Abstract
The present study was undertaken to investigate the effect of dietary supplementation with nimesulide or eugenol on N-nitrosodiethylamine (DEN)-initiated early hepatocarcinogenesis in F344 male rats. Both compounds did not alter the expression of cytochrome P450 (CYP) 2E1, the enzyme that plays a major role in the activation of DEN to genotoxic products; however, nimesulide induced the expression of CYP1A1. Western blot analysis revealed that COX-1 and COX-2 protein expressions were not modulated by DEN compared with normal controls. Furthermore, post-initiation feeding with nimesulide or eugenol did not modulate COX-2 protein expression in normal or DEN-treated rats, whereas eugenol significantly increased the liver prostaglandin E2 (PGE2) levels of DEN-injected animals compared with the DEN controls. Ultimately, nimesulide or eugenol did not modify DEN-induced hepatocarcinogenesis as evidenced by insignificant changes in the number and size of preneoplastic placental glutathione S-transferase (GST-P) positive liver foci compared with the DEN controls. These results suggest that COX-2, as well as prostaglandin E2, may play no role in the post-initiation development of DEN-induced rat hepatocarcinogenesis at an early stage.
Keywords: rat, liver, nimesulide, eugenol, cyclooxygenases
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic tumor worldwide. Over 80% of deaths due to HCC are expected to occur in Asia (Hong Kong, Singapore and Japan) and Africa. 1 Approximately 90 to 95% of these tumors are the biologic consequences of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. 2 However, epidemiological data indicate the importance of environmental factors in human liver carcinogenesis and suggest that other factors may be operative in conferring additional risk and give strong evidence that our environment plays a more dominant role in cancer etiology rather than genetics. 3
N-nitrosodiethylamine is a potent chemical carcinogen known to be activated by liver microsomal P450 enzymes in experimental animals and in humans. 4 The presence of nitroso compounds and their precursors in the human environment together with the possibility of their endogenous formation in the human body have led to suggestions of their potential involvement in human cancers. 5
Non-steroidal anti-inflammatory drugs (NSAIDs) are the principal drug treatments for inflammation, pain and fever. 6 They exert their therapeutic anti-inflammatory and antipyretic actions by inhibition of the enzyme cyclooxygenase (COX) and subsequent production of prostaglandins. 7 The two COX isozymes, COX-1 and COX-2, are both rate-limiting enzymes in the production of prostanoids, prostaglandins (PGs), thromboxanes and prostacyclins from arachidonic acid and have only approximately 60% homology, but their active site residues are almost entirely preserved. 8
Conventional NSAIDs inhibit both COX-1 and COX-2, affecting the housekeeping functions of COX-1 and hence leading to many side effects like peptic ulcers as well as gastric bleeding. These facts have provided a new rationale for the use of selective COX-2 inhibitors as anti-inflammatory agents, which have attracted a great deal of attention as more effective and safer therapeutic and cancer chemopreventive agents that have equivalent efficacy and greater gastrointestinal safety than traditional NSAIDs. 9 , 10 Nimesulide (N-(4-nitro-2-phenoxyphenyl)-methanesulfonamide) is a sulfonanilide class selective COX-2 inhibitor that appears to possess much less adverse effects on the gastrointestinal tract than non-specific NSAIDs. 8
In fact, evidence of up-regulated expression of COX-2 mRNA and protein in various human and animal tumor tissues, such as the colon, stomach, breast, head and neck, tongue, skin, pancreas, lung and urinary bladder, and prevention of carcinogenesis by specific COX-2 inhibitors as well as prevention of colon carcinogenesis by double knockout of the COX-2 gene in APC gene knockout mice strongly support the hypothesis that COX-2 could be a chemopreventive target molecule. 11 However, there have been only few studies that have examined the chemopreventive effects of COX-2 inhibitors on the liver. 8 , 12 , 13
Eugenol (1-allyl-4-hydroxy-3-methoxybenzene) is a naturally occurring phenolic compound that is used as a food flavor and fragrance agent. 14 It is found in reasonable quantities in the essential oils of different spices, such as Syzgium aromaticum (clove), Pimenta racemosa (bay leaves) and Cinnamomum verum (cinnamon leaf) and has been used as an antiseptic, antibacterial and analgesic agent in traditional medical practices in Asia as well as in dentistry in cavity-filling procedures. 15 Eugenol has been reported to act as an in vitro and in vivo antioxidant and to protect rat livers against carbon tetrachloride (CCl4) intoxication. 16 Furthermore, eugenol inhibits 7,12-dimethyl- benz(a)anthracene or benzo(a)pyrene-induced skin carcinomas and suppresses human melanoma growth through inhibition of E2F1 transcriptional activity. 15 However, it has not been systematically tested in other common cancers.
In the present study, the chemopreventive potentials of the selective COX-2 inhibitor nimesulide and the phenolic antioxidant eugenol were investigated at an early stage of DEN-induced hepatocarcinogenesis in F344 male rats.
Materials and Methods
Chemicals
N-Nitrosodiethylamine (DEN) was obtained from Tokyo Kasei Kogyo Co., Ltd. (Japan), nimesulide was obtained from Cayman Chemical Company (Japan) and eugenol was obtained from Wako Pure Chemical Industries Ltd. (Japan).
