Abstract
The taccalonolides are a class of microtubule stabilizing agents isolated from plants of the genus Tacca. In efforts to define their structure activity relationships, we isolated 5 new taccalonolides, AC-AF, and H2, from one fraction of an ethanol extract of Tacca plantaginea. The structures were elucidated using a combination of spectroscopic methods, including 1D and 2D NMR and HRESIMS. Taccalonolide AJ, an epoxidation product of taccalonolide B, was generated by semi-synthesis. Five of these taccalonolides demonstrated cellular microtubule stabilizing activities and antiproliferative actions against cancer cells, with taccalonolide AJ exhibiting the highest potency with an IC50 value of 4.2 nM. The range of potencies of these compounds, from 4.2 nM to greater than 50 µM, for the first time provides the opportunity to identify specific structural moieties crucial for potent biological activities as well as those that impede optimal cellular effects. In mechanistic assays taccalonolide AF and AJ stimulated the polymerization of purified tubulin, an activity that had not previously been observed for the taccalonolides A and B, providing the first evidence that this class of microtubule stabilizers can interact directly with tubulin/microtubules. Taccalonolides AF and AJ were able to enhance tubulin polymerization to the same extent as paclitaxel, but with a distinct kinetic profile, suggesting a distinct binding mode or the possibility of a new binding site. The potencies of taccalonolides AF and AJ, their direct interaction with tubulin, together with the previous excellent in vivo antitumor activity of this class reveal the potential of the taccalonolides as new anticancer agents.
Keywords: Taccalonolide, microtubule stabilizer, tubulin, microtubules, antimitotic
For over 40 years drugs that target microtubules have been important in oncology. In 2010, two of the four new drugs approved by the FDA for the treatment of cancer targeted microtubules, suggesting that microtubules remain a valuable target for anticancer drugs. Paclitaxel, the first microtubule stabilizer identified, was isolated from Taxus brevifolia. Although it has achieved significant clinical success, limitations of paclitaxel and the second generation analog docetaxel include intrinsic and acquired multidrug resistance and dose limiting toxicities, which prompted the development of new classes of microtubule stabilizing drugs.1,2 Recently, two microtubule stabilizers that bind within the taxane site, cabazitaxel and the epothilone ixabepilone, have been approved for clinical use. These drugs can circumvent some, but not all of the limitations of first and second generation microtubule stabilizers.3,4
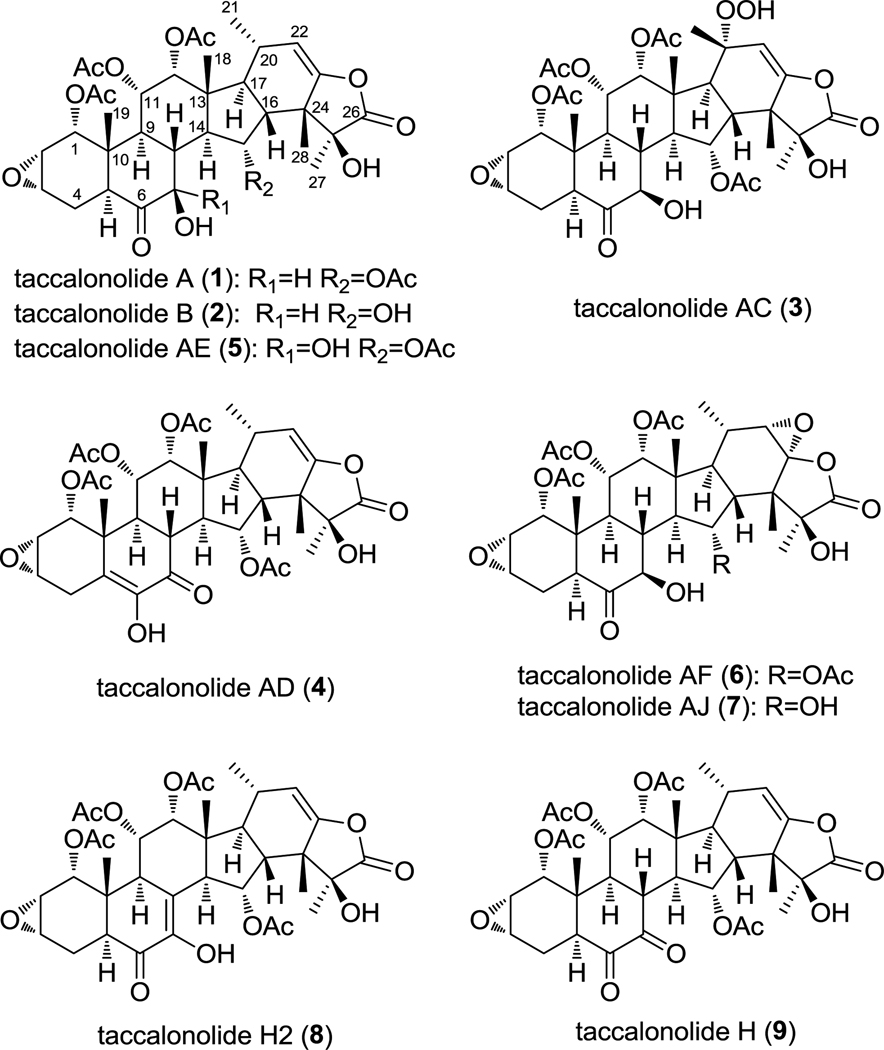
In our search for microtubule disrupting agents we identified a new class of microtubule stabilizers, the taccalonolides, from the tropical plant Tacca chantrieri.5 The taccalonolides have highly acetylated pentacyclic steroidal skeletons and they are structurally distinct from other microtubule stabilizers. The cellular effects of the most abundant taccalonolide, A (1), are almost identical to the effects of paclitaxel. They both increase the density of cellular microtubules, cause the formation of aberrant mitotic spindles leading to mitotic arrest and apoptosis, and have excellent antitumor efficacy in vivo.5,6 However, multiple experimental approaches were unsuccessful in identifying a direct