Abstract
Previously, we reported α2-macroglobulin (α2M) to be a novel marker characteristic of rat hepatocellular preneoplastic and neoplastic lesions negative for hitherto well-established markers. In the present study, we further examined other candidate markers with specificity for the same type of lesions. Glutathione S-transferase-placental form (GST-P)-negative hepatocellular altered foci (HAF) were generated using a two-stage (initiation and promotion) carcinogenesis protocol with N,N-diethylnitrosamine (DEN) and either Wy-14,643 or clofibrate, two peroxisome proliferators. Microarray analysis using total RNAs isolated from laser-microdissected GST-P-negative HAF (amphophilic cell foci) and adjacent normal tissues was conducted along with immunohistochemistry and real-time RT-PCR. Staining for glucose-regulated protein 78 (GRP78) was detected in GST-P-negative HAF and hepatocellular adenomas, and slightly increased GRP78 mRNA expression was observed in the lesions by real-time RT-PCR analysis. Thus, an early increase of GRP78 expression in hepatocarcinogenesis is likely a feature of the amphophilic subset of HAF.
Keywords: molecular marker, glucose-regulated protein 78, α2-macroglobulin, preneoplastic lesion, rat hepatocarcinogenesis
Introduction
During the process of rat hepatocarcinogenesis, a small proportion of preneoplastic lesions, hepatocellular altered foci (HAF), develop into hepatocellular adenomas (HCAs) and hepatocellular carcinomas (HCCs). While many HAF regress following withdrawal of a proliferative stimulus, 1 – 4 others may persist and demonstrate stable growth. 5 , 6 The molecular mechanisms underlying these phenomena, however, are unclear. Although foci possess some phenotypic diversity, 7 there are markers that allow detection in the majority of cases. However, while γ-glutamyl transpeptidase (γ-GT) 3 , 4 , 6 – 11 and particularly glutathione S-transferase-placental type (GST-P) 12 , 13 have become established as marker enzymes for hepatocarcinogenesis in rats, they have shortcomings regarding detection of basophilic and amphophilic cell foci, especially those induced by peroxisome proliferators. 14 – 16 Amphophilic cell foci, characterized by increased granular acidophilia and diffusely scattered cytoplasmic basophilia, demonstrate alterations in mitochondrial enzymes, 16 , 17 but cytochemical markers applicable to routine detection of γ-GT and/or GST-P-negative foci, especially small lesions not evident in routine hematoxylin-eosin (HE) staining, have been lacking.
In our previous study, α2-macroglobulin (α2M) was identified as a novel cytochemical marker characterizing preneoplastic and neoplastic rat liver lesions composed of amphophilic or basophilic cells. 18 A previous investigation revealed this typical member of the pan-proteinase inhibitor α2M family to be upregulated in human serum during HCC development. 19 It was also found overexpressed in HCCs due to hepatitis C virus infection as compared with nontumorous liver tissues. 20 Moreover, Pizzo’s group reported that an activated form of α2M (α2M*) is able to modulate cell proliferation via a distinct cell surface receptor, the 78-kDa glucose-regulated protein (GRP78). 21
GRP78 is well known as the major sensor of endoplasmic reticulum (ER) stress and recently has attracted attention as a promising target for cancer therapy, since it makes a contribution to tumor progression by inhibition of apoptosis. 22 The majority of GRP78 molecules reside in the lumen of the ER, but a subpopulation exists as an ER transmembrane protein on the surfaces of some cells, such as certain cancer cells and growth-stimulated endothelial cells. 22
In the present study, we demonstrated that GRP78 is characteristically upregulated from an early stage of rat hepatocellular carcinogenesis, specifically in GST-P-negative lesions. The results suggest that an early increase of GRP78 expression in hepatocarcinogenesis is likely a feature of the amphophilic subset of HAF.