Animals and diets
Five-week-old male F344 rats were obtained from Charles River Japan Inc. (Atsugi, Japan). They were housed in plastic cages on wood-chip bedding in an air-conditioned, specific pathogen-free (SPF) animal room at 22 ± 2°C and 55 ± 5% humidity with a 12 h light/dark cycle. The animals had free access to food (Oriental MF, Oriental Yeast, Tokyo, Japan) and water. Diets containing eugenol or nimesulide were prepared once weekly by mixing these compounds with powdered basal diet in a blender for 15 min and stored at 4°C in the dark. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Nagoya City University School of Medicine.
Experimental protocol
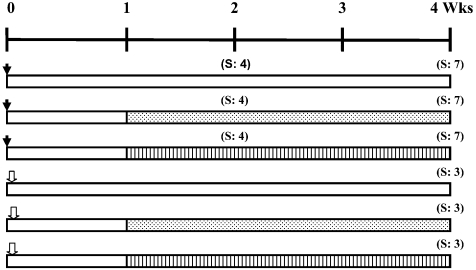
After an acclimatization period of one week, a total of 42 six-week- old male F344 rats were used in this study. Thirty-three male rats were given a single intraperitoneal injection of DEN (200 mg/Kg body weight) dissolved in sterile isotonic saline to initiate hepatocarcinogenesis. After one week on a pellet diet, the DEN-injected rats were allocated into three equally-sized groups and were fed powder diet using stainless steel containers. Group 1 received no treatment serving as the DEN control group, groups 2 and 3 were administered a powder diet containing 400 ppm nimesulide 8 , 9 or 6000 ppm eugenol, 17 respectively, for one or three weeks. The remaining nine rats, representing a normal counterpart study, received a single intraperitoneal injection of sterile isotonic saline and were further subdivided into three groups (3 rats/group) as follows; the first group remained untreated, and the animals in the second and third groups received a powder diet containing 400 ppm nimesulide or 6000 ppm eugenol, respectively, for three weeks (Fig. 1). The diets were available ad libitum and were given to the animals by freshly replenishing the feed trays twice weekly. Body weight and food consumption were recorded twice weekly.
Fig. 1.
Experimental protocol. A total of 42 six-week-old F344 male rats were
used throughout this study. A group of thirty-three rats (each animal was
injected with DEN) and a group of nine rats (each rat was injected with saline)
were further subdivided each into three equally-sized groups as shown. The rats
were sequentially sacrificed two or four weeks following DEN injection or 4
weeks following saline injection.  : Diethylnitrosamine (DEN), 200 mg/Kg b.w.,
i.p.
: Diethylnitrosamine (DEN), 200 mg/Kg b.w.,
i.p.  : Saline, i.p.
: Saline, i.p.  : Nimesulide 400 ppm in diet,
: Nimesulide 400 ppm in diet,  : Eugenol 6000 ppm in diet,
: Eugenol 6000 ppm in diet,  :
Basal diet. S: Sacrifice.
:
Basal diet. S: Sacrifice.
Blood collection and tissue sampling
At the end of the experiment period (two or four weeks), rats were anesthetized under ether, and blood was collected from the abdominal aorta into 10 mL plastic vacuum tubes, kept on ice to clot and then centrifuged. Serum samples were then analyzed for alanine aminotransferase (ALT) using a commercial kit. At autopsy, livers were immediately excised and weighed, and the liver to body weight ratio was calculated. The livers were then cut into 2-3 mm thick slices with a razor blade and fixed in 10% phosphate-buffered formalin for immunohistochemical examination of placental glutathione S-transferase (GST-P) positive foci expression, as well as routine hematoxylin and eosin staining. Other slices from the remaining livers were immediately frozen in liquid nitrogen and stored at –80°C until processed.
Immunohistochemistry for measurement of GST-P foci
Liver tissues fixed in phosphate-buffered formalin were processed into paraffin embedded sections as described previously. 18 Briefly, 3 μM thick liver sections were treated with rabbit anti-rat GST-P antibody (MBL, Nagoya, Japan) and then sequentially with secondary antibody and avidin-biotin complex reaction (Vectastain ABC Elite kit, Vector Laboratories Inc., CA, USA). The sites of peroxidase binding were visualized with diaminobenzidine. Sections were then counterstained with hematoxylin for microscopic examination. The number and area of GST-P positive foci (> 0.05 mm in diameter) in the liver sections were quantitatively measured with an Image Processor for Analytical Pathology (IPAP-WIN, Sumika Technos Co., Osaka, Japan).