interaction between 1 and tubulin/microtubules.7,8 Other studies showed that the taccalonolides A, E, B (2) and N can circumvent clinically relevant forms of taxane resistance, including expression of P-glycoprotein and βIII tubulin, suggesting that the taccalonolides offer advantages over existing microtubule-targeting agents.6 In a recent study, we isolated three additional taccalonolides with antimitotic and microtubule stabilizing properties, that we designated Z, AA and AB, and began to identify structure-activity relationships (SAR) for this class of compounds.9 This communication focuses on the isolation and characterization of five additional naturally occurring taccalonolides designated, AC (3), AD (4), AE (5), AF (6), and H2 (8), and one semi-synthetic product AJ (7), which allows significant refinement of the SAR of this class of molecules. Additionally, the single digit nanomolar potency of 7 gave us the ability to detect a direct interaction between a taccalonolide and tubulin.
The structures of the taccalonolides (Figure 1) were solved using 1D and 2D NMR spectra as well as HRMS data. All compounds were obtained as a white powder. The 1H spectra of 3, 4, 5, 6 and 8 revealed signals for five methyls, four methyls of acetyl groups, four oxygenated methines, two epoxide methines, and one olefinic methine (Supplemental Table 1), which are characteristic of the taccalonolides. The molecular formula of compound 3 was determined as C36H46O16 by HRESIMS analysis (calc. 735.2864, expt. 735.2893), which was two oxygen atoms more than taccalonolide A (1). The proton NMR of 3 showed a singlet resonance for C-21 methyl group, indicating a quaternary C-20 which requires an additional substituent at C-20 as compared to 1. This is confirmed by the HMBC correlation between H-22 and C-17, C-20, and C-24, and between H-21 and C-17, C-20, and C-22. The substitution was proposed to be a hydroperoxyl group based on the chemical shift of C-20 at 84.5 ppm and the two additional oxygen atoms required by the molecular formula. Additional support for the hydroperoxyl group was provided by the down field shift of C-20 in comparison with that of taccalonolide W, which possesses a hydroxyl group at C-20.10 All taccalonolides discovered to date possess 18β-Me and 16β-H configurations. The α-orientation of the hydroperoxyl group was deduced by the NOE correlations between Me-21 and H-16/Me-18. The other signals of 3 are similar to 1, thus the structure of 3 was proposed as depicted in Figure 1, and a trivial name taccalonolide AC was given. The LC-ESI/MS of 4 showed pseudomolecular ions at 701 [M+H]+, 718 [M+NH4]+, 723 [M+Na]+, indicating 2 Da less than that of 1. The molecular formula of 4 was determined as C36H44O14 by HRESIMS analysis (calc. 701.2807, expt. 701.2787). The 13C NMR showed two additional olefinic carbon signals at 143.9 and 127.3 ppm, suggesting the presence of one more double bond for 4 as compared to 1. The location of this double bond between C-5 and C-6 was deduced by the HMBC correlations between C-5 and H-1/H-3/H-19. In addition, an enol hydroxyl group at the C-6 position was determined by the HMBC correlations between hydroxyl proton at 6.26 ppm and C-6/C-7. The shift of ketone group to C-7 was evidenced by the HMBC correlation between C-7 at 190.3 ppm and H-8/H-9/6-OH. Therefore, the structure of compound 4 was determined as depicted and a trivial name taccalonolide AD was designated. Compound 5 gave