Abstract
The placenta grows rapidly for a short period with high blood flow during pregnancy and has multifaceted functions, such as its barrier function, nutritional transport, drug metabolizing activity and endocrine action. Consequently, the placenta is a highly susceptible target organ for drug- or chemical-induced adverse effects, and many placenta-toxic agents have been reported. However, histopathological examination of the placenta is not generally performed, and the placental toxicity index is only the placental weight change in rat reproductive toxicity studies. The placental cells originate from the trophectoderm of the embryo and the endometrium of the dam, proliferate and differentiate into a variety of tissues with interaction each other according to the development sequence, resulting in formation of a placenta. Therefore, drug- or chemical-induced placental lesions show various histopathological features depending on the toxicants and the exposure period, and the pathogenesis of placental toxicity is complicated. Placental weight assessment appears not to be enough to evaluate placental toxicity, and reproductive toxicity studies should pay more attention to histopathological evaluation of placental tissue. The detailed histopathological approaches to investigation of the pathogenesis of placental toxicity are considered to provide an important tool for understanding the mechanism of teratogenicity and developmental toxicity with embryo lethality, and could benefit reproductive toxicity studies.
Keywords: placental hypertrophy, placental pathology, rat, small placenta
Introduction
The placenta grows rapidly and exhibits marked changes in morphological structure according to fetal development. Although the placenta is a temporary organ, it is an interface between the dam and developing embryos/ fetuses and a multifaceted organ that performs a number of important functions that are modified throughout gestation. These functions include anchoring the developing fetus to the uterine wall, mediating maternal immune tolerance, O2/ CO2 exchange, providing nutrients for the fetus and removing waste products during embryonic development1. It also secures the embryo/fetus to the endometrium as a protective barrier against xenobiotics and releases a variety of steroids, hormones and cytokines. Therefore, placental dysfunction and injury have adverse effects on the maintenance of pregnancy, and fetal growth and development. Drug- or chemical-induced histopathological changes of the placenta in rats are important in safety evaluation to understand the mecha nism of teratogenicity and developmental toxicity. However, the placenta has not received proper consideration as a target organ in safety evaluation of the risks for dams and embryos/fetuses. Morphological or histopathological evaluation of placental development and abnormalities has been scarce and incomplete in experimental animals. The present review describes an overview of the normal placental structure and the histopathology of some drug- or chemical-induced placental lesions and the relationship between fetal intrauterine growth restriction (IUGR) and a small placenta in rats.
Placentation in Mammals
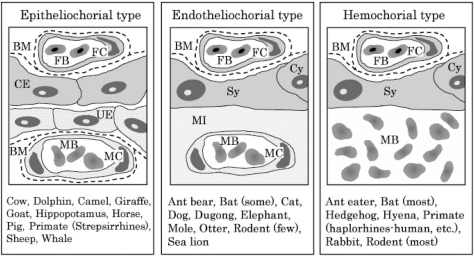
In mammals, a yolk sac placenta and chorioallantoic placenta, are present during gestation. The yolk sac actively absorbs nutrients from the chorion and chorionic cavity, transports them to the embryo through the yolk sac circulation, and plays a role as a transient placenta during early post-implantation before the allantoic circulation is established2,3. In most mammals including humans, the yolk sac placenta becomes vestigial after the first trimester. On the other hand, the inverted yolk sac eventually covers the fetus and contributes to the special functions as a yolk sac placenta before parturition in rodents and rabbits. The chorioallantoic placenta is the principal placenta in mammals during middle to late-gestation and is formed from the endometrium of the dam and the trophectoderm of the embryo. The definitive chorioallantoic placenta shows a variety of different shapes between species4, such as diffuse (horse, pig), discoid (human, rodent), zonary (dog, cat) and multicotyledonary (cow, sheep). Furthermore, the three main types are recognized according to the relationship established between the chorion and uterine wall5,6: (1) epitheliochorial type (horse, pig, cow), (2) endotheliochorial type (dog, cat) and (3) hemochorial type (human, rodent; Fig. 1).
Fig.1.
Placental classification of chorioallantoic placentas according to the relationship established between the chorion and uterine wall. The remaining fetal components include three layers (trophoblast, basement membrane and fetal capillary), whereas the maternal components are reduced step by step. BM, basement membrane; CE, chorionic epithelium (trophoblasts); Cy, cytotrophoblast; FB, fetal blood; FC, fetal capillary; MB, maternal blood; MC, maternal capillary; MI, maternal interstitium; Sy, syncytio trophoblast;UE,uterineepithelium.(Figuremodifiedfrom that of Burton et al.6)
Normal Development and Structure of the Chorioallantoic Placenta in Rats
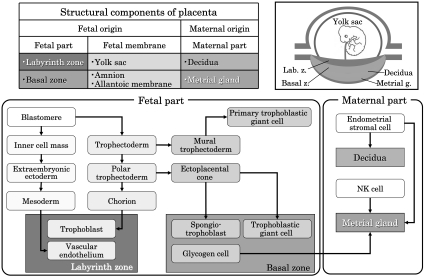
The rat chorioallantoic placenta morphologically has a discoid shape and is classified into the hemochorial type. Histologically, the maternal part of the placenta consists of the decidua and metrial gland. The fetal part of the placenta consists of the labyrinth zone and basal zone (Fig. 2). In addition, there are two distinct layers of membranes that enclose the fetus. The outer layer is the yolk sac, and the inner layer is the amnion.
Fig. 2.
Structural components and differentiation of the rat placenta.
Development of the chorioallantoic placenta
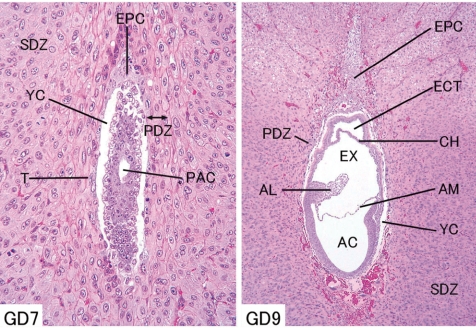
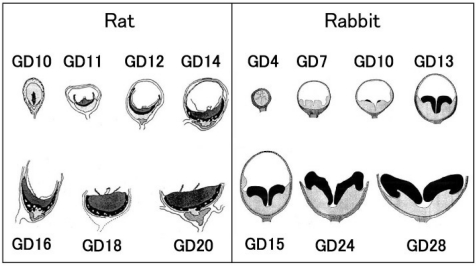
The trophectoderm, which differentiates into the placenta, consists of the mural trophectoderm and polar trophectoderm (Fig. 2). The mural trophectoderm surrounding the blastocyst cavity arrests cell division and differentiates into primary trophoblastic giant cells just after implantation. The polar trophectoderm neighboring the embryoblast forms the ectoplacental cone and invades into the decidua (Figs. 3, 4). The edge and center of the ectoplacental cone differentiate into (secondary) trophoblastic giant cells and spongiotrophoblasts, respectively, and then these cell masses form the basal zone7. The chorion, which is the embryonic-side membrane of the ectoplacental cone, fuses with the allantois derived from the embryonic hindgut and then differentiates into the labyrinth zone. In the endometrium, the decidual cells develop from the endometrial stromal cells by stimulation of blastocyst apposition (decidualization) and form the basic structural matrix of the decidua. The decidua rapidlygrowsandfillsuptheuterinelumen,causinginflammation, edema, congestion, and hemorrhages. The metrial gland is composed of nodular aggregates of heterogeneous tissue that develops in the mesometrial triangle in the uterine wall. A schematic view of placental development in rats and rabbits is shown in Fig. 58,9.
Fig. 3.
Rat embryo and placenta. (Left - GD 7, right - GD 9, HE stain) AC, amniotic cavity; AL, allantois; AM, amnion; CH, chorion; ECT, ectoplacental cavity; EPC, ectoplacental cone; EX, extraembryonic coelom; PAC, proamniotic cavity; PDZ, primary decidual zone; SDZ, secondary decidual zone; T, trophectoderm; YC, yolk sac cavity.
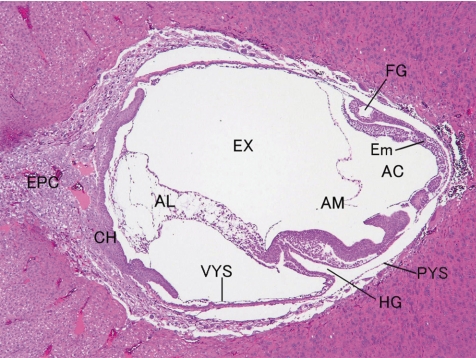
Fig. 4.
Rat embryo and placenta (GD 10, HE stain). AC, amniotic cavity; AL, allantois; AM, amnion; CH, chorion; Em, embryo; EPC, ectoplacental cone; EX, extraembryonic coelom; FG, foregut pocket; HG, hindgut pocket; PYS, parietal layer of yolk sac; VYS, visceral layer of yolk sac.
Fig. 5.
Placental development schema (Left - rat; right - rabbit). Black area shows the fetal part of the placenta. (Figure modified from those of Davies et al.8 and Hafez et al.9.)
Normal structure of the chorioallantoic placenta
(1) Fetal part of the placenta
i) Labyrinth zone
The labyrinth zone contains the maternal sinusoids and the trophoblastic septa, which are composed of the trilaminar trophoblastic epithelium and fetal capillary (Fig. 6). The maternal sinusoids full of maternal blood pass between the trophoblastic septa without an endothelium. The trophoblast epithelium, which comes into direct contact with the maternal blood, is referred to as the cytotrophoblast (Fig. 7). The cytotrophoblast can be easily discerned by its large spherical nucleus with prominent nucleolus. It displays numerous microvilli on its surface and contains many pinocytotic vesicles at the basal position. Under this trophoblast layer, there are two layers of syncytiotrophoblasts (syncytiotrophoblast I and syncytiotrophoblast II from the maternal sinusoid side). Gap junctions are present between these two syncytiotrophoblast layers. Basal laminas are located between the syncytiotrophoblast II layer and the fetal capillary endothelium. The continuity of these syncytiotrophoblasts layers provides a placental barrier10. The fetal capillaries are the fenestrated type. The pores may contribute to the high permeability of the fetal capillary11. Maternal and fetal blood come very close together, and most of the maternofetal exchange of substances is carried out in the labyrinth zone. The proliferative activity of these trophoblasts peaks in midgestation and reduces gradually toward late gestation. The labyrinth zone becomes a major part of the placenta with pregnancy progression, although other parts of the placenta regress after midgestation (Figs. 5, 8).
