Abstract
Individual variation in infection modulates both the dynamics of pathogens and their impact on host populations. It is therefore crucial to identify differential patterns of infection and understand the mechanisms responsible. Yet our understanding of infection heterogeneity in wildlife is limited, even for important zoonotic host–pathogen systems, owing to the intractability of host status prior to infection. Using novel applications of stable isotope ecology and eco-immunology, we distinguish antecedent behavioural and physiological traits associated with avian influenza virus (AIV) infection in free-living Bewick's swans (Cygnus columbianus bewickii). Swans infected with AIV exhibited higher serum δ13C (−25.3 ± 0.4) than their non-infected counterparts (−26.3 ± 0.2). Thus, individuals preferentially foraging in aquatic rather than terrestrial habitats experienced a higher risk of infection, suggesting that the abiotic requirements of AIV give rise to heterogeneity in pathogen exposure. Juveniles were more likely to be infected (30.8% compared with 11.3% for adults), shed approximately 15-fold higher quantity of virus and exhibited a lower specific immune response than adults. Together, these results demonstrate the potential for heterogeneity in infection to have a profound influence on the dynamics of pathogens, with concomitant impacts on host habitat selection and fitness.
Keywords: antibody, habitat use, host–parasite interactions, nucleoprotein, predisposition, zoonotic
1. Introduction
Pathogens can play a significant role in shaping host behaviour, fecundity, population dynamics and community composition [1,2]. Yet hosts inevitably vary in their exposure and sensitivity to pathogenic infection. The way in which infection varies between individual hosts is fundamental to the transmission and maintenance of pathogens, as well as their impact on host fitness [3–6]. It is therefore crucial to identify differential patterns of infection and understand the mechanisms responsible.
Factors driving heterogeneity in infection may manifest themselves through variation in exposure to a pathogen, or variation in susceptibility to infection once the pathogen has been encountered [7]. For example, abiotic factors such as moisture and salinity can influence the persistence and transmission of pathogens [8,9]. As a result, pathogen exposure has been suggested to vary between biomes, with arctic, alpine and marine habitats anticipated to support lower pathogen density and diversity than temperate, tropical or freshwater habitats [10–12]. Furthermore, given that hosts often encounter their pathogens while feeding [13], the habitat in which a host forages is likely to influence its exposure to various pathogens. Following exposure, the likelihood that a host becomes infected and its level of pathogenesis following infection may also vary between hosts. Host age, prior infection(s) and physiological condition, particularly immunocompetence, have been suggested to influence a host's susceptibility to infection, with ramifications for disease dynamics in host populations [3,14]. Yet quantitative data on host foraging behaviour, condition and immunocompetence prior to infection have proven largely intractable in free-living populations.
Our knowledge gap is particularly apparent in migratory species, presumably because of logistic constraints in following them throughout the annual cycle and linking behaviour and physiology over such large spatial scales [15]. Yet these migratory movements may connect pathogen populations in disparate habitats [16] and at the same time expose migratory hosts to a high diversity of pathogens [17]. Moreover, migrants play host to a number of zoonotic pathogens of importance to humans. Understanding the drivers and consequences of disease epidemics in migratory hosts therefore remains a major frontier in ecology [18].
Certain abiotic conditions have long been considered crucial to the persistence, transmission and maintenance of a number of zoonotic pathogens [19], including avian influenza virus (AIV) [20,21]. Freshwater has been found to provide an ideal medium for the indirect faecal–oral transmission of AIV, which replicates in the gastrointestinal and/or respiratory tract of their hosts [22,23]. In addition, members of the Anseriformes and Charadriiformes that occupy aquatic habitats are regarded as the reservoir for all low-pathogenic AIV strains [23]; and laboratory and field observations reveal that AIV can persist for extended periods in freshwater [21,24,25]. Together, these findings suggest that exposure to AIV may be linked to aquatic foraging behaviour of individual hosts. Furthermore, Hinshaw et al. [26] found that a higher proportion of juvenile ducks were infected with AIV than adults, leading to the suggestion that immunological naivety may also be fundamental to the epidemiology of avian influenza.
Because low-pathogenic AIV infections generally proceed without clinical signs, these viruses have historically been considered non-pathogenic to their waterfowl reservoir [23]. However, recent field evidence suggests that infection with AIV may entail significant fitness costs to individual free-living birds, including reduced body mass and food intake rates, as well as delayed migration [27,28]. In addition, AIV circulating in wild birds represent a primordial source for influenza virus infection in humans and domestic livestock [29,30]. Identification of wild birds at risk of infection and factors driving the transmission and maintenance of AIV are therefore critical to understanding the epidemiology of this zoonotic disease in its natural hosts and assessing its ecological consequences.