pseudomolecular ions at 719 [M+H]+, 736 [M+NH4]+, and 741 [M+Na]+ in LC-ESI/MS. The HRESIMS of 5 showed a [M +H]+ ion at m/z 719.2944 (calc. 719.2915), corresponding to a molecular formula of C36H46O15, differing from that of 1 by one additional oxygen atom. The 1H and 13C NMR spectra of 5 were very similar to those of 1. The only difference was the occurrence of a signal for an additional hydroxyl group at 5.01 ppm instead of H-7 at ca. 4 ppm. The location of the additional hydroxyl groups at C-7 to form a geminal diol was deduced by the chemical shift of C-7 at 92.4 ppm and confirmed by the HMBC correlations between 7-OH at 5.01 ppm and C-6/7/8, 7-OH at 3.64 ppm and C6/7, as well as H-8 and C-7. Compound 5 represent a rare example of geminal diol functionality for natural products and is designated as taccalonolide AE. It is stable during our experiments. The molecular formula C36H46O15 was established for compound 6 by a [M+H]+ ion at m/z 719.2911 (calc. 719.2915) in HRESIMS, indicating the presence of one more oxygen than that is found in 1. The 1H and 13C NMR data of 6, fully assigned through 2D NMR experiments, closely resembled those of 1. The only difference was the absence of resonances for the olefinic group at C-22,23 and the appearance of signals for one oxygenated methine (δH 3.29 ppm and δC 65.9 ppm) and one quaternary carbon at 92.2 ppm. In accordance with the molecular formula of 6, the additional oxygen should be present as an epoxide group at C-22,23. This was confirmed by the HMBC correlations between H-21 and C-20/22, H-22 and C-20/21, and H-28 and C-23/24. The relative configuration of this epoxide group was deduced based on the coupling constants. The small coupling constant of 3J(H20,22) required an equatorial (β-oriented) H-22, thus the epoxide group is α-oriented. This epoxide-containing taccalonolide was given the trivial name taccalonolide AF. Compound 8 showed the same pseudomolecular ions at 701 [M+H]+, 718 [M+NH4]+, 723 [M+Na]+ as 4. The molecular formula was determined as C36H44O14 by HRESIMS analysis (calc. 701.2809, expt. 701.2813). Similar to 4, compound 8 also showed carbon signals for an additional double bond at 140 and 126 ppm in its 13C NMR. The location of this additional double bond was determined at C-7,8 based on the HMBC correlations between H-9/H-14 and C-7 as well as H-9/H-14/H-15 and C-8. A hydroxyl group resonance at 6.27 ppm showed HMBC correlations with C-6/C-7/C-8, suggesting that this hydroxyl group was located at C-6 position. Thus, the structure of 8 was determined to be the enol of taccalonolide H (9) reported previously.11 Since 8 is stable as the enol, a trivial name taccalonolide H2 was given to distinguish it from taccalonolide H with a ketone.
Figure 1.
Taccalonolide structures
Compounds 1, 2, 3, 4, 5, and 8 were isolated in sufficient quantities to accurately determine their biological activities. Taccalonolide AF (6) was present at low abundance in the extract and more material was semi-synthesized by epoxidation of the abundant compound 1 with dimethyldioxirane.12 The reaction was carried out quantitatively under very mild conditions to yield 6 as the single product. No β-epoxide isomer was observed. After evaporation of the solvent and reagent, sufficient quantities of 6 were obtained for biological studies. Because of the potent activity of 6, taccalonolide AJ (7), the epoxidation product of taccalonolide B (2), was also produced using the same method.
