Abstract
The cross-sectional area of a nutrient foramen of a long bone is related to blood flow requirements of the internal bone cells that are essential for dynamic bone remodelling. Foramen area increases with body size in parallel among living mammals and non-varanid reptiles, but is significantly larger in mammals. An index of blood flow rate through the foramina is about 10 times higher in mammals than in reptiles, and even higher if differences in blood pressure are considered. The scaling of foramen size correlates well with maximum whole-body metabolic rate during exercise in mammals and reptiles, but less well with resting metabolic rate. This relates to the role of blood flow associated with bone remodelling during and following activity. Mammals and varanid lizards have much higher aerobic metabolic rates and exercise-induced bone remodelling than non-varanid reptiles. Foramen areas of 10 species of dinosaur from five taxonomic groups are generally larger than from mammals, indicating a routinely highly active and aerobic lifestyle. The simple measurement holds possibilities offers the possibility of assessing other groups of extinct and living vertebrates in relation to body size, behaviour and habitat.
Keywords: metabolic rate, blood flow, bone remodelling, nutrient foramen, allometry
1. Introduction
The size of blood vessels is dynamically variable, responding to the blood flow requirements through them. Vessels throughout the body react specifically to greater blood pressures by thickening and strengthening the walls, and to greater shear stress (related to the velocity of the blood) by increasing circumference [1,2]. Organs that have higher metabolic rates require higher flow rates and therefore have larger blood vessels that service them. To test the hypothesis that differences in blood perfusion rates reflect differences in metabolic capacity between species, this study examines the correlations between femoral nutrient foramen size, bone volume, body mass and resting and maximum metabolic rates in living mammals and reptiles. As bones are the only tissues remaining from non-avian dinosaurs, the relationships between nutrient foramina size and metabolic capacity developed for mammals and reptiles can indicate the levels of activity and metabolic status of dinosaurs.
Long bones of all amniotes, comprising the reptiles, mammals and birds, receive blood from three sources: (i) nutrient vessels, (ii) metaphyseal and epiphyseal vessels that combine after the cartilaginous growth plate is closed and (iii) periosteal vessels [3]. The nutrient system contributes around 50–70% of the blood supply of the cortical and medullary regions [4]. Each nutrient artery enters the long bone through a discrete foramen on the diaphysis (central shaft comprising the cortex and medullary cavity). The artery does not branch inside this canal but divides into an ascending and descending limb on reaching the medullary cavity. From these limbs arise two types of arteries—cortical branches that supply the cortical bone, and centripetal branches that enter the medullary sinusoids to supply the bone marrow before draining into the central venous sinus. The central vein exits the bone either along the artery through the foramen or as a separate emissary channel [5]. Cortical bone of all amniotes is cellular [6,7]. Some bones are well vascularized, with capillaries inside primary vascular canals, primary osteones or secondary osteones (Haversian systems). Avascular bone is found commonly in the Lepidosauria and lacks any kind of capillary network, yet all other structural components are present [6]. Bone cells are confined within lacunae and communicate via cytoplasmic extensions within canaliculi, which are involved in interstitial fluid flow in both vascular and avascular bone [7]. These cellular components are responsible for oxygen consumption within bone tissue, therefore contributing to the amount of blood flowing into a bone.
Bones are responsive to static and dynamic stresses applied to them during growth, load bearing and locomotion throughout life. Basic multicellular units, consisting of osteoclasts that resorb the matrix followed by osteoblasts that lay it down again, continually bore through cortical bone and replace old bone containing micro-fractures with new bone [8–10]. The bone cells respond to local strain by either net deposition or resorption of bone as appropriate, creating strength along the lines of stress [11,12]. Many studies indicate that loading and exercise increase micro-damage in the short term and bone remodelling over a longer period [11,13]. Bone cells have high aerobic metabolic rates, comparable with nervous tissue [14], and they are accompanied by blood capillary loops [10]. Blood flow rates are capable of oxygenating the interior of bones to a high level [15]. It is reasonable to hypothesize that animals with higher levels of locomotory activity should have higher metabolic rates of the whole body and individual organs [16], and that this should be reflected in higher blood perfusion rates through larger blood vessels. Long bones represent a convenient tissue to study, because the size of the blood vessels can be evaluated from the dimensions of the nutrient foramina that penetrate them.