We uniquely employ stable isotope ecology and ecoimmunology to divulge pre-infection status in a natural AIV host species, the Bewick's swan (Cygnus columbianus bewickii, Yarrel). Given that the stable isotope composition of an animal's tissues archive dietary isotope composition, and hence foraging habitat, during tissue synthesis [31], stable isotope analysis affords unparalleled insight into the importance of antecedent foraging behaviour to infection risk [32]. Information on the intensity of these infections, combined with the individual's ability to mount a specific immune response, is then used to reveal marked variation in host susceptibility. Together, these methods unveil unprecedented information on variation in host exposure and susceptibility to a major zoonotic disease in a natural host species.
2. Material and methods
(a). Capture and sampling
Bewick's swans were captured six to eight weeks after arrival on their Dutch wintering grounds over five successive winters (2005–2009 inclusive; electronic supplementary material, table S1). Each catch event targeted a single, cohesive flock of 200–400 swans foraging on sugar beet remains. Birds were aged as juveniles (6 months of age; n = 39), yearlings (n = 10) or adults (n = 133) on the basis of plumage, and sexed using molecular methods [33]. We sampled approximately 1 ml of whole blood from the brachial or tarsal vein, and collected cloacal and oropharyngeal swabs to test for current infection with AIV using sterile cotton swabs subsequently stored in Hank's Balanced Salt Solution. Blood samples were allowed to clot before being centrifuged approximately 6 h later. Red blood cells were stored in 70 per cent ethanol and, together with serum samples, maintained at −20°C until analysis.
(b). Virus and antibody detection
To estimate population prevalence of AIV with greater accuracy, we collected and swabbed fresh droppings from the capture site immediately after removing swans from the net (electronic supplementary material, table S1). Presence of AIV in the live bird and dropping samples was tested using a real-time reverse transcriptase polymerase chain reaction assay targeting the matrix gene [29]. The degree of viral shedding was assessed using the cycle threshold (CT) value, where CT is the first real-time amplification cycle in which matrix gene amplification was detectable. Therefore, CT value is inversely proportional to the number of virions in the sample. Three cycles represent a log10 difference in genome copies. The presence of antibodies to nucleoprotein (NP) in individual serum samples was tested using a commercially available blocking enzyme-linked immunosorbent assay (bELISA MultiS-Screen Avian Influenza Virus Antibody Test Kit, IDEXX Laboratories) with absorbance measured at 620 nm using a Tecan infinite 200 plate reader. All samples were run in duplicate, in combination with supplied positive and negative controls. Sample signal to noise ratios (sample mean absorbance divided by negative control mean absorbance) greater than 0.5 were considered negative for the presence of antibodies to NP.
(c). Stable isotope analysis
Bewick's swans forage in terrestrial and aquatic habitats throughout autumn migration and early winter [34]. In early winter, the majority of individuals rely on terrestrial food sources, with a minority also foraging on aquatic vegetation [33,35]. Importantly, the carbon stable isotope composition, δ13C (‰; where δ13C = 1000 × [(13C/12Csample ÷ 13C/12CVienna PeeDee limestone) − 1]) of food plants used by Bewick's swans in aquatic habitats (−17.2‰ ± 0.64) is significantly higher than food plants from terrestrial habitats (−27.5‰ ± 0.42) [33,35]. As a result, δ13C of Bewick's swan tissues can be used to infer an individual's habitat use across the aquatic–terrestrial continuum. Furthermore, the period required to incorporate a specific isotope pattern from diet into body tissue, known as turn-over time, depends on the speed of tissue renewal and determines the temporal window during which changes in the isotopic composition of an animal's diet can be discerned [31]. Blood components show rapid turn-over and therefore archive information on an individual's diet, and hence foraging habitat, in the foregoing days (plasma) to weeks (red blood cells) [36]. Given that infection persists for 3–8 days in free-living ducks [27], δ13C of blood components was examined to assess the importance of temporally relevant foraging behaviour to intra-population variation in AIV infection. Sub-samples of red blood cells and serum were freeze-dried before 200–500 µg was analysed for δ13C. Reproducibility based on replicate measurements of a casein standard was 0.42‰ (s.d.; n = 42). To correct for the inherent difference between diet and tissue δ13C values, discrimination factors (δ13CTissue − δ13CDiet) experimentally obtained from our captive population of Bewick's swans were subtracted: −0.69 for red blood cells and −0.09 for serum [37,38]. All tissue δ13C values were transformed to achieve normality before statistical analysis.