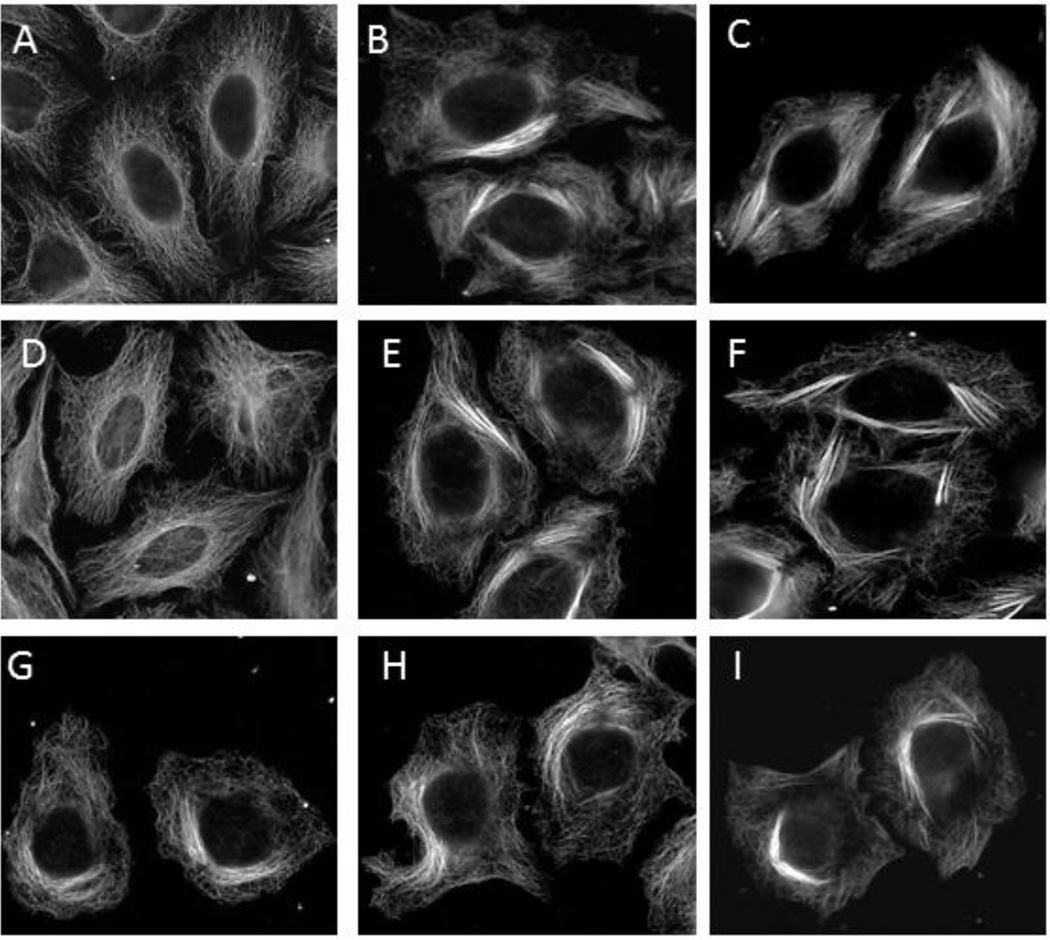
Each of the taccalonolides isolated or synthesized, with the exception of 3, inhibited the proliferation and caused cytotoxicity of HeLa cells, with IC50 values ranging from 4.2 nM for 7, to 5 µM for 1 and 5 (Table 1). In contrast, concentrations up to 50 µM of 3 had no effects on cellular proliferation. Significant increases in cellular microtubule density and microtubule bundling occurred at concentrations 5-fold greater than the IC50 for each of the active compounds, consistent with the microtubule stabilizing activities of other taccalonolides (Figure 2). In contrast, concentrations of 3 up to 20 µM showed no effects on cellular microtubules (Figure 2). In addition to increasing the density of interphase microtubules, all of these taccalonolides with the exception of 3 caused HeLa cells to arrest in the G2/M phase of the cell cycle (Supplemental Figure 2) with multiple aberrant mitotic spindles (Supplemental Figure 3), which are additional phenotypes associated with microtubule stabilizing agents. No change in cell cycle distribution was observed in cells treated with a 20 µM concentration of 3 (Supplemental Figure 2). In immunofluorescence studies 3-treated cells in mitosis exhibited normal bipolar spindles (Supplemental Figure 3) and DNA alignment (data not shown). These data are consistent with the inability of 3 to disrupt interphase microtubules or inhibit cell proliferation. Compound 3 appears relatively stable under the assay conditions, reducing the possibility that its inactivity is due to rapid decomposition.