Materials and Methods
Animals
A total of 50 male, 5-week-old F344 rats were purchased from Charles River Japan Inc. (Atsugi, Japan) and housed in suspended aluminium cages (three rats per cage) in a room kept at 24 ± 2°C and 40–70% humidity with a 12-h light/dark cycle. Drinking water was available ad libitum. They received CRF-1 Laboratory Chow (Charles River Japan Inc.) as basal diet in experiments 1 and 2 ad libitum. The animals were observed daily and were used for the experiments after a 1-week acclimation period. Body weight was measured every week. 18
Chemicals
N,N-diethylnitrosamine (DEN) and clofibrate (> 98%) were purchased from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan), and Wy-14,643 (> 98%) was obtained from ChemSyn Laboratories (Lenexa, KA, USA).
Experimental protocol
All experiments were performed in accordance with the Guide for Animal Care and Use of Sumitomo Chemical Co., Ltd. In Experiment 1, an in vivo rat liver medium-term bioassay for carcinogens was conducted, 13 with a minor modification in regard to the treatment period for the peroxisome proliferators. Briefly, at the age of 6 weeks, 20 male F344 rats were divided into four groups (5 animals per group). The animals were given a single intraperitoneal injection of DEN (200 mg/kg body weight) dissolved in saline to initiate hepatocarcinogenesis and after a 2-week recovery period received clofibrate (3,000 ppm, Group 1) or Wy-14,643 (1,000 ppm, Group 2) in the basal diet. The rats were subjected to two-thirds partial hepatectomy (PH) in week 3. The animals in Group 3 were given DEN and PH in the same manner as for Groups 1 and 2 without administration of any other chemicals, and the animals in Group 4 were treated in the same manner as Group 3, except that they were injected with saline instead of DEN. All animals were sacrificed at week 12.
In Experiment 2, an initiation-promotion model was used in which the number of administrations of DEN was increased in place of PH in Experiment 1. Judging from our experience, however, the dose of DEN was set at 100 mg/kg so as to not impose too heavy a burden on the animals. Briefly, 30 male, 6-week-old, F344 rats were divided into three groups (10 animals per group). The animals in Groups 1 and 2 were injected with DEN (100 mg/kg body weight) intraperitoneally once a week for 2 weeks and after a one-week recovery period received clofibrate (3,000 ppm, Group 1) or the basal diet (Group 2). The animals in Group 3 were injected with saline instead of DEN solution without subsequent administration of any chemicals. Sacrifice was at weeks 26 and 36.
In both experiments, all animals in each group were exsanguinated and sacrificed under ether anesthesia, and the liver tissues were obtained and treated with appropriate procedures for the following examinations.
Laser microdissection (LMD) and total RNA isolation
Frozen liver tissues embedded in OCT compound (Sakura Finetech, Tokyo, Japan) were sectioned at about 8 μm to obtain several sets of 7 serial sections, and the first and last sections in each suite were applied to routine HE staining and immunohistochemical staining for rat GST-P to identify microlesions histopathologically for microdissection. The remaining sections were treated to block degradation of RNA, i.e., the sections were fixed in 70% ethanol for 1 min, immersed in RNase-free hematoxylin for 5 min, rinsed in RNase-free water several times, immersed in RNase-free PBS for 5 min and immersed in RNase-free eosin for 1 min. In experiment 1, GST-P-positive and GST-P-egative HAF and corresponding adjacent normal tissues were microdissected using a laser microdissection system (Leica Microsystems Japan, Tokyo, Japan) from the following groups: GST-P-positive HAF in Groups 1 and 3 and GST-P-negative HAF in Group 2. In experiment 2, HAF, HCA, HCC and corresponding adjacent normal tissues were microdissected in the same manner from the following groups: GST-P-negative HAF in Group 1 and GST-P-negative and GST-P-positive HCA and HCC in Group 1. In each experiment, 4 foci from Group 1, 23 foci from Group 2 and 6 foci from Group 3 in experiment1 and 3 foci from Group 1 in the respective experiment were pooled, since the sizes of HAF were not sufficient to obtain a large enough total quantity of RNA from each lesion. Subsequently, total RNAs were isolated from the microdissected tissues in accordance with the manufacturer’s instructions for the RNeasy Protect Mini Kit (QIAGEN, Tokyo, Japan) with a minor modification consisting of use of poly (C) (Amersham Bioscience, Buckinghamshire, UK) as a carrier. The total RNA pool was used for the subsequent microarray analysis and RT-PCR assays.