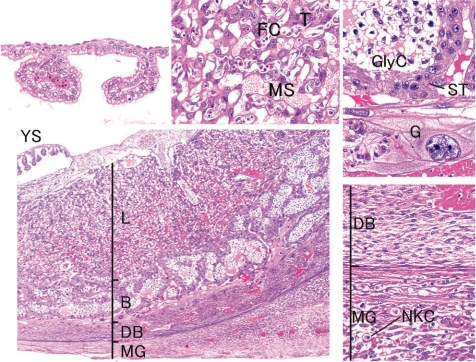
Fig. 6.
Rat placenta (GD 15, HE stain, lower left, low magnification; lower right, decidua basalis; higher left, yolk sac; higher middle, labyrinth zone; higher right, basal zone). B, basal zone; DB, decidua basalis; FC, fetal capillary; G, trophoblastic giant cell; GlyC, glycogen cell; L, labyrinth zone; MG, metrial gland; MS, maternal sinusoid; NKC, uterine natural killer cells; ST, spongiotrophoblast; T, trophoblast; YS, yolk sac.
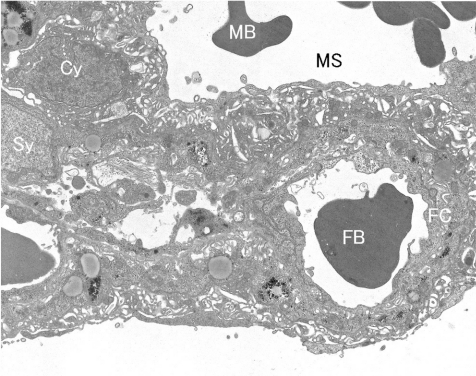
Fig. 7.
Rat placental ultrastructure (GD 17). Cy, cytotrophoblast; FB, fetal blood; FC, fetal capillary; MB, maternal blood; MS, maternal sinusoid; Sy, syncytiotrophoblast.
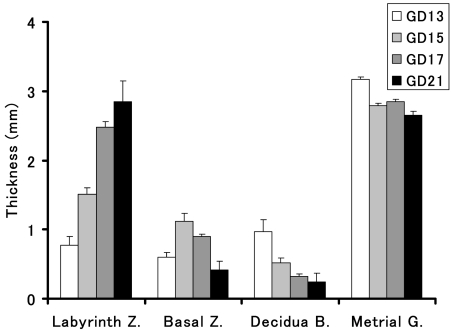
Fig. 8.
Time-dependent change in the thickness of each placental layer in the rat.
ii) Basal zone (Junctional zone)
The basal zone is comprised of three differentiated cells: (1) spongiotrophoblast cells, (2) trophoblastic giant cells and (3) glycogen cells (Figs. 2, 6). The spongiotrophoblast cells are located immediately above the trophoblastic giant cell layer and are the main structural component of the basal zone. The trophoblastic giant cells located at the maternal-placental interface are one of the major endocrine cells of the placenta. They synthesize and secrete hormones/ cytokines belonging to the prolactin family12–14 Glycogen cells have been reported to appear to be derived from the spongiotrophoblasts. However, recent evidence may suggest that glycogen cells are distinct from spongiotrophoblasts15,16. Glycogen cells are transiently detected in the basal zone in midgestation. They form multiple small cell masses and glycogen cell islands, and most of them disappear at the end of pregnancy. Their biological function is not well understood, but it appears to be related with glycogen metabolism. The trophoblasts originated from the glycogen cells invade into the decidua and metrial gland as interstitial invasion with an extensive mushroom-like spreading. The basal zone forms channels draining the maternal blood from the placenta, but fetal capillaries do not penetrate into the basal zone.
(2) Maternal part of the placenta
i) Decidua
Decidual cells surrounding the blastocyst initially form the primary decidual zone, which is avascular and densely packed with decidual cells. Subsequently, the more loosely packed decidual cells around the primary decidual zone form the secondary decidual zone17 (Fig. 3). The primary decidual zone degenerates progressively, and placental and embryonic growth slowly replace the secondary decidual zone, which is reduced to a thin layer called the decidua capsularis and decidua parietalis. The mesometrial decidual cells ultimately form only a thin layer at the base of the placenta called the decidua basalis, which is an important site for maternal angiogenesis. The decidua basalis includes newly developed blood vessels, which play essential roles in the development of vascularized decidual-placental interface18. The decidua can produce a wide range of hormones, cytokines, growth factors and immunomodulatory moleculesinvolvedintherecruitmentofthelimitedbutspecific immune cell populations and growth of the placenta19,20.
ii) Metrial gland
The metrial gland is a normal structure located in the mesometrial triangle of the pregnant uterus from early gestation and is fully developed in midgestation, leading to regression before parturition21. The metrial gland is composed of a dynamic mixed cell population of decidualized endometrial stromal cells, uterine natural killer (uNK) cells, spinal-shaped arteries and fibroblasts22 (Fig. 6). UNK cells belong to a family of natural killer (NK) cells. They are recruited after conception, rapidly divide and differentiate to a phenotype that is different from that of the circulating NK cells in the metrial gland23. UNK cells play an important immunological role in their tolerogenic form24. In addition, two types of trophoblasts invade from the fetal part into the metrial gland: endovascular trophoblasts and interstitial trophoblasts. The former enter uterine blood vessels where they can replace endothelial cells, and invade to the metrial gland from GDs 13–1425. The latter penetrate through the uterine stroma from GD 15 and are often situated in perivascular locations26. The endovascular and interstitial trophoblast migrating into the metrial gland express a subset of members of the prolactin gene family12. The invaded endovascular trophoblasts and uNK cells are necessary for spinal-shaped arterial remodeling27,28.
(3) Fetal membrane
i) Amnion
The amnion develops from the membrane that crosses between the exocoelom and amniotic cavity. Turning of the embryo induces the amnion to become enlarged, and then the amnion surrounds the whole embryo and forms the amniotic raphe after midline fusion29. The amnion consists of a single layer of flattened ectodermal cells and some connective tissue. The amniotic fluid allows free movement of the fetus during the later stages of pregnancy and protects it by diminishing the risk of injury.
ii)Yolk sac
The yolk sac develops from the membrane lining the exocoelom, becomes enlarged and surrounds the whole embryo like the amnion. It is divided into two parts: (1) the visceral yolk sac surrounding the embryo with the amnion and (2) the parietal yolk sac apposed to the chorion (Fig. 6). Because the parietal yolk sac ruptures in midgestation, the inside of the visceral yolk sac becomes exposed to the intrauterine cavity and is called a reversed yolk sac placenta. The yolk sac consists of epithelial cells and mesodermal cells. In addition, the parietal yolk sac is lined with Reichert’s membrane, which is a rodent-specific and acellular thin membrane. Blood islands are formed on the surface of the yolk sac and subsequently develop into the yolk sac circulation.
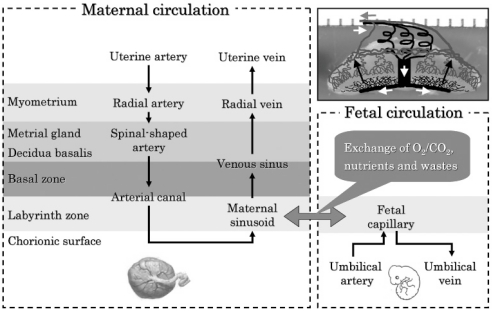
Placental circulation
The maternal circulation through the placenta is as follows (Fig. 9); the maternal arterial supply to the placenta originates from radial arteries, which enter the uterus through the myometrium on the mesometrial side of the uterus. Branches of the radial arteries either pass laterally through the myometrium or traverse the myometrium and enter the metrial gland. Then these arteries branch into several spinal-shaped arteries. After traversing the decidua basalis, the spiral arteries converge to form a small number of centrally located arterial canals. The arterial canals turn around at the surface and lead into the trophoblast-lined maternal sinusoid spaces in the labyrinth zone. Maternal blood drains from the labyrinth through venous sinuses that cross the basal zone into the decidua basalis. The venous sinuses traverse the outer region of the metrial gland and exit into the radial veins outside the myometrium18.
Fig. 9.
Placental circulation in the rat.
Placental Toxicological Evaluation
Comparison between rats and humans
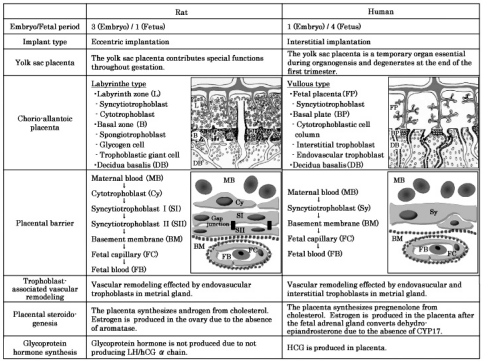
Generally, rat placental models have been useful for evaluating the potential of drugs or chemicals that affect human reproductive development, since there are several similarities between rats and humans in early placental development30. However, there are some differences between rats and humans31,32, such as the embryo/fetal period ratio, implantation type, function of the yolk sac placenta, placental structure and endocrine synthesis (Fig. 10). Particularly, it is suggested that rat placental models are unsuitable for evaluating the potential effects of drugs or chemicals on the human reproductive system and developmental toxicity induced by the alteration of placental endocrine functions, because estrogen biosynthesis during pregnancy in humans is much different from that in rats33. Thus, extrapolating data from rats to humans in drug- or chemical-induced developmental toxicity should be done based on fully understanding the differences and similarities between the rat and human placenta.