3. Results
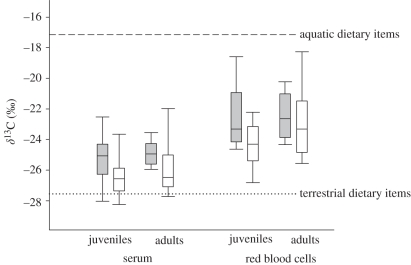
Infection differed on the basis of foraging habitat (tissue δ13C) and host age. Swans infected with AIV exhibited higher serum δ13C (least-squares mean (LSM) ± s.e.: −25.3 ± 0.4) than their non-infected counterparts (LSM: −26.3 ± 0.2; χ2 = 5.86, p = 0.016; figure 1) when the effect of age (χ2 = 7.35, p = 0.007) was taken into account (logistic regression: χ2 = 11.30, p = 0.004). Although the δ13C values for red blood cells were higher than those from serum (repeated-measures ANOVA: F1,139 = 152.31, p < 0.001; figure 1) [33], red blood cell δ13C showed patterns similar to serum δ13C with respect to infection. Infected swans showed higher red blood cell δ13C (LSM: −22.8 ± 0.6) than their non-infected counterparts (LSM: −23.9 ± 0.3; χ2 = 3.62, p = 0.057), when the effect of age (χ2 = 8.09, p = 0.005) was considered (logistic regression: χ2 = 9.88, p = 0.007; figure 1).
Figure 1.
δ13C (‰) of serum and red blood cells from juvenile and adult Bewick's swans naturally infected (grey box plots) or uninfected (white box plots) with avian influenza virus at the time of capture; whiskers indicate 2.5 and 97.5 percentiles. Horizontal lines represent mean δ13C (‰) of dietary items from aquatic (dashed) and terrestrial (dotted) habitats (data from Hoye et al. [33]).
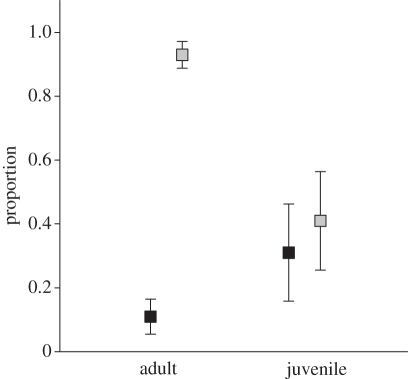
At the time of capture, 15.4 per cent (95% CI: 10.4, 20.4) of swans were found infected with AIV. Prevalence estimates were substantially higher in juveniles (30.8%; 95% CI: 17.9, 46.2) than in adults (11.3%; 95% CI: 6.0, 16.5; figure 2); only one of the 10 yearlings was infected. Parents were no more likely to be infected than were adults without offspring (χ2 = 1.66, p = 0.197). There was no effect of sex or year in any of the statistical models. Antibodies to NP were detected in 81.4 per cent of the swans (95% CI: 75.9, 86.9), with the vast majority of adults (93.0%; 95% CI: 88.8, 96.7) and yearlings (70.0%; 95% CI: 51.7, 86.2) being seropositive. In contrast, less than half of the juveniles were seropositive (41.0%; 95% CI: 43.6, 74.4; χ2 = 46.62, p < 0.001; figure 2). All but two of the 15 adults who were infected had detectable antibodies to NP, whereas only half of the infected juveniles (six of 12) were seropositive. Of those who had detectable antibodies to NP, the signal to noise ratio of the bELISA differed between adults (median: 0.19; inter-quartile range: 0.11, 0.28) and juveniles (median: 0.30; inter-quartile range: 0.25, 0.39; t = 4.27, d.f. = 138, p = 0.0003).
Figure 2.
Proportion of adult and juvenile Bewick's swans infected with avian influenza virus (AIV; black squares), and with detectable antibodies to the nucleoprotein (NP) of AIV (grey squares) when captured on their Dutch wintering grounds. Error bars represent 95% CI of the prevalence estimate.
The degree of viral shedding (CT value) also differed on the basis of age (adults: LSM 34.68 ± 0.90; juveniles: LSM 31.15 ± 1.04), indicating approximately 15-fold higher virus concentration in samples from juveniles when the effect of tract and serum δ13C were considered (GLM: r2 = 0.478, F6,18 = 2.75; electronic supplementary material, table S2). There was an interaction between age and tract: adults had lower CT from the cloaca compared with the oropharynx, whereas juveniles showed substantially lower CT in the oropharynx compared with the cloaca (figure 3), indicating approximately 150-fold higher virus concentrations in juvenile compared with adult oropharynges. Finally, there was an interaction between serum δ13C and tract, with a negative relationship between serum δ13C and CT for oropharyngeal infections, but no relationship for cloacal infections.
Figure 3.