High-density oligonucleotide microarray analysis
Rat Genome U34A Array chips containing 9,000 probes for known rat genes or Expressed sequence tags (ESTs) were purchased from Affymetrix (Santa Clara, CA, USA). For microarray probing, reverse transcription, second-strand synthesis and probe generation were all accomplished following the technical notes of the Small Sample Labeling Protocol Ver.#2 (Affymetrix). Briefly, first-strand cDNA was synthesized from 100 ng of total RNA with SuperScript II reverse transcriptase (Invitrogen, Tokyo, Japan) and a T7-(dT)24 primer (Amersham Bioscience, Buchinghamshire, UK), and then double-stranded cDNA was synthesized with E.coli RNase H, E.coli DNA polymerase I and E.coli DNA ligase (Invitrogen). From this double-stranded cDNA, cRNA was prepared using a MEGAscript T7 Kit (Ambion, Austin, TX, USA). After a second cycle of amplification and biotin-labeling with a BioArray High Yield RNA Transcript Labeling Kit (Enzo Diagnostics, Farmingdale, NY, USA), 20 μg of labeled cRNA was fragmented. The RGU34A arrays were hybridized as described in the Gene Chip Expression Analysis Technical Manual (Affymetrix) and stained for use with a GeneArray scanner (Agilent Technologies, Palo Alto, CA, USA). The derived signal value was globally normalized and targeted to all probe sets equal to 100 before comparative analysis.
Microarray data analysis
To examine gene expression differences between GST-P-negative or GST-P-positive HAF and the corresponding adjacent normal tissue, we performed a comparison analysis using the Affymetrix data suite system, MAS 5.0. The genes (probe sets) showing greater than two-fold alterations in their values, increases or decreases, were selected as changed genes.
TaqMan real-time RT-PCR of GRP78 mRNA in GST-P-negative HAF
TaqMan real-time RT-PCR of GRP 78 mRNA was performed in accordance with a standard protocol and standard TaqMan thermocycling conditions using the same RNA samples as them applied to microarray analysis. The PCR primer and TaqMan probe sequences for rat GRP78 mRNA were as follows: forward primer, 5’-CAC GTC CAA CCC GGA GAA-3’; reverse primer, 5’-TTC CAA GTG CGT CCG ATGA-3’; probe, 5’-ACC GTC TTC GAC GCC AAG CGC-3’. Primers and the TaqMan probe for rat GAPDH were purchased (Invitrogen, Tokyo, Japan).
Immunohistochemistry for GRP78 and GST-P
Immunohistochemical staining for GRP78 and GST-P was performed on consecutive 4% paraformaldehyde-fixed liver sections from animals in experiment 2. Staining for GST-P was conducted as in our previous study. 18 That for GRP78 was accomplished in accordance with the protocol provided in the CSA II Kit (Dako Japan, Tokyo), with a minor modification consisting of retrieval of antigens using Target Retrieval Solution (Dako Japan), using a primary goat anti-GRP78 antibody (1:1000, room temperature, 15 min, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The negative control was prepared by withdrawal of the 1st antibody to validate the specificity in each immunohistochemical staining.
Results
Histopathology and laser microdissection
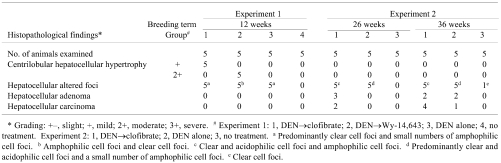
Histopathological examination was conducted with reference to earlier articles 16 , 22 and a diagnostic guide, 23 and the results are shown in Table 1. 18 Several frozen cell populations were harvested, specifically from GST-P-positive and/or GST-P-negative HAF, HCAs and HCCs, using laser microdissection, and total RNA was isolated from each lesion in experiments 1 and 2. GST-P-negative HAF, i.e., amphophilic cell foci, were not clearly detectable in the frozen sections stained by HE in Group 1 of experiment 1, so amphophilic GST-P-negative HAF were microdissected from Group 2 in experiment 1 and Group 1 in experiment 2.
Table 1. Histopathological Findings in the Liver.