Fig. 10.
Placental comparison between the rat and human.
Toxicological significance of the placenta
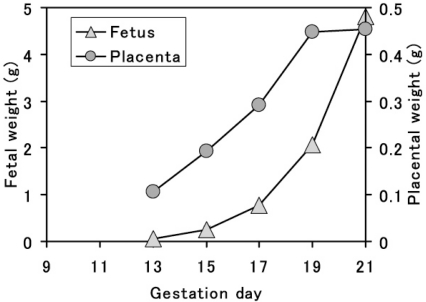
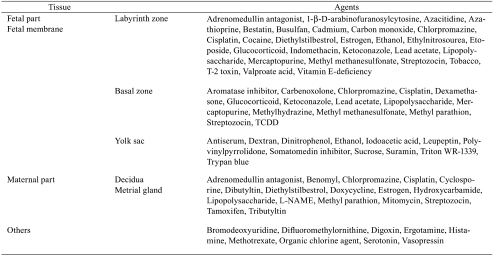
The placenta grows rapidly for a short period with high blood flow during pregnancy (Fig. 11) and has multifaceted functions, such as its barrier function, nutritional transport, drug metabolizing activity and endocrine action. Consequently, the placenta is a highly susceptible target organ for drug- or chemical-induced adverse effects, and many placenta-toxic agents have been reported (Table 1). On the other hand, the dam and fetus have a close relationship with each other via the placenta and form the maternal-fetal-placental unit in mammalian embryonic development. Drug- or chemical-induced placental functional depression and injury subsequently result in abnormal fetal growth or devel-opment leading to fetal resorption or teratogenicity. Thus, the placenta is an important organ for evaluating embryonic developmental toxicity and understanding its mechanism34. However, histopathological examination of the placenta is not generally performed, and the placental toxicity index is only the placental weight change in rat reproductive toxicity studies. As we note below, some drug- or chemical-induced placental histopathological lesions have been described from the point of view of placental weight changes.
Fig. 11.
Time-dependent change in placental and fetal weight in the rat.
Table 1. Placental Toxic Agents.
Histopathology of the Placenta in the Rat
Lesion associated with an increase in organ weight
(1) Hypertrophy
An increase in placental weight is macropathologically observed as placental hypertrophy. Firstly, placental hypertrophy is induced as a compensatory reaction to IUGR under the conditions of a slightly unfavorable maternal environment, such as maternal hemorrhage35, uterine vessel ligation36 and carbon monoxide exposure37. In spontaneously hypertensive rats, it is induced in response to a poor capacity of the uteroplacental unit for transferring glucose to fetuses38. Drug- and chemical-induced compensatory placental hypertrophy is reported in indomethacin-exposed rats39 and ethanol-exposed rats40,41. Secondly, placental hypertrophy is detected in the intact uterus with a decreased number of corpora luteum, implantation sites and fetuses (less than six fetuses in rats)42 as an implantation-related reaction. Thirdly, placental hypertrophy is induced as a hormone imbalance reaction, such as estrogen deficiency43,44 and ovariectomy with estrogen and progesterone treatment45. Pathological sequential changes of ketoconazoleinduced placental hypertrophy were recently investigated in rats46, as described below.
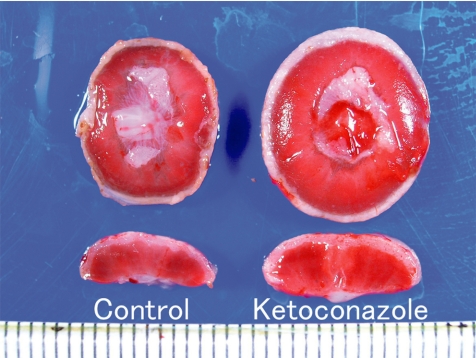
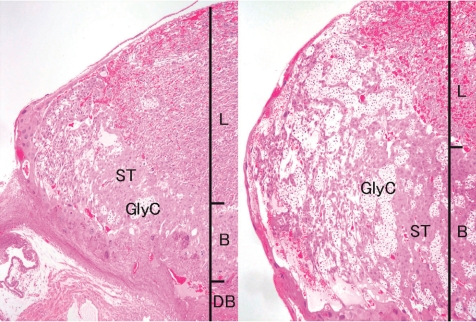
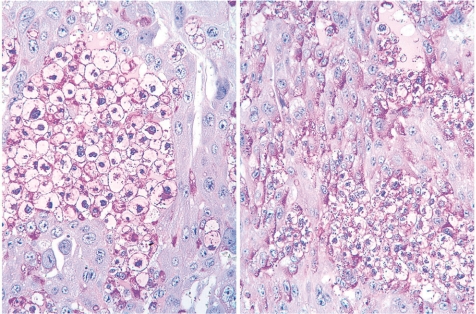
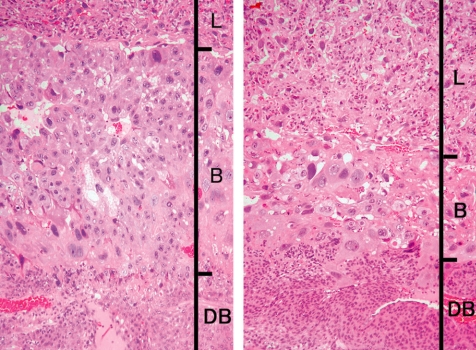
Ketoconazole, an imidazole antifungal compound, was orally administered at doses of 0 and 25 mg/kg/day during gestation days (GDs) 12 to 14, and placentas were sampled on GDs 15, 17 and 21. There were no effects on the fetal mortality rates at each sampling time. No effects on fetal weight or no macroscopic fetal abnormalities were detected on GD 21. The placentas in the ketoconazole-treated group appeared more hypertrophic with increases in their weight, diameter and/or thickness from GD 15 onward (Fig. 12). Histopathologically, increased thickness was noted in the labyrinth zone and basal zone on GDs 17 and 21, while the change was already evident in the former zone on GD 15. In the labyrinth zone, the mitotic figures of trophoblasts were elevated on GD 15. A multiple cystic dilatation of maternal sinusoids was observed in some placentas on GDs 15, 17 and 21. In the basal zone, an increase in the number of spongiotrophoblasts and clusters of glycogen cells were detected on GDs 17 and 21. These changes were particularly remarkable at the edge of the basal zone (Fig. 13). In the decidua, there were no significant changes in either histology or thickness between the control and the ketoconazole-treated group during GDs 15 to 21. Estrogen is a known inhibitor of placental growth, and its deficiency induces placental hypertrophy43,44. Ketoconazole inhibits 17α-hydroxylase/C17,20lyase and aromatase activity in the steroid biosynthesis pathway47,48. Administration of estrogen inhibits ketoconazole induced-placental hypertrophy in rats49. In addition, overgrowth of the basal zone is detected in pregnant rats ovariectomized and supplied with estrogen and progesterone45, and this may be induced as a response to hormonal imbalance. Therefore, it is suggested that ketoconazole administration in pregnant rats induces placental hypertrophy, which is attributed to the overgrowth of the labyrinth zone and basal zone by inhibition of estrogen synthesis and hormonal imbalance.
Fig. 12.
Placental hypertrophy (Rat, GD 21, left - control; right - ketoconazole). Ketoconazole treatment.
Fig. 13.
Thickening of the basal zone (Rat, GD 17, HE stain, left - control; right - ketoconazole). Ketoconazole treatment. B, basal zone; DB, decidua basalis; GlyC, glycogen cell; L, labyrinth zone; ST, spongiotrophoblast.
Lesions associated with a decrease in organ weight
A decrease in placental weight is macropathologically observed as a small placenta. Mitotic inhibition, apoptosis, degeneration and/or necrosis of trophoblasts, which are induced by direct placental injury or nonspecific effects associated with the conditions of an excessively unfavorable maternal environment, result in the inhibition of placental development, leading to a small placenta.
(1) Necrosis/degeneration of trophoblasts
Placental necrosis macroscopically shows thinning, discoloration, hemorrhage, white spots or adherence of the yolk sac on the chorion surface (Fig. 14). In animal experiments, placental necrosis is induced by such things as valproate acid50, chlorpromazine51, glucocorticoid52, streptozotocin53, cadmium54, ethanol40, lead acetate55, diethylstilbestrol, estrogen, tobacco, adrenomedullin antagonist, cocaine and vitamin E-deficiency. Histopathologically, placental necrosis appears more commonly in the trophoblasts in the labyrinth zone. There is a reduction in thickness and disruption of the trophoblastic septa and irregular dilatation of maternal sinusoids with hemorrhage, fibrin deposition and inflammatory cell migration, leading to fibrosisand calcification (Fig. 15). In the cadmium-exposed placenta, expression of metallothionein is detected in the necrotic area in the labyrinth zone (Fig. 16), although the main expression site of metallothionein is in the decidua and yolk sac surrounding the embryo/fetus throughout gestation56.
Fig. 14.
Placental necrosis. Discoloration of the placenta and adherence of the yolk sac to the chorionic surface (Rat, GD 19). Cadmium chloride treatment.
Fig. 15.
Calcificationandirregulardilatationofthematernalsinusoid in the labyrinth zone (Rat, GD 21, HE stain). Lead acetate treatment.
Fig. 16.