Table 1.
Antiproliferative potencies of the taccalonolides
| Compound | IC50 (nM) |
|---|---|
| Taccalonolide A (1) | 5,380 ± 230 |
| Taccalonolide B (2) | 3,120 ± 180 |
| Taccalonolide AC (3) | >50,000 |
| Taccalonolide AD (4) | 3,480 ± 230 |
| Taccalonolide AE (5) | 5,010 ± 210 |
| Taccalonolide AF (6) | 23 ± 3 |
| Taccalonolide AJ (7) | 4.2 ± 0.3 |
| Taccalonolide H2 (8) | 730 ± 20 |
| Paclitaxel | 1.0 ± 0.1 |
The concentrations of taccalonolides that caused 50% inhibition of cellular proliferation (IC50) were measured in HeLa cells using the SRB assay (n=3).
Figure 2.
Effects of the taccalonolides on interphase microtubules. HeLa cells were treated with vehicle or a taccalonolide for 18 h. Representative images of cells treated with: vehicle (A), 1 (B), 2 (C), 3 (D), 4 (E), 5 (F), 6 (G), 7 (H) or 8 (I). Concentrations used were 5× the IC50 for all taccalonolides except for 3, which was added at 20 µM. Microtubules were visualized by indirect immunofluorescence using a β-tubulin antibody.
The large number of naturally occurring taccalonolides distinguishes them as one of only a few natural product classes where the SAR can be identified without significant synthetic manipulation. Our previous studies with other taccalonolides provided initial indications of SAR.6,9 A bulky isovalerate at the C-1 position, found in taccalonolide T, was associated with excellent potency as compared to 1.9 Additionally, when making comparisons among 1, 2, taccalonolide E and taccalonolide N, the presence or absence of acetoxy groups at C-11 or C-15 did not have a major effects on potency.6 However, in other taccalonolides differences in potency associated with the presence or absence of the C-11 acetoxy group and the hydrolysis of the C-15 acetate were contingent on moieties present in other parts of the molecule, suggesting interrelations across the northern and southern regions.9 In the present study 8 showed a 7.4 fold increase in potency as compared to 1. The only difference between these two taccalonolides is the presence of an additional double bond at C-7,8 in 8. The location of this double bond is important since a double bond at C5–C6, as in 4, does not provide the same increase in potency. When a hydroxyl group was added to C-7 of 1 to form a rare geminal diol as in 5, the potency is also unchanged (Table 1). Surprisingly, 3, which differs from 1 only by an additional hydroperoxyl group at C-20, showed no antiproliferative activity at concentrations up to 50 µM, suggesting the importance of substituents at this site. In contrast, 6 and 7, which differ from 1 and 2 by conversion of the C-22,23 double bond to an epoxide group, increased the activity 234 to 743-fold to give the most potent taccalonolides identified to date (Table 1). These results clearly suggested that an epoxide moiety at C-22,23 provides optimal potency and we will test this further with additional taccalonolides in future studies. Together these results highlight the importance of the C-20, C22–23 region of the taccalonolide molecule and suggest that this region plays a central role in its interactions with tubulin.