Expression of mRNA
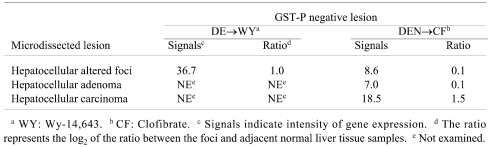
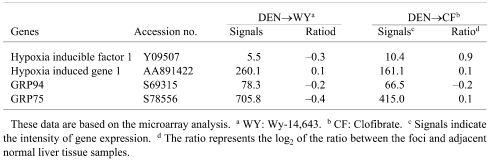
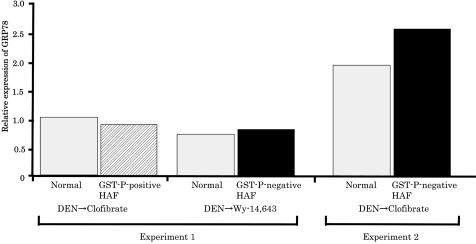
GST-P-negative HAF induced by DEN→Wy-14,643 (experiment 1) or DEN→clofibrate (experiment 2), and GST-P-positive HAF induced by DEN alone or DEN→clofibrate (Experiment 1) were compared with the corresponding respective adjacent normal tissues. As a result of microarray analysis, while α2M was successfully identified in preneoplastic and neoplastic rat liver lesions negative for GST-P, 18 fluctuation of GRP78, which can play a role as a receptor for α2M* when located on the cell surface, was not observed in the same lesions (Table 2). Furthermore, mRNAs for other genes related to ER stress and /or hypoxia, as well as GRP78, did not show abnormal expression in GST-P-negative HAF (Table 3). However, slight enhancement of GRP78 mRNA was observed only in GST-P-negative HAF induced by DEN→clofibrate by real-time RT-PCR analysis, even though the levels of GRP78 mRNA in the normal tissues were different between experiments 1 and 2 (Fig. 1).
Table 2. GRP78 mRNA Expression Based on Microarray Analysis.
Table 3. mRNA Expressions of Genes Related to Hypoxia or ER Stress in GST-P Negative HAF.
Fig. 1.
The relative expression of GRP78 mRNA in GST-P-negative HAF induced by DEN→clofibrate was slightly increased compared with the adjacent normal tissue. The vertical axis represents the ratio of GRP78 mRNA expression versus GAPDH mRNA expression in each region. The white bars represent GRP78 mRNA expression in the normal tissue, and the left hatched and black bars represent GST-P-positive and GST-P-negative HAF, respectively.
Immunohistochemical analysis: Immunohistochemical analyses revealed characteristic signals for GRP78 in GST-P-negative HAF and HCA induced by DEN→clofibrate (Figs. 2 and 3), although the positive intensity was too weak to identify the intensity clearly in some of the small GST-P-negative HAF. On the other hand, GRP78 did not appear to be upregulated in GST-P-negative HAF induced by DEN→Wy-14,643 (data not shown).
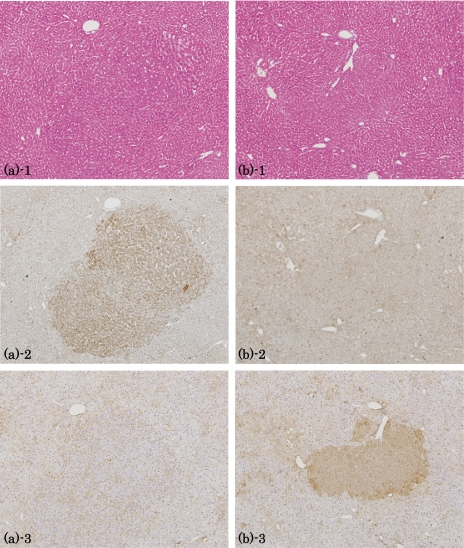
Fig. 2.
Immunohistochemistry for GRP78 and GST-P in HAF induced by DEN→clofibrate. (a) GST-P-negative lesion: 1, HE staining; 2, GRP78; 3, GST-P. (b) GST-P positive lesion: 1, HE staining; 2, GRP78; 3, GST-P. Lens × 4.