Preparation of liver homogenate and isolation of microsomal proteins
Frozen rat liver slices were washed with ice-cold saline to remove excess blood. A small piece of liver was cut on dry ice and then homogenized in 1 mL RIPA buffer [150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 50 mM Tris-HCl (pH 8.0), 0.1% sodium dodecyl sulphate and a cocktail of protease inhibitors] on ice for 30 s using a Physcotron homogenizer (Tokyo, Japan). The homogenate was then sonicated on ice at a 20 s interval for a total of 5 min using an ultrasonic cell disruptor. The sonicates were centrifuged at 15,000 g for 15 min at 4°C, and the resultant supernatants were stored at –80°C for western blot analysis. Hepatic microsomes were prepared by differential centrifugation. 19 Briefly, a piece of liver (approximately 50 mg) was homogenized on ice in 0.5 mL 0.25 M sucrose for 30 s. The liver homogenate was centrifuged at 600 g for 5 min to remove unbroken cells and nuclear debris. The supernatant was then transferred to a 1.5 mL eppendorf tube and centrifuged in a microfuge at 13,000 g for 15 min, and the pellet was then discarded. Using a Beckman TL-TB-023B ultracentrifuge with an MLS-50 Rotor (Beckman CoulterTM, CA, USA), the resulting supernatant was centrifuged for one hour at 105,000 g to yield a fraction rich in smooth endoplasmic reticula (microsomes). All procedures were performed at 4°C. The 105,000 g pellet was resuspended in 200 μL RIPA buffer and stored at –80°C for further analysis of cytochrome P450 1A1/1A2 and 2E1 proteins by western blot. Protein concentrations were determined for each fraction by the method of Bradford 20 using a Quick StartTM Bradford Protein Assay kit (Bio-Rad, Hercules, CA, USA) with bovine gamma globulin as a standard.
Western blot analysis
Supernatant samples containing 1–5 μg protein were mixed 1:1 with Laemmli Sample buffer (Bio-Rad, Hercules, CA, USA), which contained 62.5 mM Tris-HCl, pH 6.8, 25% glycerol, 2% SDS and 0.01% bromophenol blue, and 5% β-mercaptoethanol (Wako Pure Chemical Industries, Osaka, Japan). Samples were boiled for 5 min and separated by SDS-PAGE using Bio-Rad Minigel apparatus (Bio-Rad Laboratories, Hercules, CA, USA). Resolving gels were composed of 12%, 10% and 8% polyacrylamide for separation of cytochromes, PCNA and β-actin and COX-1 and COX-2, respectively, whereas the stacking gel was composed of 5% polyacrylamide, and both gels contained 0.1% SDS. Protein migration was assessed using protein standards (Kaleidoscope, Bio-Rad). Protein bands were electroblotted onto a nitrocellulose membrane (Hybond ECL membrane, Amersham Pharmacia Biotech, UK) using a Bio-Rad Trans-Blot electrophoretic transfer system. The membranes were blocked for one hour at room temperature with 5% non-fat dried milk in Tris buffered saline (TBS, pH 8.0) containing 0.1% Tween-20, followed by brief washing twice with TBS-T buffer (pH 7.5) containing 0.1 M Tris, 0.9% NaCl and 0.1% Tween-20. The membranes were then incubated overnight at 4°C with primary antibodies to cytochrome P450 1A1/1A2 (goat anti-rat CYP1A1, Daichi Pure Chemicals Co., Ltd., Japan) at 1:500 dilution in TBS-T buffer (pH 7.5), cytochrome P450 2E1 (rabbit anti-rat Cytochrome P450 IIE1, ECL Western Blotting kit, Amersham Life Science, Buckinghamshire, UK) at 1:200 dilution, cyclooxygenase 1 (rabbit anti-murine COX-1 polyclonal antibody, Cayman Chemical, Ann Arbor, MI, USA) at 1:250 dilution, cyclooxygenase 2 (rabbit anti-rat COX-2, IBL Co., Ltd., Takasaki, Gunma, Japan) at 1:50 dilution, proliferating cell nuclear antigen (mouse anti-rat PCNA monoclonal antibody, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at 1:200 dilution and β-actin (monoclonal anti-β-actin mouse IgG2a isotype, A 5316, Sigma, St Louis, MO, USA) at 1:5000 dilution. Following incubation with primary antibody, the membranes were washed three times in TBS-T buffer and incubated for one hour with horseradish peroxidase-linked donkey anti-rabbit IgG, sheep anti-mouse IgG (ECLTM, Amersham Pharmacia Biotech, Buckinghamshire, UK) or donkey anti-goat IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) secondary antibodies. The membranes were washed three times in TBS-T buffer, and protein signals were enhanced by chemiluminescence (ECL Plus Western Blotting Detection Reagents, Amersham, Buckinghamshire, UK); bands were detected on radiographic film (Amersham HyperfilmTM ECL, GE Healthcare Limited, Buckinghamshire, UK).
Measurement of PGE2
A small piece of liver (approximately 50–100 mg) was homogenized in 0.5 mL RIPA buffer (without protease inhibitors) on ice for 30 s using a Physcotron homogenizer (Tokyo, Japan), the homogenate was centrifuged at 15,000 g for 15 min at 4°C and the resultant supernatants were stored at –80°C until use. Tissue PGE2 was measured using an ELISA kit provided by R&D Systems (Minneapolis, MN, USA). Protein concentrations were determined in supernatants by the method of Bradford 20 using a Quick StartTM Bradford Protein Assay kit (Bio-Rad, Hercules, CA, USA) with bovine gamma globulin as a standard. PGE2 levels were expressed as pg/mg protein. 21
Statistical analysis
The significance of differences in the means of body, absolute and relative liver weights and serum ALT activity between the controls and treated groups was examined by ANOVA followed by Dunnett’s test. In regard to the quantitative data for liver GST-P positive foci and the PGE2 concentration, the Kruskal-Wallis test was applied followed by the Mann-Whitney U test. For western blot data, bands were scanned, and densitometry measurement of the scanned bands was performed. Data were normalized to β-actin and expressed as means ± SE. The significance of differences between the treated groups and DEN controls was examined by ANOVA followed by the LSD test. P<0.05 was considered statistically significant for all tests.