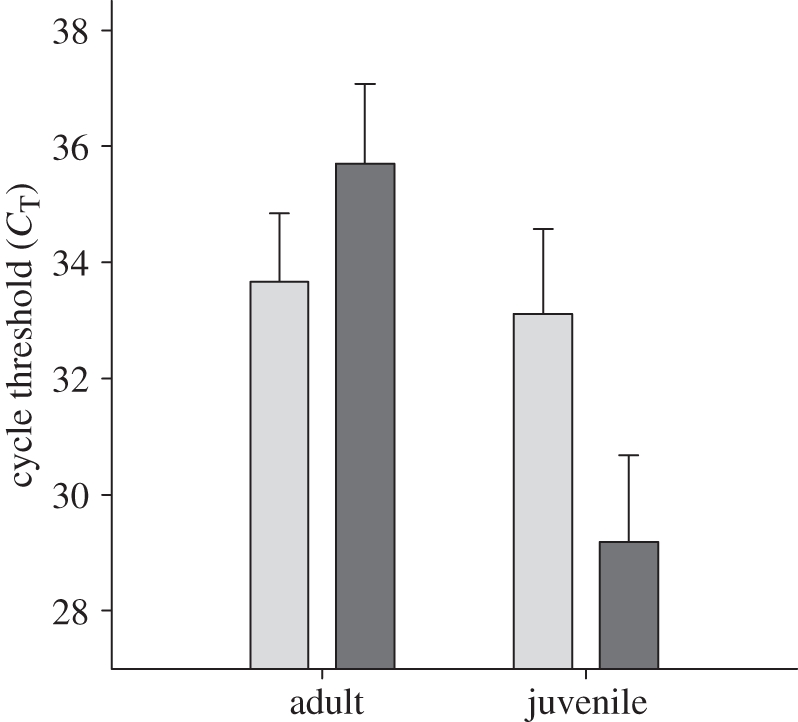
Mean avian influenza virus cycle threshold value (the first real-time amplification cycle in which target gene amplification is detectable) estimates (+s.e.) of naturally infected Bewick's swans, when the effect of serum δ13C was accounted for (GLM: r2 = 0.478, F6,18 = 2.75; electronic supplementary material, table S2). Light grey bars, cloaca; dark grey bars, oropharynx.
The infection risk of a flock of swans (a single capture event) was also related to the flock's demographic make-up. Prevalence estimated from the proportion of birds infected mirrored prevalence estimated from the proportion of droppings infected (log-transformed to achieve normality; r2 = 0.994, F1,4 = 715.79, p < 0.001; electronic supplementary material, table S1), and was therefore used as an estimate of prevalence for each catch event. Prevalence of AIV increased with an increase in the proportion of juveniles in the flock (r2 = 0.448, F1,7 = 5.69, p = 0.049), but showed no significant relationship with either the mean serum δ13C or the mean red blood cell δ13C of a flock.
4. Discussion
Environmental conditions, host condition and host infection history have been hypothesized to be just as critical to the outcome of infection as parasite traits. Environmental and host conditions are therefore of fundamental importance to the transmission, maintenance and ecological impacts of pathogen infection [3,6]. Yet analyses of disease dynamics use average quantities to describe host–pathogen systems [3,4], primarily because heterogeneity in infection at the individual level has rarely been examined in wildlife systems. Using AIV in free-living Bewick's swans as a model system, we uniquely demonstrate that differences in host behaviour, as well as age and immunocompetence, are linked to infection risk. These differences entail broad-scale ramifications for both the transmission and maintenance of the pathogen, as well as the fitness costs imposed on the host population.
(a). Age and immunocompetence
Juvenile Bewick's swans were more than three times as likely to be infected with AIV as contemporaneously sampled adults (figure 2). Similarly, at the population level, we found that flocks that contained a higher proportion of juveniles also exhibited a higher prevalence of infection. Bewick's swans show extended parental care such that juveniles remain with their parents throughout autumn migration and overwintering. However, adults with attendant juveniles were no more likely to be infected than adults without offspring, suggesting that the higher infection risk in juveniles may be more related to differential susceptibility to infection than differential exposure to AIV.
Hinshaw et al. [26] suggested that immunological naivety may play an important role in the epidemiology of AIV in wild birds. However, despite more than 30 years of additional research, the importance of age and immunocompetence has remained largely theoretical. Strikingly, we found that juveniles were less likely to exhibit a detectable specific immune response while infected than adults (figure 2). Furthermore, of the individuals who did show a detectable response, bELISA values indicated that juveniles exhibited substantially lower blocking activity in the assay. Such differences may be the result of ontogenetic differences in the immune system or age-related differences in infection history. The presence of antibodies to homo- and hetero-subtypic AIV strains has been shown to decrease the duration of subsequent infections in the laboratory [39,40]. Moreover, even if antibodies to prior infection(s) have waned, as appears to be the case for antibodies to AIV NP [41], the immune responses of individuals who have previously experienced AIV infection may differ from that of naive individuals. Individuals who have previously been infected with AIV may mount a more rapid immune response, and thus be more likely to show detectable antibodies while infected, than naive individuals. Simultaneously, individuals who have previously been infected may also mount a more robust immune response to subsequent infection, with higher binding activity in the bELISA assay, than naive individuals. As a result, individuals who have already experienced AIV infection (i.e. adults) would be expected to be infected for a shorter period, and hence show lower prevalence as a cohort, as demonstrated here.