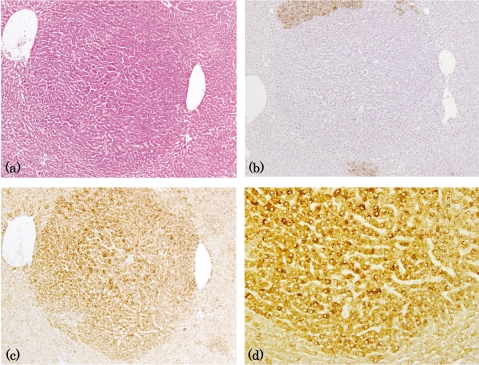
Fig. 3.
Immunohistochemistry for GRP78 in GST-P-negative HCAs induced by DEN→clofibrate. (a) HE staining. (b) GST-P. (c), (d) GRP78. (a)-(c) lens × 4; (d) lens × 10.
Discussion
In the present study, an increase in immunohistochemical staining for GRP78 was characteristically observed in GST-P-negative HAF induced by DEN→clofibrate, and slightly increased GRP78 mRNA expression was also observed in the lesions. These results are in line with previous reports showing that GRP78, a major molecular ER chaperone, is induced in a wide variety of human cancers. 24 , 27
While Hung et al. reported that ER stress can stimulate COX-2 expression through the NF-κB and p38 kinase pathways, 28 GST-P-negative HAF positive for GRP78 may not necessarily be characterized by hypoxic conditions, since mRNAs of genes related to hypoxia and/or ER stress, such as Hypoxia inducible factor 1α, Hypoxia induced gene 1, GRP94 and GRP75, were not upregulated according to the cDNA microarray analysis.
It has been reported that GRP78 located on the cell surface can play a role as a receptor for circulating autoantibodies against GRP78 and α2M* and modulate intracellular signal cascades, for instance, leading to an inositol 1,4,5-trisphosphate-dependent increase in Ca2+ and activation of Ras/mitogen-activated protein kinase-dependent signaling. 29 – 33 We previously observed upregulation of α2M mRNA in GST-P-negative HAF, 18 so control of their expression may be coordinated. Although α2M protein was not always immunohistochemically detectable in GST-P-negative foci, in contrast with α2M mRNA, 18 this might be explained by the short half-life of α2M*, i.e., <2 minutes. 34 In the present study, GRP78 was not detectable immunohistochemically in GST-P-negative HAF induced by DEN→Wy-14,643 (data not shown), in line with the result from RT-PCR, but this might be because the lesions were generally very small.
According to our preliminary data, no characteristic immnohistochemical staining of GRP78 was observed in GST-P-positive HAF induced by DEN→DDT or sodium phenobarbital, however, GRP78 is observed in HCCs occurring spontaneously in TGFα transgenic mice. This may point to a characteristic accumulation of GRP78 in ER stress due to the microenvironment of the HCC. Whatever the case, the results do suggest that accumulation during hepatocarcinogenesis is independent of the species, at least in HCCs.
In conclusion, the present study provides the first evidence that GRP78 is upregulated even in early lesions, such as HAF in rat hepatocarcinogenesis. However, much remains to be learned before we can determine the mechanistic implications and feasibility of its use as a marker.
Acknowledgments
The authors wish to express their appreciation to Ms M. Yamaguchi for her expert assistance with histotechnology.