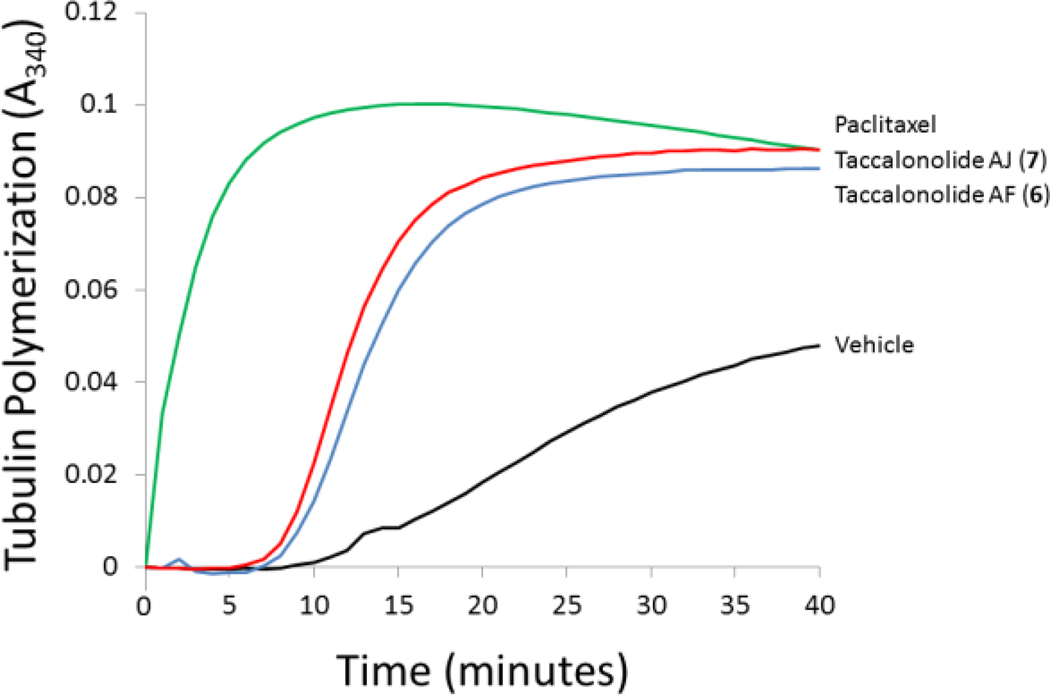
Previously, all classes of microtubule stabilizing agents that increase the density of cellular microtubules except the taccalonolides were shown to stimulate the polymerization of purified tubulin in biochemical preparations, demonstrating a direct interaction with tubulin.7 Multiple studies evaluating the interaction of 1 and 2 with purified tubulin, microtubule protein, or cellular lysates containing tubulin and microtubule associated proteins suggested that these taccalonolides were not able to bind directly to tubulin.7,8 However, these studies were all performed with 1 and 2 which have antiproliferative potencies in the low micromolar range. In this study we generated 7, the first taccalonolide to inhibit cellular proliferation at concentrations less than 5 nM, the range of potency of microtubule stabilizers in clinical use. In contrast to all previous studies with 1 and 2, we found that 7 enhanced both the rate and extent of purified tubulin polymerization (Figure 3). A direct comparison of the effects of 7 and paclitaxel on tubulin polymerization at equimolar concentrations showed that the two drugs were able to polymerize tubulin to the same extent. However, a lag in the time required for polymerization to occur was observed in the presence of 7 as compared to the immediate tubulin assembly that occurs with paclitaxel. This suggests that 7 may interact with tubulin/microtubules in a manner distinct from paclitaxel or bind to a different binding site. Studies with the other highly potent taccalonolide 6 indicated that it was also able to enhance the polymerization of purified tubulin (Figure 3), demonstrating that this is a common feature for taccalonolides with low nanomolar potency. Additional experiments to test this hypothesis and define the interaction between the taccalonolides and tubulin are ongoing and will provide important information regarding the mechanism of action of this class of microtubule stabilizers and whether they bind within the taxane site, the peloruside A/laulimalide site or a unique site on microtubules.
Figure 3.
Effects of 6 and 7 on tubulin assembly. Purified porcine brain tubulin was incubated at 37°C in the presence of vehicle, 10 µM paclitaxel, 10 µM 6 or 10 µM 7. Microtubule polymerization was monitored turbidimetrically at 340 nm.
Taccalonolides 4 and 8 are ketone/enol tautomers of 9. Additionally, 5 and 9 are potentially interconvertible germinal diol and ketone moieties (Supplemental Figure 4). It is interesting that we isolated all of them as stable isomers as suggested by the different biological potencies obtained with these compounds. Additionally, significant tautomerization or interconversions were not seen during purification or storage in the NMR solvent over three weeks. The enol and germinal diol in 4, 5, and 8 may be stabilized by the α-carbonyl and 15-acetoxy groups through intramolecular hydrogen bonds. Since quantities were limited, the conditions for tautomerization and interconversion were not evaluated, but will be investigated in future studies.
The six new taccalonolides described in this study expand the SAR of this class and identified the critical importance of the E ring constituents at C20–C23. The C-22,23 epoxide group in 6 and 7 dramatically increased the potency, while the C-20 hydroperoxyl group in 3 resulted in total loss of antiproliferative and microtubule stabilizing activities.