Once infected, juveniles also shed a greater quantity of virus than adults, especially from the oropharynx (figure 3). Costa et al. [42] experimentally demonstrated that among naive, one- to four-month old mallards (Anas platyrhynchos), shedding decreased with age. Furthermore, the intensity of viral shedding in laboratory-reared mallards has been shown to be 1–5 log10 lower following hetero-subtypic reinfection than following a primary inoculation [39,40]. When paired with these experimental results, our findings from free-living Bewick's swans uniquely demonstrate that both age and lack of prior exposure to AIV result in juveniles experiencing longer and more intense infections than adults. Such increased susceptibility to infection implies that juvenile birds are of critical importance to the transmission and maintenance of AIV, and are likely to be more vulnerable to any sub-lethal effects of infection.
(b). Host behaviour
Numerous pathogens require water and/or high humidity to complete certain phases of their life cycles, such that infection risk may be higher in moist, aquatic environments [9]. Indeed, proximity to surface water has repeatedly been linked with disease risk in humans (e.g. [12,43]). Data from wildlife are more scarce; however, species using freshwater rather than marine wintering habitats showed higher prevalence of blood parasites on the breeding grounds [44,45]. Aquatic environments are considered critical to the persistence of AIV [21,23] and are thought to play an important role in viral transmission [22,46]. Correspondingly, our stable isotope data uniquely demonstrate that the abiotic requirements of the pathogen result in heterogeneity in pathogen exposure within a species, owing to differences in host foraging behaviour.
While very few Bewick's swans forage exclusively in aquatic habitats during winter [33], those that continue to nocturnally forage in aquatic habitats while diurnally foraging in terrestrial habitats have been shown to be maximizing their foraging potential [34]. However, this foraging strategy, resulting in tissue δ13C values being higher than those for terrestrial vegetation, was associated with higher risk of infection within a given age class (figure 1). While some individuals who used aquatic habitats were not infected, the vast majority of individuals who were infected had used aquatic habitats in the foregoing days (indicated by serum δ13C) and weeks (red blood cell δ13C). These results suggest that aquatic foraging exposes individuals to a higher risk of infection with AIV, adding to the meagre body of evidence demonstrating the potential for hosts to encounter free-living pathogens or vectors while foraging [13]. Host foraging behaviour and habitat use will therefore play a fundamental role in modulating pathogen transmission.
In demonstrating a link between AIV infection and individual foraging habitat, we also underscore the potential for habitat selection to be modulated by infection risk [10,18]. Aquatic habitats provide Bewick's swans with abundant, high-quality food resources, longer foraging times and shelter from predation [34]. These habitats have also been associated with higher body condition prior to spring migration, as well as increased breeding success [33]. Such superior foraging conditions in aquatic habitats suggest that it is unlikely that individuals using aquatic foraging strategies are more susceptible to infection. Instead, our data suggest that aquatic foraging exposes individuals to a higher risk of infection with AIV. Because AIV may impose subtle reductions in host fitness [27,28], the benefits of aquatic foraging must be weighed against the cost of increased exposure to AIV infection. Bewick's swans will therefore face multi-faceted trade-offs when selecting foraging habitats during migration and overwintering. Crucially, habitat–infection risk trade-offs are likely to operate differentially among individuals within a population, modulated by the underlying condition of the host and its ability to cope with infection. As juveniles appear more susceptible to infection, infection risk may be of fundamental importance to the foraging decisions of families.
Factors governing disease epidemics in natural host populations and their long-term consequences for host behaviour, life history and biogeography remain an important frontier in ecology and epidemiology. By distinguishing host-specific behavioural and physiological traits associated with AIV infection, we reveal the potential for heterogeneity in infection to have profound influence on the dynamics of pathogens and their ecological consequences for host populations.
Acknowledgements
Swans were handled under approvals CL04.02, CL06.06 and CL08.05 from the Animal Experimentation Committee (DEC) of the Royal Netherlands Academy of Arts and Sciences (KNAW), and all efforts were made to minimize any suffering throughout the study.