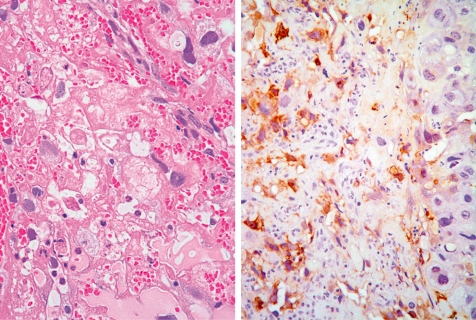
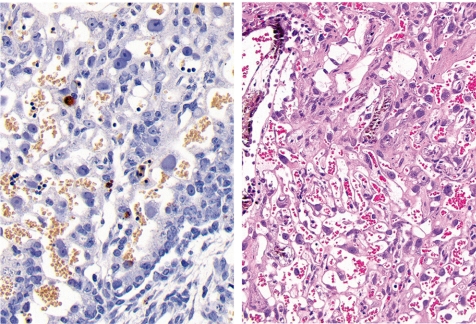
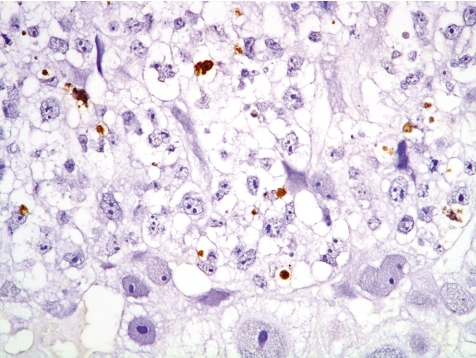
Left - extensive necrosis in the labyrinth zone (Rat, GD 19, HE stain). Right - expression of metallothionein in cad-mium-damaged trophoblasts in the labyrinth zone (Rat, GD 19, metallothionein immunostain). Cadmium chloride treatment.
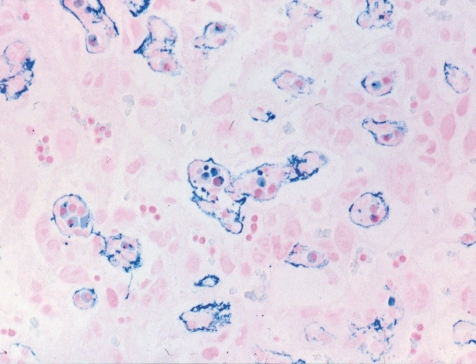
Among other specific changes, fetal sideroblastic anemia resulting from hemoglobin synthesis inhibition can induce iron deposition not only in the erythroblasts, but also in the trophoblastic septa (Fig. 17).
Fig. 17.
Iron deposition in fetal erythroblasts and trophoblastic septa in the labyrinth zone (Rat, GD 13, Berlin blue stain). Compound A treatment.
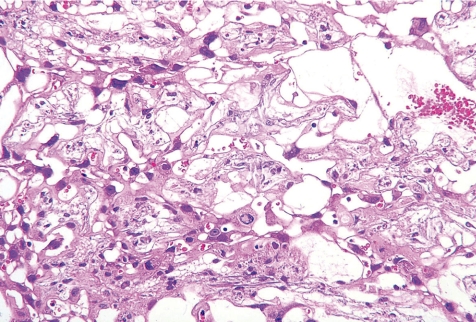
(2) Cystic degeneration of glycogen cells
Cystic degeneration of glycogen cells is the condition describing abnormal retention of extensive cytoplasmic vacuolation within glycogen cells. The vacuoles contain eosinophilic fibrinous material and polymorphs. The degenerated cells undergo cytolysis and subsequently coalesce into multiple large cysts that are filled with a homogeneous acidophilic mass and multiple clusters of residual glycogen cells, macrophages, erythrocytes and cell debris (Fig. 18). The degenerated cells did not undergo regression, although most glycogen cells disappear at the end of pregnancy in normal development. In animal experiments, cystic degeneration of glycogen cells is induced by such things as chlorpromazine51, streptozotocin53, 6-mercaptopurine (6-MP)57 and TCDD58.
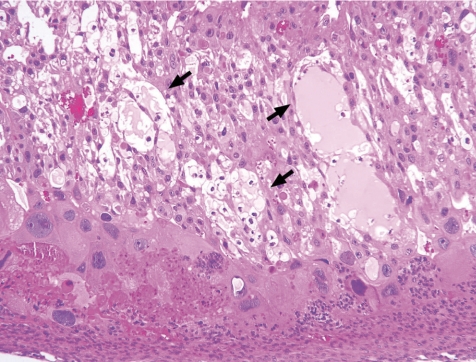
Fig. 18.
Thickening of the basal zone with cytolysis of glycogen cells(↑)(Rat, GD21, HE stain). Treatment with 6-MP.
(3) Apoptosis/mitotic inhibition of trophoblasts
Placental apoptosis can be detected in both endothelial cells, trophoblasts and stromal cells of normal placental tissue, and is believed to be a part of normal developmental placental aging59. However, placental apoptosis is also increased in spontaneous abortion, preeclamptic pregnancies, post-term pregnancies and pregnancies complicated with IUGR60–62. It is known that trophoblasts in the fetal part of the placenta are a common toxicological target tissue for some drugs and chemicals, because they have high proliferative activity and constitute a major structural component of the fetal part of the placenta. Trophoblast apoptosis leads to a lack of cell populations required for later normal histogenesis, resulting in a small placenta. In animal experiments, placental apoptosis is induced by such things as glucocorticoid52,63, lipopolysaccharide64, T-2 Toxin65, anoxia and some anticancer drugs. Particularly, anticancer drugs, such as ethylnitrosourea66 and 1-β-D-arabinofuranosylcytosine67 induce trophoblastic apoptosis and/or mitotic inhibition by an increase in p53 expression in response to DNA damage. However, anticancer drug-induced histopathological lesions differ depending on the drugs and the exposure period. The pathological sequential changes of the small placenta in rats treated with busulfan68, 6-MP57 and cisplatin (not yet published) were recently investigated, as described below.
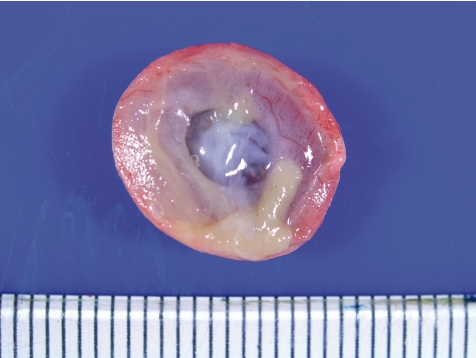
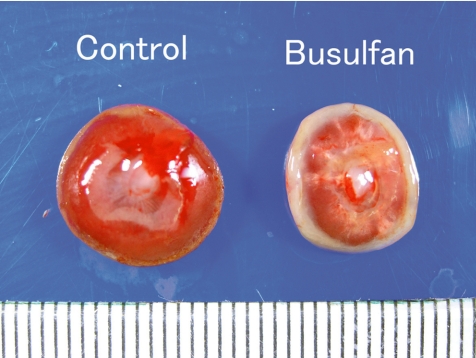
Busulfan, an alkylating agent, is a known teratogen, inducing anophthalmia, microtia, microrostellum, micrognathia, microabdomen, micromelia, oligodactylia, brachydactylia, vestigial tail, short tail, anasarca, microencephaly, microphthalmia, and cataract in rats69. In the present study, busulfan was intraperitoneally administered at doses of 0 and 10 mg/kg/day during GDs 12 to 14, and placentas were sampled on GDs 13.5, 14.5, 15, 16 and 21. There were no effects on the fetal mortality rates at each sampling time. Macroscopically, fetal dwarfism was observed with reduced body weight and kinky tail in the busulfan-treated group. The placentas decreased in weight and were shown macroscopically to be small and thin with scattered white spots and a white peripheral rim on GD 21 (Fig. 19). Histopathologically, busulfan treatment provoked increased apoptosis (Fig. 20) and decreased mitotic activities of the trophoblasts in the labyrinth zone on GDs 13.5, 14.5, 15 and 16. Degeneration and necrosis of the trophoblasts, a diminution in thickness of the trophoblastic septa with deposition of calcium and irregular dilation of the maternal sinusoids were scattered in the labyrinth zone (Fig. 20), although there were no conspicuous changes in the basal zone. A reduction in diameter in the labyrinth zone was detected on GD 21. From these results, it is suggested that busulfan induces cell cycle arrest in the G1/G2-phase and DNA damage in trophoblasts, leading to apoptosis and mitotic inhibition in the labyrinth zone. It is reported that the proliferative period of spongiotrophoblasts in the basal zone diminishes earlier and is narrower than that of trophoblasts in the labyrinth zone70. Therefore, the difference in sensitivity to busulfan between the labyrinth zone and the basal zone appears to be attributed to the difference of each cellular proliferation period with advancing pregnancy. It is considered that busulfan administration in pregnant rats induces growth arrest of the labyrinth zone, leading to a small placenta.
Fig. 19.
Small placenta (Rat, GD 21, left - control; right - busulfan). Busulfan treatment.
Fig. 20.
Left - apoptosis of trophoblasts in the labyrinth zone (Rat, GD 15, TUNEL stain). Right - degeneration and necrosis of trophoblasts with deposition of calcium in the labyrinth zone (Rat, GD 21, HE stain). Busulfan treatment.