Results
Body and organ weights, food intake and serum ALT activity
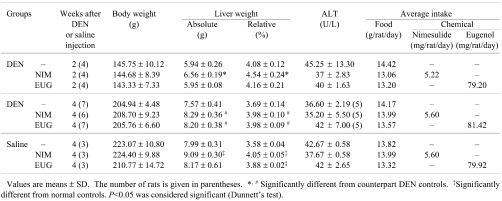
Experimental details are summarized in Table 1. Mortality during the experimental period was limited to one rat in the nimesulide-treated DEN-injected group, which died accidentally 3 days after the start of nimesulide administration. There was no significant change in the body weights of the saline- or DEN-injected rats treated with nimesulide or eugenol for three weeks, whereas the relative liver weights showed a slight significant increase, with respect to their counterpart controls. Despite the significantly increased liver weights, no explanation for such increase was found on histological analysis. Furthermore, expression of proliferating cell nuclear antigen (PCNA; a marker of cell proliferation), as determined by western blot, was unchanged (data not shown), and liver toxicity of the drugs was excluded by the insignificant change in serum ALT activity.
Table 1. Body and Liver Weights, Alanine Aminotransferase (ALT) Activity and Intake Data of DEN- and Saline-injected Rats Treated with Nimesulide (NIM) or Eugenol (EUG).
Western blot analysis and PGE2 results
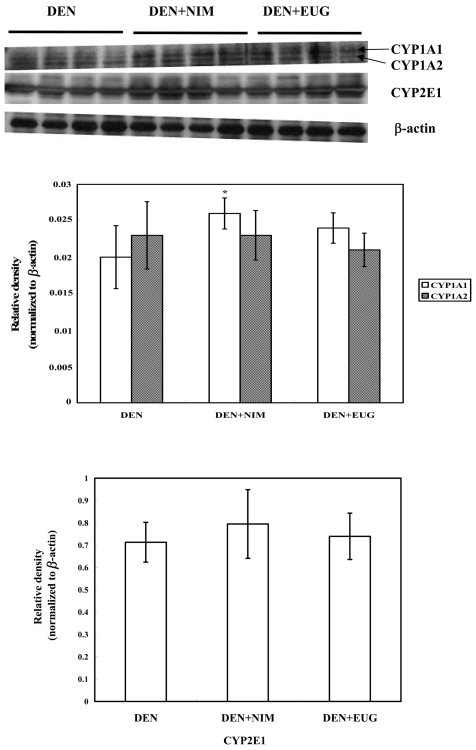
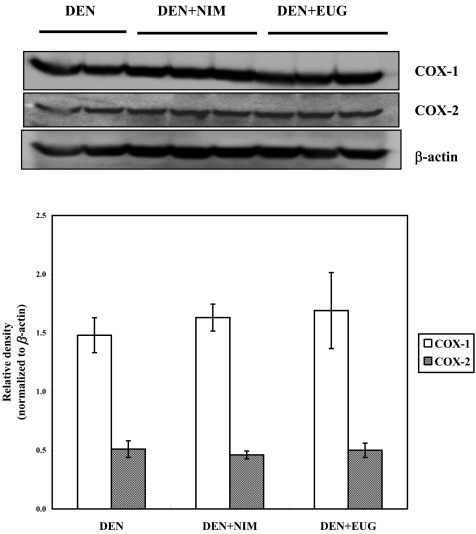
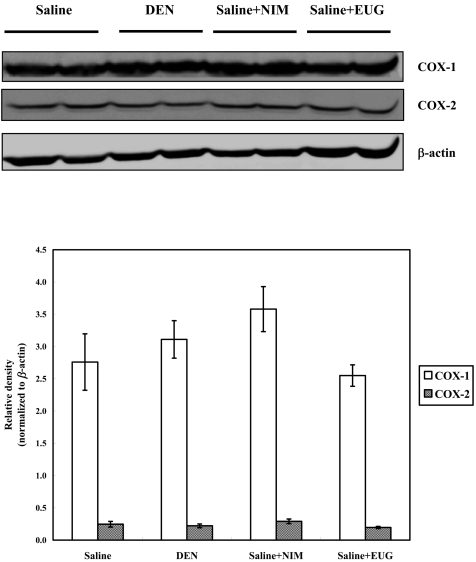
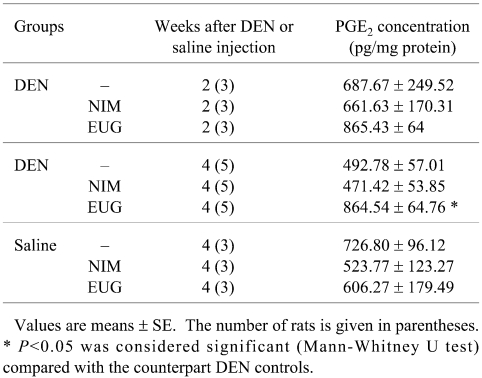
Western blot analysis revealed that oral administration of nimesulide or eugenol to the DEN-injected rats did not alter the CYP2E1 and 1A2 expressions, whereas nimesulide, but not eugenol, significantly induced CYP1A1 expression, as compared with the DEN controls (Fig. 2). Cyclooxygenases 1 and 2 (COX-1 and COX-2) were found to be expressed in normal control livers, and their expression was not modulated following DEN exposure and/or nimesulide or eugenol treatments (Figs. 3 and 4). Treatment of saline- or DEN-injected rats with nimesulide was found to insignificantly change the liver prostaglandin E2 (PGE2) level (Table 2). On the other hand, eugenol administration for 3 weeks in the DEN-injected rats produced a sharp significant increase in liver PGE2 level (75%), as compared with the DEN controls.