We would like to thank Jan van Gils, Kees Oosterbeek, Wim Tijssen, Simon Deuzeman, Naomi Huig, Thijs de Boer and Peter de Vries for valuable assistance with catching swans, Christa Mateman for molecular sexing, and Harry Korthals for stable isotope analysis. Vincent Munster, Pascal Lexmond, Oahn Vuong, Judith Guldemeester and Chantal Baas provided excellent laboratory assistance. This study was supported by the Research Council for Earth and Life Sciences (ALW) with financial aid from the Netherlands Organization for Scientific Research (NWO; grant 851.40.073), EU Framework six programme NewFluBird (044490) and contract NIAIDNIH HHSN266200700010C. This is publication 5057 of the NIOO-KNAW.
References
- 1.Hudson P., Rizzolli A., Grenfell B., Heesterbeek J., Dobson A. (eds) 2002. The ecology of wildlife diseases. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Lafferty K. D. 2008. Effects of disease on community interactions and food web structure. In Infectious disease ecology: the effect of ecosystems on disease and of disease on ecosystems (eds Ostfeld R. S., Keesing F., Eviner V. T.), pp. 205–222 Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Beldomenico P. M., Begon M. 2010. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol. 25, 21–27 10.1016/j.tree.2009.06.015 (doi:10.1016/j.tree.2009.06.015) [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Smith J. O., Schreiber S. J., Kopp P. E., Getz W. M. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359 10.1038/nature04153 (doi:10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer-Schadt S., Fernandez N., Eisinger D., Grimm V., Thulke H. H. 2009. Individual variations in infectiousness explain long-term disease persistence in wildlife populations. Oikos 118, 199–208 10.1111/j.1600-0706.2008.16582.x (doi:10.1111/j.1600-0706.2008.16582.x) [DOI] [Google Scholar]
- 6.Johnson P. T. J., Hartson R. B. 2009. All hosts are not equal: explaining differential patterns of malformations in an amphibian community. J. Anim. Ecol. 78, 191–201 10.1111/j.1365-2656.2008.01455.x (doi:10.1111/j.1365-2656.2008.01455.x) [DOI] [PubMed] [Google Scholar]
- 7.Wilson K., Bjørnstad O. N., Dobson A. P. 2002. Heterogeneities in macroparasite infections: patterns and processes. In The ecology of wildlife diseases (ed. Hudson P. J., Rizzoli A., Grenfell B. T., Heesterbeek H., Dobson A. P.), pp. 6–44 Oxford, UK: Oxford University Press [Google Scholar]
- 8.Guernier V., Hochberg M. E., Guegan J. F. O. 2004. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2, 740–746 10.1371/journal/pbio.0020141 (doi:10.1371/journal/pbio.0020141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Combes C. 2001. Parasitism: the ecology and evolution of intimate interactions. Chicago, IL: University of Chicago Press [Google Scholar]
- 10.Piersma T. 1997. Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80, 623–631 10.2307/3546640 (doi:10.2307/3546640) [DOI] [Google Scholar]
- 11.Balls M. J., Bodker R., Thomas C. J., Kisinza W., Msangeni H. A., Lindsay S. W. 2004. Effect of topography on the risk of malaria infection in the Usambara Mountains, Tanzania. Trans. R. Soc. Trop. Med. Hyg. 98, 400–408 10.1016/j.trstmh.2003.11.005 (doi:10.1016/j.trstmh.2003.11.005) [DOI] [PubMed] [Google Scholar]
- 12.Foley D. H., Torres E. P., Mueller I., Bryan J. H., Bell V. 2003. Host-dependent Anopheles flavirostris larval distribution reinforces the risk of malaria near water. Trans. R. Soc. Trop. Med. Hyg. 97, 283–287 10.1016/S0035-9203(03)90143-X (doi:10.1016/S0035-9203(03)90143-X) [DOI] [PubMed] [Google Scholar]
- 13.Hall S. R., Sivars-Becker L., Becker C., Duffy M. A., Tessier A. J., Cáceres C. E. 2007. Eating yourself sick: transmission of disease as a function of foraging ecology. Ecol. Lett. 10, 207–218 10.1111/j.1461-0248.2006.01011.x (doi:10.1111/j.1461-0248.2006.01011.x) [DOI] [PubMed] [Google Scholar]
- 14.Johnson P. T. J., Kellermanns E., Bowerman J. 2011. Critical windows of disease risk: amphibian pathology driven by developmental changes in host resistance and tolerance. Funct. Ecol. 25, 726–734 10.1111/j.1365-2435.2010.01830.x (doi:10.1111/j.1365-2435.2010.01830.x) [DOI] [Google Scholar]