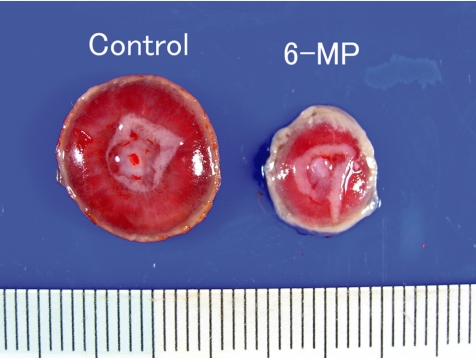
The purine antimetabolite 6-MP is a known teratogen, inducing limb defects, micrognathia, ventral hernia, skeletal, urogenital, CNS and ocular anomalies, cleft palate and diaphragmatic hernia. In the present study, 6-MP was intraperitoneally administered at doses of 0 and 60 mg/kg/day during GDs 11 to 12, and placentas were sampled on GDs 13, 15 and 21. The fetal mortality rates increased up to 40%, and some malformations of fetuses (as referred to above) were detected on GD 21 in the 6-MP-treated group. The fetal and placental weights were decreased on GDs 15 and 21. Macroscopically, the placentas on GD 21 were small, brittle and thin with a white peripheral rim (Fig. 21). Histopathologically, 6-MP treatment mainly evoked decreased mitosis on GDs 13 and 15, and increased apoptotic cells on GDs 13, 15 and 21 in the labyrinth zone (Fig. 22). There were decreased trophoblasts, a diminution in the thickness of the trophoblastic septa and irregular dilatation of maternal si nuses with deposition of fibrin on GD 21 (Fig. 22). In the basal zone, there were decreased mitotic spongiotrophoblasts on GD 13 and increased apoptotic cells on GD 21. PAS-positive material in the spongiotrophoblasts on GD 15 was still detected in the 6-MP-treated group, but not in the control group (Fig. 23). The clusters of glycogen cells consisted of small and irregular-shaped cells as compared with the controls. Because most of the PAS-positive material in the spongiotrophoblasts disappears after GD 14 in normal development8, spongiotrophoblast development and differentiation appear to be delayed in the 6-MP-treated group. Furthermore, cytolysis of glycogen cells (cystic degeneration of glycogen cells), apoptosis and a subinvolution of spongiotrophoblasts were observed on GD 21 (Fig. 18). The thickness of the basal zone was increased on GD 21, as a result of the cystic degeneration of glycogen cells, although the labyrinth zone was reduced in diameter on GDs 15 and 21. Therefore, it is considered that 6-MP administration in pregnant rats induces growth arrest of the labyrinth zone, leading to a small placenta. In addition, 6-MP provokes a delay in the developmental process of the basal zone and cystic degeneration of glycogen cells.
Fig. 21.
Small placenta (Rat, GD 21, left - control; right - 6-MP). Treatment with 6-MP.
Fig. 22.
Left - Apoptosis of trophoblasts in the labyrinth zone (Rat, GD 13, HE stain). Right - decreased number of trophoblasts, a reduction in thickness of trophoblastic septa and irregular dilatation of maternal sinusoids with deposition of fibrin (Rat, GD21, HE stain). Treatment with 6-MP.
Fig. 23.
Increased PAS-positive material in spongiotrophoblasts around clusters of glycogen cells (Rat, GD 15, PAS stain, left - control; right - 6-MP). Treatment with 6-MP.
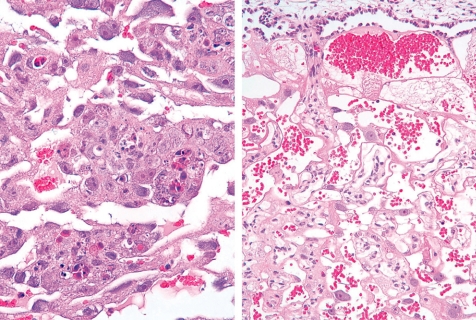
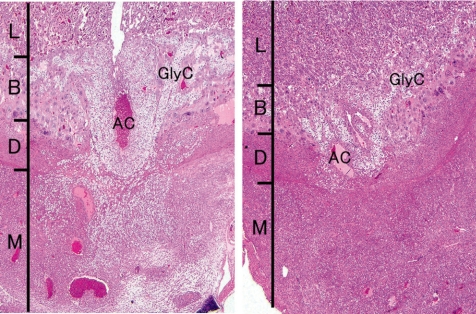
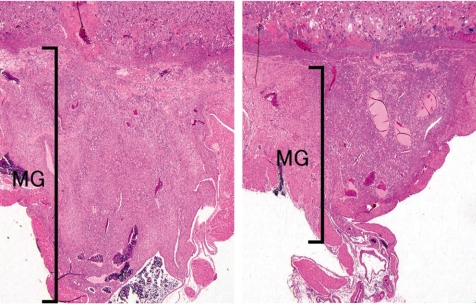
Cisplatin, a platinating agent, is considered to be highly embryo lethal and growth retardant but to not be a teratogen in rats and rabbits71. Cisplatin can pass through the placental barrier and is a transplacental carcinogen for the fetal liver, kidney, nervous system and lung in pregnant rats72. In the present study, cisplatin was intraperitoneally administered at 2 mg/kg/day during GDs 11 to 12 (GD11,12-treated group) or GDs 13 to 14 (GD13,14-treated group), and the placentas were sampled on GDs 13, 15, 17 and 21. Fetal mortality rates were increased up to 65% from GD 17 onward, and fetal weights were decreased on GD 21 in the GD11,12-treated group. However, there were no effects on fetal mortality rates and fetal weight in the GD13,14-treated group. There were no macroscopic fetal abnormalities on GD 21 in either treated groups. A reduction in placental weight was detected from GD 15 onward, and the placentas on GD 21 were macroscopically small and thin with a white peripheral rim in both treated groups. Histopathologically, an increase in apoptotic cells was detected in the labyrinth zone during the experimental period and in the basal zone on GD 21, and then labyrinth zone hypoplasia was induced in the GD13,14-treated group. By contrast, an increase in apoptotic cells was detected on GDs 13, 15 and 17 in the labyrinth zone, and during the experimental period in the basal zone (Fig. 24), and then hypoplasia of the labyrinth and basal zones was induced in the GD11,12-treated group (Fig. 25). In addition, a marked decrease in glycogen cell islands in the basal zone was also detected on GDs 15 and 17 in this group (Fig. 26). There was a reduction in interstitial invasion of glycogen cell-like trophoblasts along the arterial canal into the metrial gland on GD 15 (Fig. 26) and metrial gland hypoplasia from GD 17 onward (Fig. 27). Consequently, trophoblastic apoptosis in the basal zone, including pre-glycogen cells leads to a lack of the cell populations required for normal development into glycogen cells and then inhibits the interstitial invasion of glycogen cells into the decidua and metrial gland, resulting in metrial gland hypoplasia. On the other hand, in the 6-MP-administrated rats on GDs 11 and 12, there was no detected basal zone hypoplasia, although labyrinth zone hypoplasia was induced57. These results suggest that the basal zone has not only an earlier and narrower sensitive period but also higher specificity for the toxicity of anticancer drugs, compared with the labyrinth zone. Therefore, it is considered that cisplatin administration in pregnant rats induces trophoblast apoptosis in the labyrinth and basal zones, leading to a small placenta. In addition, metrial gland hypoplasia occurs secondary to the failure of glycogen cell island development.
Fig. 24.
Apoptosis of spongiotrophoblasts in the basal zone (Rat, GD 15, TUNEL stain). Cisplatin treatment.
Fig. 25.
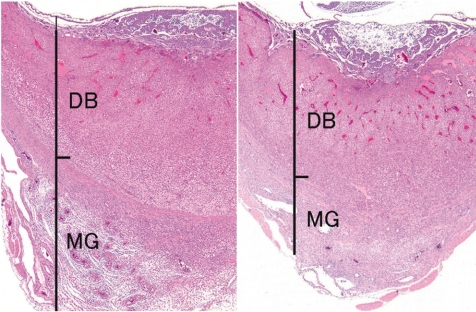
Basal zone hypoplasia (Rat, GD 21, HE stain, left - control; right - cisplatin). Cisplatin treatment. B, basal zone; DB, decidua basalis; L, labyrinth zone.
Fig. 26.
Decrease in glycogen cell islands and inhibition of interstitial invasion of glycogen cell-like trophoblasts into metrial glands (Rat, GD 15, HE stain, left - control; right - cisplatin). Cisplatin treatment. AC, arterial canal; B, basal zone; D, decidua basalis; GlyC, glycogen cell; L, labyrinth zone; M, metrial gland.
Fig. 27.
Metrial gland hypoplasia (Rat, GD 21, HE stain, left - control; right - cisplatin). Cisplatin treatment. MG, metrial gland.
Lesions associated without changes in organ weight
(1) Hypoplasia of the decidua/metrial gland
Hypoplasia of the decidua and metrial gland is not reflected in the placental weight, because placentas are stripped off between the basal zone and the decidua basalis at the time of placental weight measurement in developmental toxicity studies. Hypoplasia of the decidua and metrial gland is induced by such things as decidualization inhibition, reduction in the proliferative activity of uNK cells and inhibition of interstitial trophoblast invasion into the metrial gland. In animal experiments, hypoplasia of the decidua and metrial gland is induced as a result of inhibition of matrix metalloproteinases by doxycycline73, suppression of decidual cell proliferation by benzimidazole fungicides74, hydroxyurea75, diethylstilbestrol, mitomycin and ovariectomy76, decreased progesterone levels resulting from by tributyltin77 and inhibition of interstitial trophoblast invasion by cisplatin (described above). The pathological sequential changes in tamoxifen-induced metrial gland hypoplasia78 were recently investigated in rats, as described below.