Fig. 2.
A representative western blot showing the expression of cytochrome P450 (CYP) 1A1/1A2 and 2E1 proteins in the livers of F344 male rats two weeks after DEN injection and treatment with nimesulide (NIM) or eugenol (EUG). The histogram shows the relative densities of bands normalized to β-actin (means ± SE). * Significantly different from the DEN controls at P<0.05 by LSD test.
Fig. 3.
A representative western blot showing the expression of cyclooxygenase (COX)-1 and COX-2 proteins in the livers of F344 male rats four weeks following DEN injection and treatment with nimesulide (NIM) or eugenol (EUG). The histogram shows the relative densities of bands normalized to β-actin (means ± SE).
Fig. 4.
A representative western blot showing the expression of COX-1 and COX-2 proteins in the livers of normal F344 males, livers of those treated with nimesulide or eugenol and livers of DEN-injected rats. The histogram shows the relative densities of bands normalized to β-actin (means ± SE).
Table 2. Statistical Significance of the Liver Prostaglandin E2 (PGE2) Concentration of DEN- and Saline-injected Rats Treated with Nimesulide (NIM) or Eugenol (EUG).
Histopathological findings and assessment of GST-P positive foci
An assay system was established 22 in which the carcinogenic potential of chemicals can be detected by measurement of altered liver foci as an end-point marker within a relatively short period. The preneoplastic nature of altered hepatic foci and usefulness of such lesions as early indicators of hepatocarcinogenicity are now well accepted, and the validity of foci in assays for detection of carcinogenic agents has been emphasized. Their phenotypic characteristics have been extensively studied using enzyme histochemical and immunohistochemical approaches, and the glutathione S-transferase placental form (GST-P) has been found to be an optimal marker for clear visualization of even very small lesions. 23 , 24
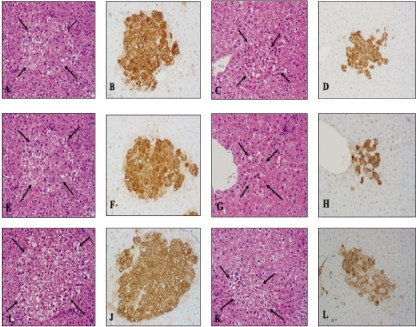
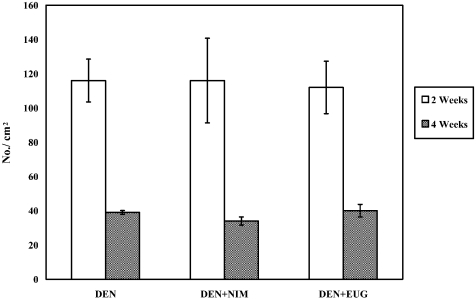
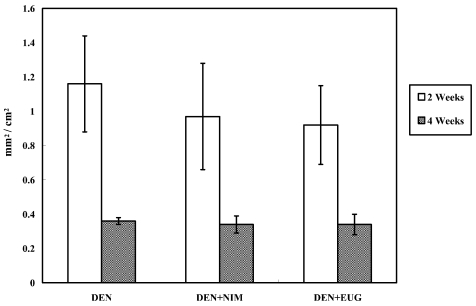
Two weeks after DEN injection, hematoxylin and eosin staining of F344 rat livers showed periportal foci containing hepatocytes with large clear cytoplasmic vacuoles and prominent nucleoli. These foci clearly regressed after 4 weeks. No histopathologic changes were observed regarding the size of hepatic foci after treatment of DEN-injected rats with nimesulide or eugenol for one or three weeks, as compared with the age-matching DEN controls (Fig. 5). Assessment of GST-P positive liver foci showed that nimesulide or eugenol treatment did not exert any suppressive or promotional effect on DEN-induced hepatocarcinogenesis in rats, compared with the DEN controls, as evidenced by the insignificant change in the number and area of GST-P positive preneoplastic liver foci (Figs. 6 and 7).