References
- 1.Ito N, Hananouchi M, Sugihara S, Shirai T, Tsuda H, Fukushima S, Nagasaki H. Reversibility and irreversibility of liver tumors in mice induced by the α isomer of 1,2,3,4,5,6-hexachlorocyclohexane. Cancer Res. 1976;36:2227–2234. [PubMed] [Google Scholar]
- 2.Moore MA, Hacker HJ, Bannasch P. Phenotypic instability in focal and nodular lesions induced in a short-term system in the rat liver. Carcinogenesis. 1983;4:595–603. doi: 10.1093/carcin/4.5.595. [DOI] [PubMed] [Google Scholar]
- 3.Tatematsu M, Nagamine Y, Farber E. Redifferentiation as a basis for remodeling of carcinogen-induced hepatocyte nodules to normal appearing liver. Cancer Res. 1983;43:5049–5058. [PubMed] [Google Scholar]
- 4.Takahashi S, Lombardi B, Shinozuka H. Progression of carcinogen-induced foci of gamma-glutamyltranspeptidase-positive hepatocytes to hepatomas in rats fed a choline-deficient diet. Int J Cancer. 1982;29:445–450. doi: 10.1002/ijc.2910290414. [DOI] [PubMed] [Google Scholar]
- 5.Scherer E, Emmelot P. Kinetics of induction and growth of precancerous liver-cell foci, and liver tumour formation by diethylnitrosamine in the rat. Eur J Cancer. 1975;11:689–696. doi: 10.1016/0014-2964(75)90042-0. [DOI] [PubMed] [Google Scholar]
- 6.Moore MA, Mayer D, Bannasch P. The dose depedence and sequential appearance of putative preneoplastic populations induced in the rat liver by stop experiments with N-nitrosomorpholine. Carcinogenesis. 1982;3:1429–1436. doi: 10.1093/carcin/3.12.1429. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa K, Solt DB, Farber E. Phenotypic diversity as an early property of putative preneoplastic hepatocyte populations in liver carcinogenesis. Cancer Res. 1980;40:725–733. [PubMed] [Google Scholar]
- 8.Enomoto K, Farber E. Kinetics of phenotypic maturation of remodelling of hyperplastic nodules during liver carcinogenesis. Cancer Res. 1982;42:2330–2335. [PubMed] [Google Scholar]
- 9.Solt DB, Medline A, Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977;88:595–618. [PMC free article] [PubMed] [Google Scholar]
- 10.Ito N, Tsuda H, Hasegawa R, Imaida K. Sequential observation of pathomorphologic alterations in preneoplastic lesions during the promotion stage of hepatocarcinogenesis and the development of a short-term test system for hepatopromoters and hepatocarcinogens. Toxicol Pathol. 1982;10:37–49. doi: 10.1177/019262338201000207. [DOI] [PubMed] [Google Scholar]
- 11. Tatematsu M, Kaku T, Medline A, Frikson L, Roomi W, Sharma RN, Murray RK, Farber E.Markers of liver neoplasm-real or fictional? In: Application of Biological Markers to Carcinogen Testing. H Milman and S Sell (eds). Plenum Publishing, New York. 25–42.1983.
- 12.Sato K, Kitahara A, Satoh K, Ichikawa T, Tatematsu M, Ito N. The placental form of glutathione S-transferase as a new marker protein for preneoplasia in rat chemical carcinogenesis. Gann. 1984;75:199–202. [PubMed] [Google Scholar]
- 13.Ito N, Tamano S, Shirai T. A medium-term rat liver bioassay for rapid in vivo detection of carcinogenic potential of chemicals. Cancer Sci. 2003;94:3–8. doi: 10.1111/j.1349-7006.2003.tb01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao MS, Tatematsu M, Subbarao V, Ito N, Reddy JK. Analysis of peroxisome proliferator-induced preneoplastic and neoplastic lesions of rat liver for placental form of glutathione S-transferase and gamma-glutamyltranspeptidase. Cancer Res. 1986;46:5287–5290. [PubMed] [Google Scholar]
- 15.Rao MS, Nemali MR, Usuda N, Scarpelli DG, Makino T, Pitot HC, Reddy JK. Lack of expression of glutathione-S-transferase P, gamma-glutamyl transpeptidase, and alpha-fetoprotein messenger RNAs in liver tumors induced by peroxisome proliferators. Cancer Res. 1988;48:4919–4925. [PubMed] [Google Scholar]
- 16.Weber E, Moore MA, Bannasch P. Enzyme histochemical and morphological phenotype of amphophilic foci and amphophilic/tigroid cell adenomas in rat liver after combined treatment with dehydroepiandrosterone and N-nitrosomorpholine. Carcinogenesis. 1988;9:1049–1054. doi: 10.1093/carcin/9.6.1049. [DOI] [PubMed] [Google Scholar]