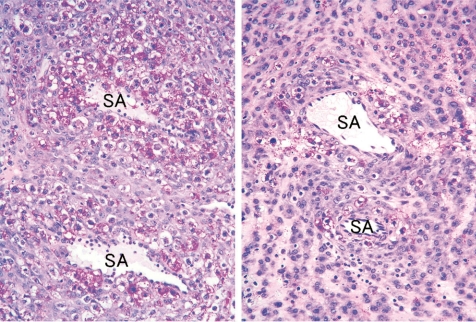
Tamoxifen, a nonsteroid selective estrogen receptor modulator, has been widely used for therapy of estrogen-receptor-positive breast cancer. In the present study, tamoxifen was intraperitoneally administered at doses of 0 and 2 mg/ kg/day during GDs 8 to 10, and placentas were sampled on GDs 11, 13, 15, 17 and 21. The fetal mortality rates in the tamoxifen-treated group were increased up to approximately 50% from GD 15 onward. However, there were no effects on the weights of live embryos/fetuses and their placentas at each sampling time, and there were no macroscopic abnormalities in the fetuses and placentas on GD 21. Histopathologically, the size of the metrial gland in the tamoxifentreated group was reduced at all sampling times compared with the control group (Fig. 28). The spiral arteries appeared less well developed in the hypoplastic metrial gland (Fig. 28). The uNK cells around the spiral arteries were decreased from GD 13 onward in the tamoxifen-treated group (Fig. 29). The number of mitotic cells that appeared to be uNK cells was lower on GDs 11 and 13 in the tamoxifen-treated group. There were no obvious changes in the labyrinth zone, basal zone or decidua basalis. It is known that the development of the metrial gland is a part of decidualization, which is a sequential process of growth and differentiation of uterine stromal cells and uNK cells, and remodeling of the extracellular matrix and maternal vasculature79. In NK gene knock-out mice (TgE26 mice), there was no development of the mesometrial triangle area into the metrial gland, and the reproductive performance was very poor (mortality: 40%), suggesting that uNK cells are necessary for placental growth and gestational success80. The uNK cells are involved in a role of regulation and restructuring of spiral arteries in the metrial gland23,81, and maternal immune tolerance forms toward invading trophoblast cells at the maternal-fetal interface24,27. Alterations of uNK cell function and inadequate remodeling of spiral arteries play an important role in preeclampsia, which leads to high maternal blood pressure, elevated concentrations of urinary protein and poor fetal growth82. Therefore, it is suggested that the antiestrogen effect of tamoxifen inhibits the proliferation of decidualized endometrial stromal cells in the metrial gland and leads to metrial gland hypoplasia resulting from inhibition of proliferative activity of uNK cells and defective development of spiral arteries. Tamoxifen-induced embryo/ fetus-toxicity might be associated with the immune toler ance deficiency caused by decreased uNK cells in metrial gland hypoplasia and/or preeclampsia caused by defective development of spiral arteries.
Fig. 28.
Marked metrial gland hypoplasia with less well development of spiral arteries (Rat, GD 11, HE stain, left - control; right - tamoxifen). Tamoxifen treatment. DB, decidua basalis; MG, metrial gland.
Fig. 29.
Decrease in uNK cells with clear cytoplasm and PAS-positive granules around spiral arteries (Rat, GD 13, PAS stain, left - control; right - tamoxifen). Tamoxifen treatment. SA, spiral artery.
(2) Vacuolar degeneration of the yolk sac epithelium
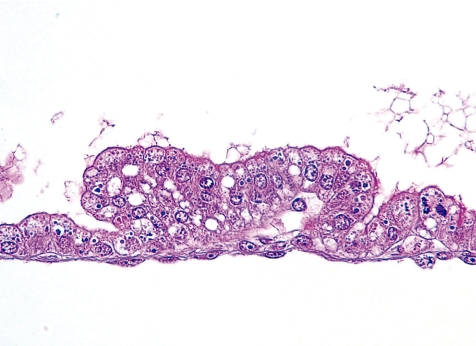
Vacuolar degeneration of the yolk sac epithelium is induced and caused by the accumulation of indigestible material in the vacuolar system by inhibition of intralysosomal proteolysis83. Histopathologically, the visceral yolk sac epithelium contains numerous accumulated vacuoles (Fig. 30). In animal experiments, the vacuolar degeneration of the yolk sac epithelium is induced by such things as trypan blue83, Triton WR-133984, polyvinylpyrrolidone84, dextran84, sucrose84, leupeptin85, somatomedin inhibitor86, ethanol87 and dinitrophenol88. Before the formation of the chorioallantoic placenta, the yolk sac plays a role in the uptake and transport of nutrients from the dam to the developing embryo2,3. Particularly, in the rodent, the functions of the yolk sac placenta are maintained until just before parturition. Thus, yolk sac epithelial damage is correlated with embryonic malformations and fetal developmental toxicity.
Fig. 30.
Vacuolar degeneration of the yolk sac epithelium (Rat, GD 15, HE stain). Trypan blue treatment.
Relationship between Fetal Intrauterine Growth Restriction and Small Placenta
Placenta size, architecture, developmental and pathological processes, and metabolic interaction with the fetus cooperate with placental transport and metabolic mechanisms to qualitatively and quantitatively affect placental-fetal nutrient exchange89. In humans, a positive correlation between placental weight and birth weight is observed in normal and large-for-gestational-age infants90. Thus, it is commonly believed that placental size and fetal weight are directly interrelated91. However, it has not been clear how the changes in placental size and function relate to changes in fetal metabolic demands92. The effect of placental size on IUGR was recently investigated in the placentas of rats exposed to 6-MP at various points of gestation93, as described below.
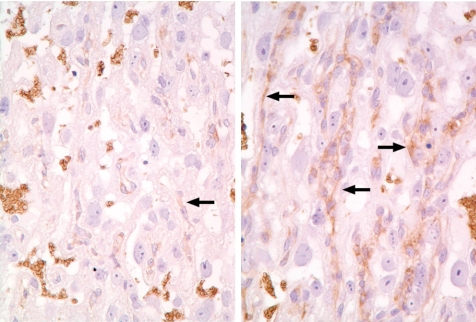
In the present study, 6-MP was administered orally at 0 and 60 mg/kg/day on GD 9, 11, 13 or 15, and the placentas were sampled on GDs 17 and 21. The main pathological findings in all treated groups were fetal resorption and IUGR with or without some malformations and a small placenta caused by mitotic inhibition and apoptosis of trophoblasts in the labyrinth zone. Complete fetal resorption was observed in most litters with the treatment on GD 9. The most remarkable response of small placenta and fetal abnormalities to 6-MP treatment occurred in the litters with the treatment on GD 11. However, the litters in a quarter of the dams with the treatment on GD 11 showed no fetotoxicity despite a 25% decline in placental weight and an increased fetal–placental weight ratio. Histopathologically, the expression of glucose transporter GLUT3 was increased in the trophoblastic septa in all treated groups, and this was particularly remarkable considering the proliferation of trophoblasts in the above litters, which showed an increased fetal-placental weight ratio (Fig. 31). It is known that one of the major nutrient transport functions of the placenta is to ensure adequate transfer of glucose from the maternal to fetal circulation. Glucose transfer across the placental barrier is crucial for fetal development. A common characteristic of pregnancies with IUGR is relative fetal hypoglycemia, and a small placenta per se is the major limitation on placental glucose transfer from the dam to the fetus94. Placental functions are highly adaptable and can change in response either to the maternal environment or to defects within the placenta itself, indicating either the capacity for placental nutrient transport to increase or the capacity for the fetus to extract nutrients from the umbilical circulation95,96. From these results, it is suggested that the elevated GLUT3 expression may reflect an attempt to increase the maternal-to-fetal glucose transport supply in order to compensate for the deterioration of placental function in the 6-MP-exposed small placenta and contribute to normal fetal growth and development. Therefore, it is considered that normal fetal growth and development can be maintained as a result of an increase in the expression of glucose transporter as adaptive change, even if the placental weight decreases by approximately 25% in 6-MP exposed rats.
Fig. 31.
Increased in expression of GLUT3 (↑) along trophoblastic septa (Rat, GD 17, GLUT3 immunostain, left - control; right - 6MP). Treatment with 6MP.
Conclusion
The fully formed placenta plays a major role in the maintenance of nutrition for the fetus and in the secretory and essential regulatory functions for the maintenance of pregnancy during the fetal period. However, despite the placenta being one of the important organs for evaluation of risks for the dam and embryo, the placental toxicity index in developmental toxicity studies is the placental weight change alone. As previously described, the pathogenesis of placental lesions show various and complex features, because the constitutive cells of the placenta originate from embryonic and maternal tissue, proliferate rapidly, differentiate and undergo morphological changes in close relation to each other according to the development sequence in a short pregnancy period. Even if the placental weight is reduced, the induced lesions are histopathologically different depending on the toxicants and the exposure period. In addition, normal fetal growth and development can be maintained as a result of the adaptive change, as long as the placental growth inhibition is within the allowable range. It is difficult to detect pathological changes in the decidua and metrial gland by placental weight assessment. Thus, placental weight assessment appears not to be enough to evaluate placental toxicity, and reproductive toxicity studies should pay more attention to placental histopathological evaluation on a case-by-case basis. Moreover, placental histopathological evaluation should comprehensively reveal the time-dependent changes in each placental tissue in view of the drug or chemical-exposure period and normal placental development. These detailed histopathological approaches to the pathogenesis of placental toxicity are considered to provide an important tool for understanding the mechanism of teratogenicity and developmental toxicity with particular regard to embryo lethality and delayed development, and could benefit reproductive toxicity studies.
Acknowledgments
The authors would like to thank Mr. Kiyoshi Kobayashi, Ms. Kaori Maejima, Ms. Hiromi Asako, Mr. Atsushi Funakoshi, Mr. Yoshinori Tanaka, Ms. Yuko Shimizu and Mr. Shigeru Iimura for their excellent technical assistance.