- 15.Harrison X. A., Blount J. D., Inger R., Norris D. R., Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18 10.1111/j.1365-2656.2010.01740.x (doi:10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 16.Ricklefs R., Fallon S., Latta S., Swanson B., Bermingham E. 2005. Migrants and their parasites. In Birds of two worlds: the ecology and evolution of migration (eds Greenburg R., Marra P.), pp. 210–221 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 17.Figuerola J., Green A. J. 2000. Haematozoan parasites and migratory behaviour in waterfowl. Evol. Ecol. 14, 143–153 10.1023/A:1011009419264 (doi:10.1023/A:1011009419264) [DOI] [Google Scholar]
- 18.Altizer S., Bartel R., Han B. A. 2011. Animal migration and infectious disease risk. Science 331, 296–302 10.1126/science.1194694 (doi:10.1126/science.1194694) [DOI] [PubMed] [Google Scholar]
- 19.Harvell C. D., Mitchell C. E., Ward J. R., Altizer S., Dobson A. P., Ostfeld R. S., Samuel M. D. 2002. Ecology: climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162 10.1126/science.1063699 (doi:10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 20.Webster R. G., Morita M., Pridgen C., Tumova B. 1976. Ortho- and paramyxoviruses from migrating feral ducks: characterization of a new group of influenza A viruses. J. Gen. Virol. 32, 217–225 10.1099/0022-1317-32-2-217 (doi:10.1099/0022-1317-32-2-217) [DOI] [PubMed] [Google Scholar]
- 21.Stallknecht D. E., Kearney M. T., Shane S. M., Zwank P. J. 1990. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Av. Dis. 34, 412–418 10.2307/1591429 (doi:10.2307/1591429) [DOI] [PubMed] [Google Scholar]
- 22.VanDalen K. K., Franklin A. B., Mooers N. L., Sullivan H. J., Shriner S. A. 2010. Shedding light on avian influenza H4N6 infection in mallards: modes of transmission and implications for surveillance. PLoS ONE 5, e12851. 10.1371/journal.pone.0012851 (doi:10.1371/journal.pone.0012851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster R. G., Bean W. J., Gorman O. T., Chambers T. M., Kawaoka Y. 1992. Evolution and ecology of influenza-A viruses. Microbiol. Rev. 56, 152–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito T., Okazaki K., Kawaoka Y., Takada A., Webster R. G., Kida H. 1995. Perpetuation of influenza-A viruses in Alaskan Waterfowl Reservoirs. Arch. Virol. 140, 1163–1172 10.1007/BF01322743 (doi:10.1007/BF01322743) [DOI] [PubMed] [Google Scholar]
- 25.Nazir J., Haumacher R., Ike A., Stumpf P., Bohm R., Marschang R. E. 2010. Long-term study on tenacity of avian influenza viruses in water (distilled water, normal saline, and surface water) at different temperatures. Av. Dis. 54, 720–724 10.1637/8754-033109-ResNote.1 (doi:10.1637/8754-033109-ResNote.1) [DOI] [PubMed] [Google Scholar]
- 26.Hinshaw V. S., Webster R. G., Turner B. 1980. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol. 26, 622–629 10.1139/m80-108 (doi:10.1139/m80-108) [DOI] [PubMed] [Google Scholar]
- 27.Latorre-Margalef N., et al. 2009. Effects of influenza A virus infection on migrating mallard ducks. Proc. R. Soc. B 276, 1029–1036 10.1098/rspb.2008.1501 (doi:10.1098/rspb.2008.1501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Gils J. A., Munster V. J., Radersma R., Liefhebber D., Fouchier R. A. M., Klaassen M. 2007. Hampered foraging and migratory performance in swans infected with low-pathogenic avian influenza A virus. PLoS ONE 2, e184. 10.1371/journal.pone.0000184 (doi:10.1371/journal.pone.0000184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munster V. J., Wallensten A., Baas C., Rimmelzwaan G. F., Schutten M., Olsen B., Osterhaus A., Fouchier R. A. M. 2005. Mallards and highly pathogenic avian influenza ancestral viruses, northern Europe. Emerg. Infect. Dis. 11, 1545–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okazaki K., et al. 2000. Precursor genes of future pandemic influenza viruses are perpetuated in ducks nesting in Siberia. Arch. Virol. 145, 885–893 10.1007/s007050050681 (doi:10.1007/s007050050681) [DOI] [PubMed] [Google Scholar]
- 31.Hobson K. A. 2008. Tracking animal migration with stable isotopes. In Terrestrial ecology, vol. 2 (eds Hobson K. A., Wassenaar L. I.), pp. 45–78 London, UK: Academic Press [Google Scholar]