Perfusion through blood vessels is normally laminar and can be described by the Hagen–Poiseuille equation: 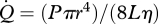 , where
, where 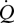 is flow rate (cm3 s−1), P is blood pressure difference (dynes cm−2), L is vessel segment length (cm) and η is blood viscosity (dynes cm−2 s−1). If blood pressure is not known, blood flow rates are proportional to an index of blood flow, Qi = r4/L (cm3), which is calculated from effective foramen radius and the length of the bone. We use Qi to compare species groups.
is flow rate (cm3 s−1), P is blood pressure difference (dynes cm−2), L is vessel segment length (cm) and η is blood viscosity (dynes cm−2 s−1). If blood pressure is not known, blood flow rates are proportional to an index of blood flow, Qi = r4/L (cm3), which is calculated from effective foramen radius and the length of the bone. We use Qi to compare species groups.
2. Material and methods
Foramen area was measured with callipers either directly or from digital photographs of the surface of the bone (figure 1). The femur was chosen as it is a large bone, important for support and locomotion and consistent in blood supply [17]. Morphometric data included foramen size, femur volume, mass and length from 59 mammal, 32 non-varanid reptile, eight varanid reptile and 10 dinosaur species. The dinosaurs included representatives of Theropoda, Ornithopoda, Sauropodomorpha, Ceratopsidae and Stegosauridae. Major and minor diameters of foramina were measured with callipers either directly by aligning the jaws across the diameter of the foramen below the tapered superficial opening or indirectly from microscopic images of both the foramen and calliper tips. Because it was not always possible to see the direction of nutrient vessel penetration, only the minor diameter was used to calculate foramen radius and area. Volume could not be reliably measured in dinosaur specimens owing to lateral crushing during burial. Correlative data on adult body mass (Mb, g), basal metabolic rate (BMR, ml O2 h−1), maximal metabolic rate (MMR) and absolute aerobic scope (AAS = MMR – BMR) were obtained from the literature for extant mammals and reptiles (see the electronic supplementary material for detailed methods, list of specimens and sources of data).
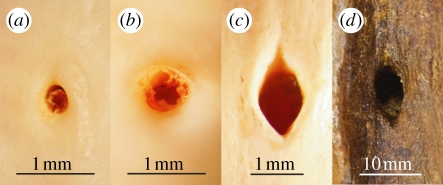
Figure 1.
Femoral nutrient foramina of (a) a non-varanid reptile, Lepidochelys olivacea; (b) a varanid reptile, Varanus mertensi; (c) a mammal, Macropus robustus; and (d) a ceratopsian dinosaur, Centrosaurus apertus.
All data were analysed allometrically, according to the equation Y = aXb, where Y is the variable of interest, X is a length, volume or mass, a is the scaling factor (elevation) of the curve and b is the exponent (slope of the log-transformed equation). Statistics include ordinary least-squares regressions, 95% confidence intervals of the regression mean, stepwise multiple regression, ANCOVA and Akaike's Information Criterion (see the electronic supplementary material for statistics and interpretations).