Fig. 5.
Representative hematoxylin and eosin staining (A, C, E, G, I and K) and immunohistochemical findings of GST-P positive liver preneoplastic foci (B, D, F, H, J and L) of the DEN controls two weeks (A and B) and four weeks (C and D) after DEN injection (200 mg/Kg b.w.) and following treatment with nimesulide (E and F, G and H) or eugenol ( I and J, K and L) for one or three weeks , respectively (×20). Arrows indicate foci.
Fig. 6.
Statistical comparison of the number of GST-P positive liver cell foci (No./cm2) in F344 male rats two and four weeks after DEN injection and treatment with nimesulide (NIM) or eugenol (EUG), as compared with their counterpart DEN controls (values are means ± SE).
Fig. 7.
Statistical comparison of the areas of GST-P positive liver cell foci (mm2/cm2) in F344 male rats two and four weeks following DEN injection and treatment with nimesulide (NIM) or eugenol (EUG), as compared with their counterpart DEN controls (values are means ± SE).
Discussion
The present study demonstrated that dietary supplementation with nimesulide or eugenol did not alter the formation of GST-P positive foci at an early stage of nitrosodiethylamine-induced hepatocarcinogenesis in F344 male rats.
It is well documented that cytochrome P450 2E1 enzyme plays a major role in the activation of nitrosodiethylamine (DEN) into genotoxic products; moreover, other cytochromes like 1A2, 2B1, 2D1 and 1A1 are also involved. 4 To the best of our knowledge, the literature implies no evidence regarding the effect of nimesulide on phase I enzymes, whereas eugenol appears to act as a better inducer of phase II rather than phase I enzymes. 16 On the basis of the present findings, nimesulide, but not eugenol, was reported herein for the first time to induce cytochrome P450 1A1 expression. In spite of the increased expression of CYP1A1 by nimesulide, CYP2E1 expression was not modulated by either eugenol or nimesulide, and as a consequence, the formation of enzyme-altered placental glutathione S-transferase (GST-P) preneoplastic foci, an end-point marker for hepatocarcinogenesis, was unchanged.
It has been reported that COX-2 protein expression was upregulated following daily administration of N-nitrosodiethylamine in drinking water (0.01%) for 15 weeks to male Wistar rats during hepatocellular carcinoma induction, as compared with normal controls. 5 In contrast, our data revealed that COX-2 expression was not modulated 4 weeks post injection of rats with a single dose of DEN compared with normal controls. It is well known that administration of a single i.p. dose of DEN (200 mg/Kg body weight) to rats is carcinogenic but only after a year or more 25 ; it is therefore possible that COX-2 plays no role in the post-initiation development of DEN-induced rat hepatocarcinogenesis at an early stage (4 weeks).
Nimesulide administration for 12 weeks at doses 200-800 ppm in diet has been reported to significantly reduce the number and size of enzyme-altered preneoplastic GST-P positive liver foci during hepato- carcinogenesis induced by a choline-deficient, L-amino acid-defined diet in F344 male rats, and direct evidence of the involvement of COX-2 in the early stage of hepatocarcinogenesis associated with fatty change, fibrosis and cirrhosis has been provided. 8 In contrast, another study 13 demonstrated that oral administration of nimesulide (10 ppm in diet for 6 weeks) to DEN-injected F344 male rats in a medium-term liver carcinogenesis bioassay did not induce any significant reduction in GST-P positive liver foci, as compared with DEN controls; however, the authors attributed this lack of effect to a low nimesulide dose that was unable to induce any chemopreventive potential. According to our results, an average daily intake of 400 ppm nimesulide corresponds to 28 mg/Kg body weight, which is approximately 8 times the respective maximum tolerated dose in humans that is 200 mg per person per day. 9 , 11 Even though a high dose was given to the rats in the present study, nimesulide did not significantly modulate COX-2 protein expression or PGE2 levels, suggesting that a three week period of nimesulide administration was insufficient to alter both parameters and that chronic administration of COX inhibitors is needed in long-term carcinogenesis study.
Several lines of evidence show that the anti-inflammatory and anticarcinogenic actions of eugenol compounds depend on inhibition of COX-2 expression. 26 , 27 Expression of COX-2 is stimulated by nitric oxide through the c-AMP response element. Eugenol has been reported to reduce nitric oxide (NO) production in lipopolysaccharide-treated macrophages by inhibiting the expression of inducible nitric oxide synthase (iNOS) protein and further decreases the expression of COX-2 protein. 28 In the present study, a significant sharp increase in the liver PGE2 level, which reached 75.4%, was recorded following oral administration of DEN-injected rats with eugenol for three weeks, as compared with the DEN controls, even though administration of eugenol per os to normal rats did not significantly alter the liver PGE2 level, as compared with normal rats. We therefore suggest that 1) eugenol induced COX-1 or COX-2 enzyme activities without modifying their protein expression, causing a differential contribution of COXs to the PGE2 level to occur in the DEN-injected animals, or 2) reactive intermediates may have been formed during microsomal metabolism of eugenol following DEN exposure, which may have induced the activity of prostaglandin E synthase, or may have even altered the activity of the liver prostaglandin dehydrogenase and cytochrome P450 monooxygenases involved in PGE2 inactivation. However, the effect of eugenol on PGE2 metabolism remains to be elucidated.