- 17.Mayer D, Metzger C, Loenetti P, Beier K, Benner A, Bannasch P. Differential expression of key enzymes of energy metabolism in preneoplastic and neoplastic rat liver lesions induced by N-nitrosomorpholine and dehydroepiandrosterone. Int J Cancer. 1998;79:232–240. doi: 10.1002/(sici)1097-0215(19980619)79:3<232::aid-ijc4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Sukata T, Uwagawa S, Ozaki K, Sumida K, Kikuchi K, Kushida M, Saito K, Morimura K, Oeda K, Okuno Y, Mikami N, Fukushima S. α2-Macroglobulin: A novel cytochemical marker characterizing preneoplastic and neoplastic rat liver lesions negative for hitherto established cytochemical markers. Am J Pathol. 2004;165:1479–1488. doi: 10.1016/s0002-9440(10)63406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kar P, Gandhi BM, Irshad M, Gupta H, Tandon BN. Alpha-2 macroglobulin: an additional marker for diagnosis of hepatocellular carcinoma. J Assoc Physicians India. 1987;35:288–289. [PubMed] [Google Scholar]
- 20.Kotaka M, Chen GG, Lai PB, Lau WY, Chan PK, Leung TW, Li AK. Analysis of differentially expressed genes in hepatocellular carcinoma with hepatitis C virus by suppression subtractive hybridization. Oncol Res. 2002;13:161–167. [PubMed] [Google Scholar]
- 21.Misra UK, Gonzalez-Gronow M, Gawdi G, Wang F, Pizzo SV. A novel receptor function for the heat shock protein Grp78: silencing of Grp78 gene expression attenuates α2M*-induced signaling. Cell Signal. 2004;16:929–938. doi: 10.1016/j.cellsig.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Bánhegyi G, Baumeister P, Benedetti A, Dong D, Fu Y, Lee AS, Li J, Mao C, Margittai E, Ni M, Paschen W, Piccirella S, Senesi S, Sitia R, Wang M, Yang W. Endoplasmic reticulum stress. Ann NY Acad Sci. 2007;1113:58–71. doi: 10.1196/annals.1391.007. [DOI] [PubMed] [Google Scholar]
- 23. Jones TC, Mohr U, Hunt RD.Monographs on Pathology of Laboratory Animals Sponsored by the International Life Sciences Institute, Digestive System. Springer, Berlin,1985. [Google Scholar]
- 24.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5:741–744. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 26.Ranganathan AC, Zhang L, Adam AP, Aquirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–1711. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 28.Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC, Lai MD. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004;279:46384–46392. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- 29.Misra UK, Chu CT, Gawdi G, Pizzo SV. The relationship between low density lipoprotein-related protein/alpha 2-macroglobulin (alpha 2M) receptors and the newly described alpha 2M signaling receptor. J Biol Chem. 1994;269:18303–18306. [PubMed] [Google Scholar]
- 30.Odom AR, Misra UK, Pizzo SV. Nickel inhibits binding of alpha2-macroglobulin-methylamine to the low-density lipoprotein receptor-related protein/alpha2-macroglobulin receptor but not the alpha2-macroglobulin signaling receptor. Biochemistry. 1997;36:12395–12399. doi: 10.1021/bi970806k. [DOI] [PubMed] [Google Scholar]
- 31.Misra UK, Pizzo SV. Ligation of alpha 2M receptors with alpha 2M-methylamine stimulates the activities of phospholipase C, phospholipase A2, and protein kinase C in murine peritoneal macrophages. Ann NY Acad Sci. 1994;737:486–489. doi: 10.1111/j.1749-6632.1994.tb44347.x. [DOI] [PubMed] [Google Scholar]
- 32.Misra UK, Pizzo SV. Ligation of the alpha2M signalling receptor elevates the levels of p21Ras-GTP in macrophages. Cell Signal. 1998;10:441–445. doi: 10.1016/s0898-6568(97)00171-x. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Gronow M, Cuchacovich M, Llanos C, Urzua C, Gawdi G, Pizzo SV. Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 2006;66:11424–11431. doi: 10.1158/0008-5472.CAN-06-1721. [DOI] [PubMed] [Google Scholar]
- 34. Hart JP, Pizzo SV.α-Macroglobulins and kunins. In: Hemostasis and Thromboses: Basic Principles and Clinical Practice. RW Colman, AW Clowes, SZ Goldhaber, VJ Marder, JN George (eds). Lippincott, Williams & Wilkins, Baltimore. 367–379.2006.