3. Results
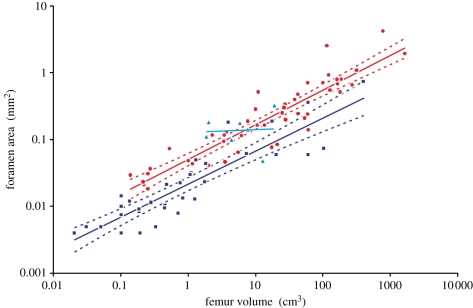
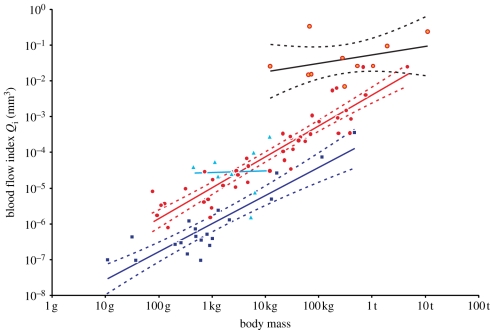
Foramen area is significantly correlated with femur volume for both mammals and reptiles, with exponents not significantly different at about 0.50, but the mammals are about twice as high as reptiles (table 1 and figure 2). Varanids show no significant correlation between foramen area and femur volume, and are not significantly different from mammals, but are significantly higher than other reptiles. The index of blood flow, Qi, is significantly correlated with body mass for mammals and reptiles, but not for varanids or dinosaurs (table 1 and figure 3). Mammals are not significantly different from reptiles in slope, but are significantly higher. Mammals and varanids are significantly different in slope, but differ little in elevation. Non-varanid reptiles and varanids are not significantly different in slope, but varanids are significantly higher. Qi, calculated from minimum foramen radius and femur length in 10 species of dinosaur, including representatives of Theropoda, Ornithopoda, Sauropodomorpha, Ceratopsidae and Stegosauridae, is significantly higher than in mammals, with the exception of the largest sauropod, Giraffatitan (i.e. Brachiosaurus) brancai (table 1 and figure 3). Because the Qi data for varanids show no significant correlation with body size, and they are all smaller than dinosaurs, comparison of their allometric equations is not meaningful. Other combinations of foramen area and Qi in relation to Mb, femur length and femur mass are displayed and analysed allometrically in the electronic supplementary material.
Table 1.
Allometric relationships between foramen area (A, mm2) and femur volume (VF, cm3), and between Qi (cm3) and body mass (Mb, g), in mammals, non-varanid reptiles, varanid reptiles and dinosaurs. Equations are in the form A = aXb, where a is the scaling factor and b is the exponent. Exponents are expressed as mean ± 95% CI. n = number of species. Analyses of covariance for these relationships are shown below each femur variable. Significant differences are bold.
| group | area versus volume 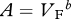 |
flow versus body mass  |
||||||
|---|---|---|---|---|---|---|---|---|
| n | equation | r2 | p | n | equation | r2 | p | |
| mammals | 49 | 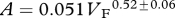 |
0.849 | <0.0001 | 44 | 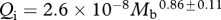 |
0.858 | <0.0001 |
| reptiles | 32 | 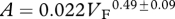 |
0.803 | <0.0001 | 20 | 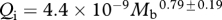 |
0.813 | <0.0001 |
| varanids | 7 | 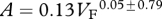 |
0.006 | 0.8734 | 8 |  |
0.001 | 0.945 |
| dinosaurs | 10 | 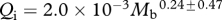 |
0.014 | 0.282 | ||||
| ANCOVA | slope | elevation | slope | elevation | ||||
| F | p | F | p | F | p | F | p | |
| mammal versus reptile | 0.207 | 0.650 | 35.7 | <0.001 | 0.524 | 0.472 | 64.8 | <0.001 |
| reptile versus varanid | 2.29 | 0.139 | 13.4 | <0.001 | 3.03 | 0.095 | 22.5 | <0.001 |
| mammal versus varanid | 3.40 | 0.071 | 0.200 | 0.656 | 4.61 | 0.037 | — | — |
| dinosaur versus mammal | 11.7 | <0.001 | a | a | ||||
aJohnson–Neyman test shows all dinosaur data are significantly higher than mammals, except Giraffatitan brancai, which is not significantly different.
Figure 2.
Relationship between femur volume (VF) and foramen area (A) for mammals (red dots, 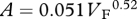 ), non-varanid reptiles (dark blue squares,
), non-varanid reptiles (dark blue squares, 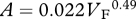 ) and varanid reptiles (light blue triangles,
) and varanid reptiles (light blue triangles, 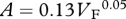 ), plotted on log–log axes. 95% confidence intervals of the regression mean are displayed. Regressions are compared in table 1.
), plotted on log–log axes. 95% confidence intervals of the regression mean are displayed. Regressions are compared in table 1.
Figure 3.