In conclusion, the present study clearly demonstrates that the early carcinogenic response of DEN in the rat liver remains unchanged in the presence of nimesulide, a selective COX-2 inhibitor, and the phenolic antioxidant eugenol. This lack of modification is partly interpreted as a result of the inability of both compounds to modulate CYP2E1 expression. Furthermore, the COXs and PGE2 are not involved in the early stage of DEN-induced hepatocarcinogenesis in the present model.
Acknowledgments
This work was performed through collaboration between the Japanese Government, represented by Professor Tomoyuki Shirai, and the Egyptian Ministry of Higher Education and State for Scientific Research (MHESR), represented by Dr. Mahmoud Mohamed Said.
References
- 1.Sakata K, Hara A, Hirose Y, Yamada Y, Kuno T, Katayama M, Yoshida K, Zheng Q, Murakami A, Ohigashi H, Ikemoto K, Koshimizu K, Tanaka T, Mori H. Dietary supplementation of the citrus antioxidant auraptene inhibits N,N-diethylnitrosamine-induced rat hepatocarcinogenesis. Oncology. 2004;66:244–252. doi: 10.1159/000078001. [DOI] [PubMed] [Google Scholar]
- 2.Motola-Kuba D, Zamora-Valdés D, Uribe M, Méndez-Sánchez N. Hepatocellular carcinoma. An overview. Ann Hepatol. 2006;5:16–24. [PubMed] [Google Scholar]
- 3.Fahim FA, Chipman JK. Illustration of the antioxidant and/or antimutagenic effect of eugenol on HepG2 cells treated with AF-B1 using single cell gel electrophoresis (comet assay) and electron microscopy (E.M.) Cell Mol Biol. 2001;8:1599–1611. [Google Scholar]
- 4.Yamazaki H, Oda Y, Funae Y, Imaoka S, Inui Y, Guengerich FP, Shimada T. Participation of rat liver cytochrome P450 2E1 in the activation of N-nitrosodimethylamine and N-nitrosodiethyl-amine to products genotoxic in an acetyltransferase-overexpressing Salmonella typhimurim strain (NM2009) Carcinogenesis. 1992;13:979–985. doi: 10.1093/carcin/13.6.979. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishnan G, Elinos-Báez CM, Jagan S, Augustine TA, Kamaraj S, Anandakumar P, Devaki T. Silymarin downregulates COX-2 expression and attenuates hyperlipidemia during NDEA-induced rat hepatocellular carcinoma. Mol Cell Biochem. 2008;313:53–61. doi: 10.1007/s11010-008-9741-5. [DOI] [PubMed] [Google Scholar]
- 6.Bennett A, Tavares IA. COX-2 inhibitors compared and contrasted. Expert Opin Pharmacother. 2001;2:1859–1867. doi: 10.1517/14656566.2.11.1859. [DOI] [PubMed] [Google Scholar]
- 7.Brown WA, Skinner SA, Malcontenti-Wilson C, Vogiagis D, O’Brien PE. Non-steroidal anti-inflammatory drugs with activity against either cyclooxygenase 1 or cyclooxygenase 2 inhibit colorectal cancer in a DMH rodent model by inducing apoptosis and inhibiting cell proliferation. Gut. 2001;48:660–666. doi: 10.1136/gut.48.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denda A, Kitayama W, Murata A, Kishida H, Sasaki Y, Kusuoka O, Tsujiuchi T, Tsutsumi M, Nakae D, Takagi H, Konishi Y. Increased expression of cyclooxygenase-2 protein during rat hepatocarcinogenesis caused by a choline-deficient, L-amino acid-defined diet and chemopreventive efficacy of a specific inhibitor, nimesulide. Carcinogenesis. 2002;23:245–256. doi: 10.1093/carcin/23.2.245. [DOI] [PubMed] [Google Scholar]
- 9.Okajima E, Denda A, Ozono S, Takahama M, Akai H, Sasaki Y, Kitayama W, Wakabayashi K, Konishi Y. Chemopreventive effects of nimesulide, a selective cyclooxygenase-2 inhibitor, on the development of rat urinary bladder carcinomas initiated by N-butyl-N-(4-hydroxybutyl)nitrosamine. Cancer Res. 1998;58:3028–3031. [PubMed] [Google Scholar]
- 10.Khanduja KL, Sohi KK, Pathak CM, Kaushik G. Nimesulide inhibits lipopolysaccharide-induced production of superoxide anions and nitric oxide and iNOS expression in alveolar macrophages. Life Sci. 2006;78:1662–1669. doi: 10.1016/j.lfs.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Shiotani H, Denda A, Yamamoto K, Kitayama W, Endoh T, Sasaki Y, Tsutsumi M, Sugimura M, Konishi Y. Increased expression of cyclooxygenase-2 protein in 4-nitroquinoline-1-oxide-induced rat tongue carcinomas and chemopreventive efficacy of a specific inhibitor, nimesulide. Cancer Res. 2001;61:1451–1456. [PubMed] [Google Scholar]