Most notable, is the finding that taccalonolides with single digit nanomolar potency can be identified and that compounds 6 and 7, the most potent taccalonolides isolated to date, afforded us the ability to detect a direct interaction between these taccalonolides and purified tubulin, consistent with their actions in cells. The rationale for why 6 and 7 interact with purified tubulin whereas 1 and 2 were not able to affect tubulin polymerization is likely related to differences in potency. The ability of microtubule targeted agents to affect the polymerization of purified tubulin in biochemical assays requires concentrations that are orders of magnitude higher than the concentration required to observe changes in microtubule density and antiproliferative and antimitotic effects in cells. A number of factors contribute to this discrepancy, including the ability of these compounds to be concentrated hundreds of fold across the cell membrane.13,14 Additionally, in intact cells microtubule associated proteins modulate the effects of these agents. The current findings demonstrate that the potent taccalonolides, 6 and 7 interact directly with tubulin. Future studies to identify the binding site and nature of interactions of the taccalonolides with tubulin and/or microtubules will be evaluated. It is interesting to speculate the possibility of a third microtubule stabilizer binding site. The potent biological activities of 6 and 7, their interaction with tubulin and the excellent in vivo antitumor effects of other taccalonolides, together with the proven value of microtubule stabilizing drugs, clearly reveal the potential of the taccalonolides as anticancer agents.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by NCI CA121138 (SLM), COSTAR Program NIDCR DE 14318 (JL), DOD-CDMRP Postdoctoral Award BC087466 (ALR), and the NCI P30 CA054174 (SLM). Support from the Mass spectrometry and NMR Cores are gratefully acknowledged. We extend sincere thanks to Prof. Doug Frantz for his critical reading of this manuscript and Prof. Cong-Gui Zhao for providing dimethyldioxirane.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental details, 1D and 2D NMR spectra as well as additional bioassay data is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Dumontet C, Jordan MA. Nat. Rev. Drug Discov. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kavallaris M. Nat. Rev. Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 3.Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Nat. Rev. Drug Discov. 2010;9:677–678. doi: 10.1038/nrd3254. [DOI] [PubMed] [Google Scholar]
- 4.Morris PG, Fornier MN. Clin. Cancer Res. 2008;14:7167–7172. doi: 10.1158/1078-0432.CCR-08-0169. [DOI] [PubMed] [Google Scholar]
- 5.Tinley TL, Randall-Hlubek DA, Leal RM, Jackson EM, Cessac JW, Quada JC, Jr, Hemscheidt TK, Mooberry SL. Cancer Res. 2003;63:3211–3220. [PubMed] [Google Scholar]
- 6.Risinger AL, Jackson EM, Polin LA, Helms GL, LeBoeuf DA, Joe PA, Hopper-Borge E, Luduena RF, Kruh GD, Mooberry SL. Cancer Res. 2008;68:8881–8888. doi: 10.1158/0008-5472.CAN-08-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buey RM, Barasoain I, Jackson E, Meyer A, Giannakakou P, Paterson I, Mooberry SL, Andreu JM, Díaz JF. Chem. Biol. 2005;12:1269–1279. doi: 10.1016/j.chembiol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Risinger AL, Mooberry SL. Cell Cycle. 2011;10:2162–2171. doi: 10.4161/cc.10.13.16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng J, Risinger AL, Fest GA, Jackson EM, Helms G, Polin LA, Mooberry SL. J. Med. Chem. 2011;54:6117–6124. doi: 10.1021/jm200757g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JY, Zhao RH, Chen CX, Ni W, Teng F, Hao XJ, Liu HY. Helvetica Chimica Acta. 2008;91:1077–1082. [Google Scholar]
- 11.Chen Z-L, Shen J-H, Gao Y-S, Wichtl M. Planta Medica. 1997;63:40–43. doi: 10.1055/s-2006-957600. [DOI] [PubMed] [Google Scholar]
- 12.Adam W, Saha-Moller CR, Zhao CG. Org. Reactions. 2002;61:219–516. [Google Scholar]
- 13.Jordan MA, Toso RJ, Thrower D, Wilson L. Proc. Natl. Acad. Sci. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuh HJ, Jang SH, Wientjes MG, Au JLS. J. Pharm. Exp. Ther. 2000;293:761–770. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.