Relationship between the index of blood flow rate (Qi) and body mass (Mb, g) in mammals (red dots,  ), non-varanid reptiles (dark blue squares,
), non-varanid reptiles (dark blue squares, 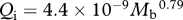 ), varanid reptiles (light blue triangles,
), varanid reptiles (light blue triangles, 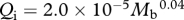 ) and dinosaurs (orange/red circles,
) and dinosaurs (orange/red circles, 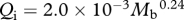 ). 95% confidence intervals of the regression mean are displayed. Regressions are compared in table 1.
). 95% confidence intervals of the regression mean are displayed. Regressions are compared in table 1.
Among the 19 species of mammals and reptiles with data for all of Qi, Mb, BMR, MMR and AAS, Qi is best related to MMR and AAS according to Akaike's Information Criterion (AICc) and Akaike weight (wi). (See the electronic supplementary material for interpretation of this analysis.) Log Qi is best predicted either by log AAS (AICc = −5.5, wi = 0.35) or log MMR (ΔAICc = 0.2, wi = 0.31). The probability that either log MMR or log AAS is the best predictor of log Qi is 0.65. Log Qi is poorly associated with log Mb (ΔAICc = 2.9, wi = 0.08) and log BMR (ΔAICc = 3.5, wi = 0.06). For mammals, stepwise multiple regression also showed better correlation between Qi and MMR and AAS than BMR, as did analysis of residuals.
4. Discussion
The results of this study indicate that the blood flow to femora is higher in mammals than in non-varanid reptiles. In relation to a body mass of 1 kg, Qi is about 9.6 times higher in mammals than in reptiles. But the real difference in bone oxygenation rate must also account for differences in blood pressure and oxygen carrying capacity of the blood. Mean arterial blood pressure is about 14 kPa in mammals and about 5 kPa in reptiles, differing by a factor of 2.8 [18]. Furthermore, the carrying capacity of mammalian blood is generally higher than that of reptiles, owing to an average twofold greater haemoglobin concentration [19]. Thus, we estimate that the difference in oxygenation of the interior of bones is potentially of the order of 9.6 × 2.8 × 2 ≈ 54 times higher in mammals than in reptiles.
It appears that bone oxygenation in mammals is disproportionately higher than the approximately 10-fold difference in temperature-corrected BMR of mammals and reptiles [20]. Because bone foramina are fixed in size, however, it is expected that they accommodate maximum blood flow rates, rather than resting rates. This idea is supported by comparison of allometric exponents (figure 3). The scaling exponent of Qi on body mass for mammals is 0.86, which is significantly higher than the exponent of 0.69 for BMR in over 600 species of mammals [21]. Because diastolic and systolic arterial blood pressure in mammals increases significantly with body mass to the 0.05 power [22], it follows that maximum bone blood flow should be proportional to body mass to the 0.86 + 0.05 = 0.91 power. This value is indistinguishable from the 0.87 scaling exponent of mammalian exercise-induced MMR [23]. Akaike's Information Criterion, stepwise multiple regression and analysis of residuals also showed better correlations of Qi with MMR and AAS than with BMR, confirming that blood flow is related to activity, not rest.
One unifying explanation for the high scaling exponents and the difference in Qi between mammals and reptiles is the requirement for bone remodelling in response to stresses put on the bones during locomotion. It is very well established that mammals increase remodelling of their bones in response to mechanical loading during exercise [24,25], and measurements indicate that this requires increased blood flow in long bones [26]. Remodelling increases cellular activity within the bone cortex, presumably increasing metabolic rate and thus accounting for the increased oxygenation [11]. However, crocodilians do not remodel their bones in response to mechanical loading of exercise [27,28], which is consistent with a low blood flow requirement.
