854070X00707 (doi:10.1163/156854070X00707) [DOI] [Google Scholar]
- 27.Maddocks R. F. 1972. Two new living species of Saipanetta (Ostracoda, Podocopida). Crustaceana 23, 28–42 10.1163/156854072X00048 (doi:10.1163/156854072X00048) [DOI] [Google Scholar]
- 28.Schornikov E. I., Gramm M. N. 1974. Saipanetta McKenzie, 1967 (Ostracoda) from the northern Pacific and some problems of classification. Crustaceana 27, 92–102 10.1163/156854074X00262 (doi:10.1163/156854074X00262) [DOI] [Google Scholar]
- 29.Wouters K. 1988. Two interesting new marine interstitial Ostracoda (Crustacea) from Comoros, with the description of Danipussella gen. nov. Bull. Inst. R. Sci. Nat. Belg. 58, 85–93 [Google Scholar]
- 30.Tsukagoshi A., Okada R., Horne D. J. 2006. Appendage homologies and the first record of eyes in platycopid ostracods, with the description of a new species of Keijcyoidea (Crustacea: Ostracoda) from Japan. Hydrobiologia 559, 255–274 10.1007/s10750-005-1139-8 (doi:10.1007/s10750-005-1139-8) [DOI] [Google Scholar]
- 31.Olempska E. 2001. Palaeozoic roots of the sigilliid ostracods. Mar. Micropaleontol. 41, 109–123 10.1016/S0377-8398(00)00062-1 (doi:10.1016/S0377-8398(00)00062-1) [DOI] [Google Scholar]
- 32.Kesling R. V., Chilman R. B. 1978. Ostracods of the Middle Devonian Silica Formation, vols 1–2 Papers on Paleontology, no. 18 Ann Arbor, MI: University of Michigan Museum of Paleontology [Google Scholar]
- 33.McKenzie K. G. 1967. Ostracod living fossils: new finds in the Pacific. Science 155, 1005. 10.1126/science.155.3765.1005 (doi:10.1126/science.155.3765.1005) [DOI] [PubMed] [Google Scholar]
- 34.Kesling R. V. 1981. Taxonomy of Ostracoda and the position of Microcheilinella Geis, vol. 25, pp. 289–315 Ann Arbor, MI: University of Michigan Museum of Paleontology [Google Scholar]
- 35.Schön I. K., Martens K., van Doninck K., Butlin R. K. 2003. Evolution in the slow lane: molecular rates of evolution in sexual and asexual ostracods (Crustacea: Ostracoda). Biol. J. Linn. Soc. 79, 93–100 10.1046/j.1095-8312.2003.00186.x (doi:10.1046/j.1095-8312.2003.00186.x) [DOI] [Google Scholar]
- 36.Schön I. K., Rossetti G., Martens K. 2009. Darwinulid ostracods: ancient asexual scandals or scandalous gossip? In Lost sex: the evolutionary biology of parthenogenesis (eds Schön I., Martens K., van Dijk P.), ch. 11, pp. 217–240 Berlin, Germany: Springer [Google Scholar]
- 37.Schön I. K., Martens K., Halse S. 2010. Genetic diversity in Australian ancient asexual Vestalenula (Ostracoda, Darwinulidae): little variability down under. Hydrobiologia 641, 59–70 10.1007/s10750-009-0057-6 (doi:10.1007/s10750-009-0057-6) [DOI] [Google Scholar]
- 38.Martens K., Rossetti G., Butlin R. K., Schön I. 2005. Molecular and morphological phylogeny of the ancient asexual Darwinulidae (Crustacea, Ostracoda). Hydrobiologia 538, 153–165 10.1007/s10750-004-4945-5 (doi:10.1007/s10750-004-4945-5) [DOI] [Google Scholar]
- 39.Rossetti G., Martens K. 1998. Taxonomic revision of the Recent and Holocene representatives of the family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bull. Inst. R. Sci. Nat. Belgique, Biol. 68, 55–110 [Google Scholar]
- 40.Horne D. J. 2003. Key events in the ecological radiation of the Ostracoda. In Bridging the gap: trends in the ostracode biological and geological sciences (eds Park L. E., Smith A. J.), pp. 181–201 Paleontology Society Paper no 9 New Haven, CT: Yale University Press [Google Scholar]
- 41.Adamczak F. J. 2005. The Superfamily Healdiacea Harlton, 1933, Family Healdiidae Harlton, 1933. Palaeozoic genera. N. Jb. Geol. Paläont. Abh. 238, 1–32 [Google Scholar]
- 42.Lord A. 1971. Revision of some Lower Lias Ostracoda from Yorkshire. Palaeontology 14, 642–665 [Google Scholar]
- 43.Hessland I., Adamczak F. 1974. On the taxonomic position of Steusloffina Teichert (Ostracoda). Geosci. Man 6, 59–64 [Google Scholar]
- 44.Schallreuter R., Hinz-Schallreuter I. 1999. Altpaläozoische Ostrakoden mit stoppern. N. Jb. Geol. Paläont. Mh. 4, 227–242 [Google Scholar]
- 45.Sohn I. G. 1985. Latest Mississippian (Namurian) nonmarine ostracodes from West Virginia and Virginia. J. Paleont. 59, 446–460 [Google Scholar]
- 46.Martens K., Rossetti G. 2002. On the Darwinulidae (Crustacea, Ostracoda) from Oceania, with the description of Vestalenula matildae n. sp. Invert. System. 16, 195–208 10.1071/IT01022 (doi:10.1071/IT01022) [DOI] [Google Scholar]
- 47.Olempska E. 2008. Soft body-related features of the carapace and the lifestyle of Paleozoic beyrichioidean ostracodes. J. Paleont. 82, 717–736 10.1666/07-096.1 (doi:10.1666/07-096.1) [DOI] [Google Scholar]