References
- 1.Bauer MK, Harding JE, Bassett NS, Breier BH, Oliver MH, Gallaher BH, Evans PC, Woodall SM, Gluckman PD. Fetal growth and placental function. Mol Cell Endocrinol. 140: 115–120 1998 [DOI] [PubMed] [Google Scholar]
- 2.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: Key pieces of the development puzzle. Science. 266: 1508–1518 1994 [DOI] [PubMed] [Google Scholar]
- 3.Jollie WP. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology. 41: 361–381 1990 [DOI] [PubMed] [Google Scholar]
- 4.Slikker W, Miller RK. Placental metabolism and transfer role in developmental toxicology. In: Developmental Toxicology, 2nd ed. CA Kimmel, and J Buelke-Sam (eds). Raven Press, New York. 245–283. 1994 [Google Scholar]
- 5.Carter AM, Martin RD. Comparative anatomy and placental evolution. In: Placental bed disorders, 1st ed. R Pijenenborg, I Brosens, and R Romero (eds). Cambridge University Press, Cambride. 109–126. 2010 [Google Scholar]
- 6.Burton GJ, Kaufmann P, Huppertz B. Anatomy and genesis of the placenta. In: Knobil and Neill’s Physiology of reproductive, 3rd ed. JD Neill (ed). Academic press, Amsterdam. 189–243. 2006 [Google Scholar]
- 7.Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice-a review. Placenta. 26Suppl A:S3–9 2005 [DOI] [PubMed] [Google Scholar]
- 8.Davies J, Glasser SR. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel). 69: 542–608 1968 [DOI] [PubMed] [Google Scholar]
- 9.Hafez ES, Tsutsumi Y. Changes in endometrial vascularity during implantation and pregnancy in the rabbit. Am J Anat. 118: 249–282 1966 [DOI] [PubMed] [Google Scholar]
- 10.Wooding P, Burton G. Haemochorial placentation: mouse, rabbit, man, apes, monkeys. In: Comparative placentation. Structures, functions and evolution, 1st ed. P Wooding, and G Burton (eds). Springer-Verlag, Heidelberg. 185–230. 2008 [Google Scholar]
- 11.Takata K, Fujikura K, Shin B. Ultrastructure of the rodent placental labyrinth: A site of barrier and transport. J Repro Dev. 43: 13–24 1997 [Google Scholar]
- 12.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 260: 176–190 2003 [DOI] [PubMed] [Google Scholar]
- 13.Soares MJ, Konno T, Alam SM. The prolactin family: Effectors of pregnancy-dependent adaptations. Trends Endocrinol Metab. 18: 114–121 2007 [DOI] [PubMed] [Google Scholar]
- 14.Soares MJ, Chapman BM, Rasmussen CA, Dai G, Kamei T, Orwig KE. Differentiation of trophoblast endocrine cells. Placenta. 17: 277–289 1996 [DOI] [PubMed] [Google Scholar]
- 15.Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 235: 3280–3294 2006 [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues-Machado Mda G, Silva GC, Pinheiro MB, Caliari MV, Borges EL. Effects of sepsis-induced acute lung injury on glycogen content in different tissues. Exp Lung Res. 36: 302–306 2010 [DOI] [PubMed] [Google Scholar]
- 17.Dey SK, Lim H. Implantation. In: Knobil and Neill’s Physiology of reproductive, 3rd ed. JD Neill (ed). Academic press, Amsterdam. 147–188. 2006 [Google Scholar]
- 18.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 250: 358–373 2002 [DOI] [PubMed] [Google Scholar]
- 19.Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction. 132: 217–232 2006 [DOI] [PubMed] [Google Scholar]
- 20.Jones RL, Kaitu’u-Lino TJ, Nie G, Sanchez-Partida LG, Findlay JK, Salamonsen LA. Complex expression patterns support potential roles for maternally derived activins in the establishment of pregnancy in mouse. Reproduction. 132: 799–810 2006 [DOI] [PubMed] [Google Scholar]
- 21.Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 115: 1–112 1989 [DOI] [PubMed] [Google Scholar]
- 22.Picut CA, Swanson CL, Parker RF, Scully KL, Parker GA. The metrial gland in the rat and its similarities to granular cell tumors. Toxicol Pathol. 37: 474–480 2009 [DOI] [PubMed] [Google Scholar]
- 23.Croy BA, Chantakru S, Esadeg S, Ashkar AA, Wei Q. Decidual natural killer cells: Key regulators of placental development (a review). J Reprod Immunol. 57: 151–168 2002 [DOI] [PubMed] [Google Scholar]
- 24.Riley JK, Yokoyama WM. NK cell tolerance and the maternal-fetal interface. Am J Reprod Immunol. 59: 371–387 2008 [DOI] [PubMed] [Google Scholar]
- 25.Caluwaerts S, Vercruysse L, Luyten C, Pijnenborg R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta. 26: 574–584 2005 [DOI] [PubMed] [Google Scholar]
- 26.Vercruysse L, Caluwaerts S, Luyten C, Pijnenborg R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta. 27: 22–33 2006 [DOI] [PubMed] [Google Scholar]
- 27.Bilinski MJ, Thorne JG, Oh MJ, Leonard S, Murrant C, Tayade C, Croy BA. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 16: 218–226 2008 [DOI] [PubMed] [Google Scholar]
- 28.Pijnenborg R, Vercruysse L. Animal models of deep trophoblast invasion. In: Placental bed disorders, 1st ed. R Pijnenborg, I Brosens, and R Romero (eds). Cambridge University Press, Cambridge. 127–140. 2010 [Google Scholar]
- 29.Slack JMW. The mouse. In: Essential Developmental Biology, 2nd ed. JMW Slack (ed). Blackwell, Malden. 112–138. 2006 [Google Scholar]
- 30.Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article: Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 2: 71–91 1981 [DOI] [PubMed] [Google Scholar]
- 31.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placenta. Placenta. 23: 3–19 2002 [DOI] [PubMed] [Google Scholar]
- 32.Maranghi F, Macri C, Ricciardi C, Stazi AV, Mantovani A. Evaluation of the placenta: Suggestions for a greater role in developmental toxicology. Adv Exp Med Biol. 444: 129–136 1998 [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi T. The problem of species comparison of developmental toxicity: Can we extrapolate human developmental toxicity induced by environmental chemicals from the data of rodent. Yakugaku Zasshi. 127: 491–500 2007 [DOI] [PubMed] [Google Scholar]
- 34.Goodman DR, James RC, Harbison RD. Placental toxicology. Fd Chem Toxic. 20: 123–128 1982 [DOI] [PubMed] [Google Scholar]
- 35.Bruce NW, Cabral DA. Effects of maternal blood loss on embryonic and placental development in the rat. J Re-prod Fertil. 45: 349–365 1975 [DOI] [PubMed] [Google Scholar]
- 36.Bruce NW. The effect of ligating a uterine artery on fetal and placental development in the rat. Biol Reprod. 14: 246–247 1976 [DOI] [PubMed] [Google Scholar]
- 37.Lynch AM, Bruce NW. Placental growth in rats exposed to carbon monoxide at selected stages of pregnancy. Biol Neonate. 56: 151–157 1989 [DOI] [PubMed] [Google Scholar]
- 38.Lewis RM, Batchelor DC, Bassett NS, Johnson BM, Napier J, Skinner SJM. Perinatal growth disturbance in the spontaneously hypertensive rat. Ped Res. 42: 758–764 1997 [DOI] [PubMed] [Google Scholar]
- 39.Wellstead JR, Bruce NW, Rahima A. Effects of indomethacin on spacing of conceptuses within the uterine horn and on fetal and placental growth in the rat. Anat Rec. 225: 101–105 1989 [DOI] [PubMed] [Google Scholar]
- 40.Akay MT, Kockaya EA. The effects of alcohol on rat placenta. Cell Biochem. Funct. 23: 435–445 2005 [DOI] [PubMed] [Google Scholar]
- 41.Eguchi Y, Yamamoto M, Arishima K, Shirai M, Wakabayashi K, Leichter J, Lee M. Histological changes in the placenta induced by maternal alcohol consumption in the rat. Biol Neonate. 56: 158–164 1989 [DOI] [PubMed] [Google Scholar]
- 42.Csapo AI, Wiest WG. Plasma steroid levels and ovariectomy-induced placental hypertrophy in rats. Endocrinology. 93: 1173–1177 1973 [DOI] [PubMed] [Google Scholar]
- 43.Bartholomeusz RK, Bruce NW, Lynch AM. Embryo survival, and fetal and placental growth following elevation of maternal estradiol blood concentrations in the rat. Biol Reprod. 61: 46–50 1999 [DOI] [PubMed] [Google Scholar]
- 44.Csapo A, Dray F, Erdos T. Letter: Oestradiol 17beta: Inhibitor of placental growth. Lancet. 6: 51–52 1974 [DOI] [PubMed] [Google Scholar]
- 45.Chan SW, Leathem JH. Placental steriodogenesis in the rat: Comparison of normal and giant placentae. Endocrinology. 100: 1418–1422 1977 [DOI] [PubMed] [Google Scholar]
- 46.Furukawa S, Hayashi S, Usuda K, Abe M, Ogawa I. Histopathological effect of ketoconazole on rat placenta. J Vet Med Sci. 70: 1179–1184 2008 [DOI] [PubMed] [Google Scholar]
- 47.Mason JI, Murry BA, Olcott M, Sheets JJ. Imidazole antimycotics: Inhibitors of steroid aromatase. Biochem Pharmacol. 34: 1087–1092 1985 [DOI] [PubMed] [Google Scholar]
- 48.Rajfer J, Sikka SC, Rivera F, Handelsman DJ. Mechanism of inhibition of human testicular steroidogenesis by oral ketoconazole. J Clin Endocrinol Metab. 63: 1193–1198 1986 [DOI] [PubMed] [Google Scholar]
- 49.Ichikawa A, Seki N, Sugimoto T, Ooshima Y. Effects of ketoconazole on placental weights in rats. Con Anom. 46: A23 2006 [Google Scholar]