Varanid lizards are clearly exceptional reptiles, with nutrient foramen areas and Qi values significantly above other reptiles, but not significantly different from mammals (figures 2,3 and table 1). It is relevant that, like mammals, larger varanids possess cortical vascular canals in their long bones [29], and, in contrast to crocodilians, bone remodelling occurs in Varanus exanthematicus in response to daily exercise [29]. As active hunters, varanids are considered to fill the niche otherwise occupied by mammalian predators [30], and they have adapted their metabolism to suit this active lifestyle. During maximum activity, varanids increase their oxygen consumption far above the upper limits of aerobic capacity of other reptiles, with small species exceeding half the maximum metabolic rate of mammals [31,32]. However, in contrast to mammals, varanid lizards maintain a BMR not significantly different from other reptiles [33]. The similarity between the aerobic scopes of mammals and varanids suggests once again that femoral blood flow is influenced by oxygen consumption of bone during activity rather than rest. Finally, it is significant that the highest factorial aerobic scopes occur in smaller varanids [34], consistent with the flat allometry of Qi in varanids of this study (figures 2 and 3). The slopes of allometric relationships of foramen area in varanids are not significantly different from zero when related to bone size (figure 2 and table 1), but are significantly different from the slopes for mammals on the basis of body mass (electronic supplementary material, table S2).
The foramina of dinosaurs were all large with respect to body size and femur length (figures 1 and 3 and electronic supplementary material, figures S1 and S2 and tables S1 and S2). Several lines of evidence, including vertical distances above the heart level and a four-chambered heart among extant archosaurs, indicate that blood pressures in dinosaurs were high, at least comparable with mammals [18]. We believe high Qi values and blood pressures indicate that dinosaur long bones received high perfusion rates characteristic of highly active animals with appreciable requirement for bone remodelling in response to stress. Perhaps the high blood flow is related to high growth rates of some dinosaurs as evident from bone histology; dinosaur fibro-lamellar bone is generally characterized by dense vascular canals, while most reptile bone is not [35]. However, our samples were largely from adult animals in which the immediate needs of bone remodelling owing to stress would seem more important than growth. These data support the ever-growing evidence that some dinosaurs were highly active animals with high aerobic metabolic rates, high food requirements and fast growth [36,37]. In general, such levels of aerobic activity are associated with endothermy, but the varanids of this study show that ectothermy and high aerobic scopes for activity are possible.
The range of dinosaur body size, from 12 kg in an unidentified hypsilophodontid to 10.9 tonnes for Giraffatitan, may appear somewhat light compared with older estimates, but newer restorations of dinosaurs take a more ‘bird-like’ view of their body shapes, producing more slender, lightly built animals, while older estimates tended to highlight the largest. Nevertheless, errors in body mass estimation would have to be greater than an order of magnitude just to coincide with the mammalian data. It might be argued that the true size of the dinosaur foramina was overestimated because of diagenetic effects or tapering from the surface to deeper in the shaft. However, we chose only femur fossils with intact and depressed nutrient foramina (e.g. figure 1) and we took the minor radius of the opening to calculate the area of a circle, which, if anything, would underestimate true size. The possibility that the femoral foramina represent pneumatic openings is unlikely, because there is no evidence of any pneumatization of long bones in dinosaurs, including Archaeopteryx; skeletal pneumaticity is confined to the axial skeleton and, in some theropod dinosaurs, to the appendicular girdles [38,39].
This preliminary study shows that the dimensions of nutrient vessel foramina can be used as a proxy for the metabolic intensity of mammals and reptiles and that the large foramina of dinosaurs are indicative of high maximum aerobic metabolic rates. The approach has been an allometric comparison of major groups that are statistically distinct. Analysis on a species-by-species basis inevitably shows some overlap, and there are exceptions, but they do not disprove the trend. Our separation of varanid lizards from other reptiles is based post hoc on physiology and foramen size, not on an a priori taxonomic decision. Birds are not included in the current analysis not only because of complications arising from pneumatization of long bones, as the pneumatic foramen is large and accompanied by nutrient vessels [40], but also because they generally fly rather than run, and we lack maximum metabolic rates for most species. Nevertheless, values of Qi estimated from non-pneumatic femoral foramina in 54 species of bird ranging in mass from 14 g to 40 kg have a significantly higher slope than mammals and are not significantly different from mammals in species weighing more than 3.9 kg. Smaller birds are significantly lower than mammals, but we emphasize that the contribution of blood flow from pneumatic foramina is not included, and until it is accounted for, the results are not comparable.