- 32.Carrete M., Serrano D., Illera J. C., Lopez G., Vogeli M., Delgado A., Tella J. L. 2009. Goats, birds, and emergent diseases: apparent and hidden effects of exotic species in an island environment. Ecol. Appl. 19, 840–853 10.1890/07-2134.1 (doi:10.1890/07-2134.1) [DOI] [PubMed] [Google Scholar]
- 33.Hoye B. J., Hahn S., Nolet B. A., Klaassen M. Submitted Habitat use throughout migration: linking individual consistency, prior breeding success and future breeding potential. J. Anim. Ecol. [DOI] [PubMed] [Google Scholar]
- 34.Nolet B. A., Bevan R. M., Klaassen M., Langevoord O., Van der Heijden Y. 2002. Habitat switching by Bewick's swans: maximization of average long-term energy gain? J. Anim. Ecol. 71, 979–993 10.1046/j.1365-2656.2002.00662.x (doi:10.1046/j.1365-2656.2002.00662.x) [DOI] [Google Scholar]
- 35.Nolet B. A., Beekman J. H., Klaassen M., Langevoord O., Santamaria L. 2000. Waterplanten en Kleine Zwanen Cygnus columbianus bewickii: een wederzijdse afhankelijkheid? Limosa 73, 105–108 [In Dutch.] [Google Scholar]
- 36.Podlesak D. W., McWilliams S. R., Hatch K. A. 2005. Stable isotopes in breath, blood, feces and feathers can indicate intra-individual changes in the diet of migratory songbirds. Oecologia 142, 501–510 10.1007/s00442-004-1737-6 (doi:10.1007/s00442-004-1737-6) [DOI] [PubMed] [Google Scholar]
- 37.Hahn S., Hoye B. J., Korthals H., Klaassen M. Submitted From food to offspring down: tissue-specific discrimination and turn-over of stable isotopes in herbivorous waterbirds and other avian foraging guilds. PLoS ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoye B. J. In press Variation in post-sampling treatment of avian blood affects ecophysiological interpretations. Methods Ecol. Evol. (doi:10.1111/j.2041-210X.2011.00135.x) [Google Scholar]
- 39.Costa T. P., Brown J. D., Howerth E. W., Stallknecht D. E. 2010. Effect of a prior exposure to a low pathogenic avian influenza virus in the outcome of a heterosubtypic low pathogenic avian influenza infection in Mallards (Anas platyrhynchos). Av. Dis. 54, 1286–1291 10.1637/9480-072210-Reg.1 (doi:10.1637/9480-072210-Reg.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jourdain E., et al. 2010. Influenza virus in a natural host, the mallard: experimental infection data. PLoS ONE 5, e8935. 10.1371/journal.pone.0008935 (doi:10.1371/journal.pone.0008935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoye B. J., Munster V. J., Nishiura H., Fouchier R. A. M., Madsen J., Klaassen M. 2011. Reconstructing an annual cycle of interaction: natural infection and antibody dynamics to avian influenza along a migratory flyway. Oikos 120, 748–755 10.1111/j.1600-0706.2010.18961.x (doi:10.1111/j.1600-0706.2010.18961.x) [DOI] [Google Scholar]
- 42.Costa T. P., Brown J. D., Howerth E. W., Stallknecht D. E. 2010. The effect of age on avian influenza viral shedding in mallards (Anas platyrhynchos). Av. Dis. 54, 581–585 10.1637/8692-031309-ResNote.1 (doi:10.1637/8692-031309-ResNote.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali M., Emch M., Donnay J. P., Yunus M., Sack R. B. 2002. Identifying environmental risk factors for endemic cholera: a raster GIS approach. Health Place 8, 201–210 10.1016/S1353-8292(01)00043-0 (doi:10.1016/S1353-8292(01)00043-0) [DOI] [PubMed] [Google Scholar]
- 44.Mendes L., Piersma T., Lecoq M., Spaans B., Ricklefs R. E. 2005. Disease-limited distributions? Contrasts in the prevalence of avian malaria in shorebird species using marine and freshwater habitats. Oikos 109, 396–404 10.1111/j.0030-1299.2005.13509.x (doi:10.1111/j.0030-1299.2005.13509.x) [DOI] [Google Scholar]
- 45.Yohannes E., Krizanauskiene A., Valcu M., Bensch S., Kempenaers B. 2009. Prevalence of malaria and related haemosporidian parasites in two shorebird species with different winter habitat distribution. J. Ornithol. 150, 287–291 10.1007/s10336-008-0349-z (doi:10.1007/s10336-008-0349-z) [DOI] [Google Scholar]
- 46.Rohani P., Breban R., Stallknecht D. E., Drake J. M. 2009. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl Acad. Sci. USA 106, 10 365–10 369 10.1073/pnas.0809026106 (doi:10.1073/pnas.0809026106) [DOI] [PMC free article] [PubMed] [Google Scholar]