- 50.Khera KS. Valproic acid-induced placental and teratogenic effects in rats. Teratology. 45: 603–610 1992 [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Padmanabhan R. Placental changes in chlorpromazine induced teratogenesis in rats - a histochemical study. Indian J Exp Biol. 18: 344–350 1980 [PubMed] [Google Scholar]
- 52.Graf R, Gossrau R, Frank HG. Placental toxicity in rats after administration of synthetic glucocorticoids. A morphological, histochemical and immunohistochemical investigation. Anat Embryol (Berl). 180: 121–130 1989 [DOI] [PubMed] [Google Scholar]
- 53.Padmanabhan R, Al-Zuhair AGH, Aida Hussein ALI. Histopathological changes of the placenta in diabetes induced by maternal administration of streptozotocin during pregnancy in the rat. Con Anom. 28: 1–15 1988 [Google Scholar]
- 54.Di Sant’Agnese PA, Jensen KD, Levin A, Miller RK. Placental toxicity of cadmium in the rat: An ultrastructural study. Placenta. 4: 149–163 1983 [DOI] [PubMed] [Google Scholar]
- 55.Fuentes M, Torregrosa A, Mora R, Götzens V, Corbellla J, Domingo JL. Placental effects of lead in mice. Placenta. 17: 371–376 1996 [DOI] [PubMed] [Google Scholar]
- 56.Furukawa S, Usuda K, Abe M, Hayashi S, Ogawa I. Histological expression of metallothionein in the developing rat placenta. J Toxoicol Pathol. 21: 223–227 2008 [Google Scholar]
- 57.Furukawa S, Usuda K, Abe M, Hayashi S, Ogawa I. Effect of 6-mercaptopurine on rat placenta. J Vet Med Sci. 70: 551–556 2008 [DOI] [PubMed] [Google Scholar]
- 58.Ishimura R, Kawakami T, Ohsako S, Nohara K, Tohyama C. Suppressive effect of 2,3,7,8-tetrachlorodibenzop-dioxin on vascular remodeling that takes place in the normal labyrinth zone of rat placenta during late gestation. Toxicol Sci. 91: 265–274 2006 [DOI] [PubMed] [Google Scholar]
- 59.Smith SC, Baker PN, Symonds EM. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 177: 57–65 1997 [DOI] [PubMed] [Google Scholar]
- 60.Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 177: 1395–1401 1997 [DOI] [PubMed] [Google Scholar]
- 61.Smith SC, Baker PN. Placental apoptosis is increased in post-term pregnancies. Br J Obstet Gynaecol. 106: 861–862 1999 [DOI] [PubMed] [Google Scholar]
- 62.Erel CT, Dane B, Calay Z, Kaleli S, Aydinli K. Apoptosis in the placenta of pregnancies complicated with IUGR. Int J Gynecol Obstet. 73: 229–235 2001; . [DOI] [PubMed] [Google Scholar]
- 63.Waddell BJ, Hisheh S, Dharmarajan AM, Burton PJ. Apoptosis in rat placenta is zone-dependent and stimulated by glucocorticoids. Biol Reprod. 63: 1913–1917 2000 [DOI] [PubMed] [Google Scholar]
- 64.Ejima K, Koji T, Tsuruta D, Nanri H, Kashimura M, Ikeda M. Induction of apoptosis in placentas of pregnant mice exposed to lipopolysaccharides: Possible involvement of Fas/Fas ligand system. Biol Reprod. 62: 178–185 2000 [DOI] [PubMed] [Google Scholar]
- 65.Doi K, Ishigami N, Sehata S. T-2 toxin-induced toxicity in pregnant mice and rats. Int J Mol Sci. 9: 2146–2158 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katayama K, Ueno M, Takai H, Ejiri N, Uetsuka K, Nakayama H, Doi K. Ethylnitrosourea induced apoptosis and growth arrest in the trophoblastic cells of rat placenta. Biol Reprod. 67: 431–435 2002 [DOI] [PubMed] [Google Scholar]
- 67.Yamauchi H, Katayama K, Ueno M, Uetsuka K, Nakayama H, Doi K. Involvement of p53 in 1-b-D-arabinofuranosylcytosine-induced trophoblastic cell apoptosis and impaired proliferation in rat placenta. Biol Reprod. 70: 1762–1767 2004 [DOI] [PubMed] [Google Scholar]
- 68.Furukawa S, Usuda K, Abe M, Hayashi S, Ogawa I. Busulfan-induced apoptosis in rat placenta. Exp Toxicol Pathol. 59: 97–103 2007 [DOI] [PubMed] [Google Scholar]
- 69.Furukawa S, Usuda F, Abe M, Ogawa I. Microencephaly and microphthalmia in rat fetuses by busulfan. Histol Histopathol. 22: 389–397 2007 [DOI] [PubMed] [Google Scholar]
- 70.Iguchi T, Tani N, Sato T, Fukatsu N, Ohta Y. Developmental changes in mouse placental cells from several stages of pregnancy in vivo and in vitro. Biol Reprod. 48: 188–196 1993 [DOI] [PubMed] [Google Scholar]
- 71.Anabuki K, Kitazima S, Koda S, Takahashi N. Reproductive studies on cisplatin. Yakuri to Chiryo. 10:673–694, 698–701 1982 [Google Scholar]
- 72.Diwan BA, Anderson LM, Ward JM, Henneman JR, Rice JM. Transplacental carcinogenesis by cisplatin in F344/NCr rats: promotion of kidney tumors by postnatal administration of sodium barbital. Toxicol Appl Pharmacol. 132: 115–121 1995 [DOI] [PubMed] [Google Scholar]
- 73.Rechtman MP, Zhang J, Salamonsen LA. Effect of inhibition of matrix metalloproteinases on endometrial decidualization and implantation in mated rats. J Reprod Fertil. 117: 169–177 1999 [DOI] [PubMed] [Google Scholar]
- 74.Spencer F, Chi L, Zhu MX. Effect of benomyl and carbendazim on steroid and molecular mechanisms in uterine decidual growth in rats. J Appl Toxicol. 16: 211–214 1996 [DOI] [PubMed] [Google Scholar]
- 75.Spencer F, Chi L, Zhu MX. Hydroxyurea inhibition of cellular and developmental activities in the decidualized and pregnant uteri of rats. J Appl Toxicol. 20: 407–412 2000 [DOI] [PubMed] [Google Scholar]
- 76.Peel S, Bulmer D. The effects of late ovariectomy on the proliferation and differentiation of the uterus of the pregnant rat. J Anat. 119: 569–578 1975 [PMC free article] [PubMed] [Google Scholar]
- 77.Harazono A, Ema M. Suppression of decidual cell response induced by tributyltin chloride in pseudopregnant rats: a cause of early embryonic loss. Arch Toxicol. 74: 632–637 2000 [DOI] [PubMed] [Google Scholar]
- 78.Furukawa S, Hayashi S, Usuda K, Abe M, Ogawa I. The impairment of metrial gland development in tamoxifen exposed rats. Exp Toxic Pathol. (in printing). [DOI] [PubMed] [Google Scholar]
- 79.Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 21: 693–702 2000 [DOI] [PubMed] [Google Scholar]
- 80.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 56: 169–179 1997 [DOI] [PubMed] [Google Scholar]
- 81.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 192: 259–270 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eriksson M, Basu S, Sentman CL. NK cells and pregnancy. In: Immunology of pregnancy, 1st ed, G Mor (ed). Springer-Verlag, New York. 84–95. 2006 [Google Scholar]
- 83.Rogers JM, Daston GP, Ebron MT, Carver B, Stefanadis JG, Grabowski CT. Studies on the mechanism of trypan blue teratogenicity in the rat developing in vivo and in vitro. Teratology. 31: 389–399 1985 [DOI] [PubMed] [Google Scholar]
- 84.Roberts AV, Nicholls SE, Griffiths PA, Williams KE, Lloyd JB. A quantitative study of pinocytosis and lysosome function in experimentally induced lysosomal storage. Biochem J. 160: 621–629 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freeman SJ, Lloyd JB. Inhibition of proteolysis in rat yolk sac as a cause of teratogenesis. Effects of leupeptin in vitro and in vivo. J Embryol Exp Morphol. 78: 183–193 1983 [PubMed] [Google Scholar]
- 86.Hunter ES, 3rd, Phillips LS, Goldstein S, Sadler TW. Altered visceral yolk sac function produced by a lowmolecular-weight somatomedin inhibitor. Teratology. 43: 331–340 1991 [DOI] [PubMed] [Google Scholar]
- 87.Jollie WP. Effects of sustained dietary ethanol on the ultra-structure of the visceral yolk-sac placenta of the rat. Teratology. 42: 541–552 1990 [DOI] [PubMed] [Google Scholar]
- 88.Beaudoin AR, Fisher DL. An in vivo/in vitro evaluation of teratogenic action. Teratology. 23: 57–61 1981 [DOI] [PubMed] [Google Scholar]
- 89.Hay WW. The placenta. Not just a conduit for maternal fuels. Diabetes. 40: 44–50 1991 [DOI] [PubMed] [Google Scholar]
- 90.Ruangvutilert P, Titapant V, Kerdphoo V. Placental ratio and fetal growth pattern. J Med Assoc Thai. 85: 488–495 2002 [PubMed] [Google Scholar]
- 91.Cetin I, Taricco E. Clinical causes and aspects of placental insufficiency. In: The placenta and human developmental programming, 1st ed. GJ Burton, DJP Barker, A Moffett and K Thornburg (eds). Cambridge University Press, Cambridge. 114–125. 2011 [Google Scholar]
- 92.Battaglia FC, Meschia G. Fetal and placental growth. In: An introduction to fetal physiology, 1st ed. FC Battaglia and G Meschia (eds). Academic Press, Orlando. 1–27. 1986 [Google Scholar]
- 93.Furukawa S, Hayashi S, Hayashi S, Usuda K, Abe M, Ogawa I. The relationship between fetal growth restriction and small placenta in 6-mercaptopurine exposed rat. Exp Toxicol Pathol. 63: 89–95 2011 [DOI] [PubMed] [Google Scholar]
- 94.Hay WW. Placental-fetal glucose exchange and fetal glucose metabolism. Trans Am Clin Climatol Assoc. 117: 321–340 2006 [PMC free article] [PubMed] [Google Scholar]
- 95.Hay WW. Metabolic interrelationships of placenta and fetus. Placenta. 16: 19–30 1995 [DOI] [PubMed] [Google Scholar]
- 96.Cross JC, Mickelson L. Nutritional influences on implantation and placental development. Nutr Rev. 64: 72–91 2006 [DOI] [PubMed] [Google Scholar]