Further investigations are clearly necessary to understand the functional significance of bone foramen size among vertebrates, especially those involving phylogenetically informed analyses. The specimens in this study are not representative of mammals and reptiles of the world, but are highly biased to Australian species, so a larger dataset may be able to outline the relationships more precisely and permit correlations on a finer scale within these two groups. For instance, foramen size may be influenced not only by body size and activity levels, but also other features that affect bone remodelling, including mode of locomotion and environment. The number, size and orientation of supporting legs may influence bone blood flow, with possibly greater stress in bipeds compared with quadrupeds and runners compared with fliers. Locomotion in the terrestrial environment, compared with the gravity-free aquatic one, may result in meaningful differences bone remodelling and blood flow. Greater knowledge of the relationship between foramen size, actual blood flow rates and actual bone metabolic rates are essential to underpin the observed correlations of this study. Finally, ontogenetic changes in size, locomotion ability, metabolic rate and bone remodelling could be related to nutrient foramen size in natural or manipulative experimental regimes to understand the factors that determine nutrient vessel size within a species.
Acknowledgements
We thank Catherine Kemper, David Stemmer and Mark Hutchinson from the South Australian Museum, Andrew Amey and Kristen Spring from the Queensland Museum and Ross Sadlier, Sandy Inglegy and Yong Yi Zhen from the Australian Museum for access to their collections. Two referees offered many constructive criticisms of manuscript, for which we are grateful.
References
- 1.Langille B. L. 1996. Arterial remodeling: relation to hemodynamics. Can. J. Physiol. Pharmacol. 74, 834–841 10.1139/cjpp-74-7-834 (doi:10.1139/cjpp-74-7-834) [DOI] [PubMed] [Google Scholar]
- 2.Lehoux S., Tedgui A. 2003. Cellular mechanics and gene expression in blood vessels. J. Biomech. 36, 631–643 10.1016/S0021-9290(02)00441-4 (doi:10.1016/S0021-9290(02)00441-4) [DOI] [PubMed] [Google Scholar]
- 3.Rhinelander F. W. 1972. Circulation in bone. In The biochemistry and physiology of bone (ed. Bourne G. H.), pp. 1–77 2nd edn New York, NY: Academic Press [Google Scholar]
- 4.Trueta J. 1963. The role of the vessels in osteogenesis. J. Bone Joint Surg. 45, 402–418 10.1097/00006534-196402000-00034 (doi:10.1097/00006534-196402000-00034) [DOI] [Google Scholar]
- 5.Singh I. J., Sandhu H. S., Herskovits M. S. 1991. Bone vascularity. In Bone (ed. Hall B. K.), pp. 141–164 Boca Raton, FL: CRC Press [Google Scholar]
- 6.Enlow D. H. 1969. The bone of reptiles. In Biology of the reptilia (ed. Gans C.), pp. 45–80 London, UK: Academic Press [Google Scholar]
- 7.Knothe Tate M. L. 2003. ‘Whither flows the fluid in bone?’ An osteocyte's perspective. J. Biomech. 36, 1409–1424 10.1016/S0021-9290(03)00123-4 (doi:10.1016/S0021-9290(03)00123-4) [DOI] [PubMed] [Google Scholar]
- 8.Currey J. D. 2003. The many adaptations of bone. J. Biomech. 36, 1487–1495 10.1016/S0021-9290(03)00124-6 (doi:10.1016/S0021-9290(03)00124-6) [DOI] [PubMed] [Google Scholar]
- 9.Jaworski Z. F. G. 1991. Haversian systems and haversian bone. In Bone (ed. Hall B. K.), pp. 21–45 Boca Raton, FL: CRC Press [Google Scholar]