- 12.Denda A, Endoh T, Kitayama W, Tang Q, Noguchi O, Kobayashi Y, Akai H, Okajima E, Tsujiuchi T, Tsutsumi M, Nakae D, Konishi Y. Inhibition by piroxicam of oxidative DNA damage, liver cirrhosis and development of enzyme-altered nodules caused by a choline-deficient, L-amino acid-defined diet in rats. Carcinogenesis. 1997;18:1921–1930. doi: 10.1093/carcin/18.10.1921. [DOI] [PubMed] [Google Scholar]
- 13.Yokohira M, Takeuchi H, Yamakawa K, Saoo K, Matsuda Y, Zeng Y, Hosokawa K, Maeta H, Imaida KA. COX-2 inhibitor, SC58125, promotes liver carcinogenesis in a rat medium-term liver bioassay, possibly due to induction of CYP 2B1 and 3A1. J Toxicol Pathol. 2006;19:37–45. [Google Scholar]
- 14.Thompson D, Norbeck K, Olsson LI, Constantin-Teodosiu D, Vane der Zee J, Moldeus P. Peroxidase-catalyzed oxidation of eugenol: formation of a cytotoxic metabolite(s) J Biol Chem. 1989;264:1016–1021. [PubMed] [Google Scholar]
- 15.Ghosh R, Nadiminty N, Fitzpatrick JE, Alworth WL, Slaga TJ, Kumar AP. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem. 2005;280:5812–5819. doi: 10.1074/jbc.M411429200. [DOI] [PubMed] [Google Scholar]
- 16.Kumaravelu P, Dakshinamoorthy DP, Subramaniam S, Devaraj H, Devaraj NS. Effect of eugenol on drug-metabolizing enzymes of carbon tetrachloride-intoxicated rat liver. Biochem Pharmacol. 1995;49:1703–1707. doi: 10.1016/0006-2952(95)00083-c. [DOI] [PubMed] [Google Scholar]
- 17. NTP Carcinogenesis studies of eugenol (CAS No. 97-53-0) in F344/N rats and B6C3F1 mice (food studies), TR No. 223. National Toxicology Program, Research Triangle Park, NC. 1983. [PubMed] [Google Scholar]
- 18.Suzuki S, Asamoto M, Tsujimura K, Shirai T. Specific differences in gene expression profile revealed by cDNA microarray analysis of glutathione S-transferase placental form (GST-P) immunohistochemically positive rat liver foci and surrounding tissue. Carcinogenesis. 2004;25:439–443. doi: 10.1093/carcin/bgh030. [DOI] [PubMed] [Google Scholar]
- 19.Norton ID, Apte MV, Haber PS, McCaughan GW, Pirola RC, Wilson JS. Cytochrome P4502E1 is present in rat pancreas and is induced by chronic ethanol administration. Gut. 1998;42:426–430. doi: 10.1136/gut.42.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Kawabe M, Shibata MA, Sano M, Takesada Y, Tamano S, Ito N, Shirai T. Decrease of prostaglandin E2 and 5-bromo-2’-deoxyuridine labeling but not prostate tumor development by indomethacin treatment of rats given 3,2’-dimethyl-4-aminobiphenyl and testosterone propionate. Jpn J Cancer Res. 1997;88:350–355. doi: 10.1111/j.1349-7006.1997.tb00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatematsu M, Shirai T, Tsuda H, Miyata Y, Shinohara Y. Rapid production of hyperplastic liver nodules in rats treated with carcinogenic chemicals: a new approach for an in vivo short-term screening test for hepatocarcinogens. Gann. 1977;68:499–507. [PubMed] [Google Scholar]
- 23.Shirai T. A medium-term rat liver bioassay as a rapid in vivo test for carcinogenic potential: a historical review of model development and summary of results from 291 tests. Toxicol Pathol. 1997;25:453–460. doi: 10.1177/019262339702500504. [DOI] [PubMed] [Google Scholar]
- 24.Ito N, Tamano S, Shirai T. A medium-term rat liver bioassay for rapid in vivo detection of carcinogenic potential of chemicals. Cancer Sci. 2003;94:3–8. doi: 10.1111/j.1349-7006.2003.tb01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pilot HC, Dragan YP.Chemical carcinogenesis. In: Toxicology; The Basic Science of Poisons, Chapter 8. 6th ed. CD Klaassen (ed). New York, McGraw-Hill, 295–296.2001.
- 26.Kim SS, Oh OJ, Min HY, Park EJ, Kim Y, Park HJ, Nam HY, Lee SK. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003;73:337–348. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 27.Okada N, Hirata A, Murakami Y, Shoji M, Sakagami H, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression by eugenol-related compounds. Anticancer Res. 2005;25:3263–3269. [PubMed] [Google Scholar]
- 28.Li W, Tsubouchi R, Qiao S, Haneda M, Murakami K, Yoshino M. Inhibitory action of eugenol compounds on the production of nitric oxide in RAW264.7 macrophages. Biomed Res. 2006;27:69–74. doi: 10.2220/biomedres.27.69. [DOI] [PubMed] [Google Scholar]