- 10.Parfitt A. M. 1994. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J. Cell. Biochem. 55, 273–286 10.1002/jcb.240550303 (doi:10.1002/jcb.240550303) [DOI] [PubMed] [Google Scholar]
- 11.Robling A. G., Castillo A. B., Turner C. H. 2006. Biomedical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 8, 455–498 10.1146/annurev.bioeng.8.061505.095721 (doi:10.1146/annurev.bioeng.8.061505.095721) [DOI] [PubMed] [Google Scholar]
- 12.Klein-Nulend J., Bacabac R. G., Mullender M. G. 2005. Mechanobiology of bone tissue. Pathol. Biol. 53, 576–580 10.1016/j.patbio.2004.12.005 (doi:10.1016/j.patbio.2004.12.005) [DOI] [PubMed] [Google Scholar]
- 13.Lieberman D. E., Pearson O. M., Polk J. D., Demes B., Crompton A. W. 2003. Optimization of bone growth and remodeling in response to loading in tapered mammalian limbs. J. Exp. Biol. 206, 3125–3128 10.1242/jeb.00514 (doi:10.1242/jeb.00514) [DOI] [PubMed] [Google Scholar]
- 14.Schirrmacher K., Lauterbach S., Bingmann D. 1997. Oxygen consumption of calvarial bone cells in vitro. J. Orthop. Res. 15, 558–562 10.1002/jor.1100150411 (doi:10.1002/jor.1100150411) [DOI] [PubMed] [Google Scholar]
- 15.Bingmann D., Wiemann M. 2007. O2-consumption, blood flow and PO2 in bone. Materialwissenschaft und Werkstofftechnik 38, 950–954 10.1002/mawe.200700227 (doi:10.1002/mawe.200700227) [DOI] [Google Scholar]
- 16.Weibel E. R., Bacigalupe L. D., Schmitt B., Hoppeler H. 2004. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respiration Physiol. Neurobiol. 140, 115–132 10.1016/j.resp.2004.01.006 (doi:10.1016/j.resp.2004.01.006) [DOI] [PubMed] [Google Scholar]
- 17.Brookes M., Revell W. 1998. Blood supply of bone. London, UK: Springer [Google Scholar]
- 18.Seymour R. S., Bennett-Stamper C. L., Johnston S. D., Carrier D. R., Grigg G. C. 2004. Evidence for endothermic ancestors of crocodiles at the stem of archosaur evolution. Physiol. Biochem. Zool. 77, 1051–1067 10.1086/422766 (doi:10.1086/422766) [DOI] [PubMed] [Google Scholar]
- 19.Withers P. C. 1992. Comparative animal physiology. Sydney, Australia: Saunders College Publishing [Google Scholar]
- 20.White C. R., Phillips N. F., Seymour R. S. 2006. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2, 125–127 10.1098/rsbl.2005.0378 (doi:10.1098/rsbl.2005.0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White C. R., Blackburn T. M., Seymour R. S. 2009. Phylogenetically informed analysis of the allometry of mammalian basal metabolic rate supports neither geometric nor quarter-power scaling. Evolution 63, 2658–2667 10.1111/j.1558-5646.2009.00747.x (doi:10.1111/j.1558-5646.2009.00747.x) [DOI] [PubMed] [Google Scholar]
- 22.Seymour R. S., Blaylock A. J. 2000. The principle of Laplace and scaling of ventricular wall stress and blood pressure in mammals and birds. Physiol. Biochem. Zool. 73, 389–405 10.1086/317741 (doi:10.1086/317741) [DOI] [PubMed] [Google Scholar]
- 23.White C. R., Seymour R. S. 2005. Allometric scaling of mammalian metabolism. J. Exp. Biol. 208, 1611–1619 10.1242/jeb.01501 (doi:10.1242/jeb.01501) [DOI] [PubMed] [Google Scholar]
- 24.Rubin C. T., Lanyon L. E. 1984. Regulation of bone formation by applied dynamic loads. J. Bone Joint Surg. 66, 397–402 [PubMed] [Google Scholar]
- 25.Beverly M. C., Rider T. A., Evans M. J., Smith R. 1989. Local bone mineral response to brief exercise that stresses the skeleton. Br. Med. J. 299, 233–235 10.1016/0378-5122(90)90071-D (doi:10.1016/0378-5122(90)90071-D) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sim F. H., Kelly P. J. 1970. Relationship of bone remodeling, oxygen consumption, and blood flow in bone. J. Bone Joint Surg. 52, 1377–1389 [PubMed] [Google Scholar]
- 27.Meers M. 2002. Cross-sectional geometric properties of the crocodylian humerus: an exception to Wolff's Law? J. Zool. 258, 405–418 10.1017/S0952836902001553 (doi:10.1017/S0952836902001553) [DOI] [Google Scholar]
- 28.Owerkowicz T., Tsai H. P., Sanchez L., Felbinger K., Andrade F., Blank J. M., Eme J., Gwalthney J., Hicks J. W. 2010. Chronic exercise does not alter limb bone morphology or microstructure in the American alligator. FASEB J. 24, 637.419843712 [Google Scholar]
- 29.Owerkowicz T., Crompton A. W. 1997. Effects of exercise and diet on bone-building: a monitor case. J. Morphol. 232, 306 [Google Scholar]
- 30.Andrews R. M., Pough F. H. 1985. Metabolism of squamate reptiles: allometric and ecological relationships. Physiol. Zool. 58, 214–231 [Google Scholar]
- 31.Taylor C. R., Maloiy G. M. O., Weibel E. R., Langman V. A., Kamau J. M. Z., Seeherman H. J., Heglund N. C. 1981. Design of the mammalian respiratory system. III. Scaling maximum aerobic capacity to body mass: wild and domestic mammals. Respiration Physiol. 44, 25–37 10.1016/0034-5687(81)90075-X (doi:10.1016/0034-5687(81)90075-X) [DOI] [PubMed] [Google Scholar]
- 32.Clemente C. J., Withers P. C., Thompson G. G. 2009. Metabolic rate and endurance capacity in Australian varanid lizards (Squamata: Varanidae: Varanus). Biol. J. Linn. Soc. 97, 664–676 10.1111/j.1095-8312.2009.01207.x (doi:10.1111/j.1095-8312.2009.01207.x) [DOI] [Google Scholar]
- 33.Christian K. A., Conley K. E. 1994. Activity and resting metabolism of varanid lizards compared with typical lizards. Australian J. Zool. 42, 185–193 10.1071/ZO9940185 (doi:10.1071/ZO9940185) [DOI] [Google Scholar]
- 34.Thompson G. G., Withers P. C. 1997. Standard and maximal metabolic rates of goannas (Squamata: Varanidae). Physiol. Zool. 70, 307–323 [DOI] [PubMed] [Google Scholar]
- 35.Padian K., de Ricqlés A., Horner J. R. 2001. Dinosaurian growth rates and bird origins. Nature 412, 405–408 10.1038/35086500 (doi:10.1038/35086500) [DOI] [PubMed] [Google Scholar]
- 36.O'Connor P. M., Claessens L. P. A. M. 2005. Basic avian pulmonary design and flow-through ventilation in non-avian theropod dinosaurs. Nature 436, 253–256 10.1038/nature03716 (doi:10.1038/nature03716) [DOI] [PubMed] [Google Scholar]
- 37.Sander P. M., et al. 2011. Biology of the sauropod dinosaurs: the evolution of gigantism. Biol. Rev. 86, 117–155 10.1111/j.1469-185X.2010.00137.x (doi:10.1111/j.1469-185X.2010.00137.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sereno P. C., Martinez R. N., Wilson J. A., Varricchio D. J., Alcober O. A., Larsson H. C. E. 2008. Evidence for avian intrathoracic air sacs in a new predatory dinosaur from Argentina. PLoS ONE 3, e3303. 10.1371/journal.pone.0003303 (doi:10.1371/journal.pone.0003303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connor P. M. 2006. Postcranial pneumaticity: an evaluation of soft-tissue influences on the postcranial skeleton and the reconstruction of pulmonary anatomy in archosaurs. J. Morphol. 267, 1199–1226 10.1002/jmor.10470 (doi:10.1002/jmor.10470) [DOI] [PubMed] [Google Scholar]
- 40.Beaumont G. D. 1968. Vascular factors in pneumatization. J. Laryngol. Otol. 82, 1067–1082 10.1017/S0022215100069899 (doi:10.1017/S0022215100069899) [DOI] [PubMed] [Google Scholar]