Abstract
Two cases of spontaneous focal hepatic hyperplasia were observed in young female cynomolgus macaques (Macaca fascicularis). Grossly, a single raised nodule was observed in the left hepatic lobe. Histopathologically, the nodule compressed surrounding normal tissue; however, the hepatic cords within the nodule continued to those in the nor mal area except in part. Extensive fibrosis and absence of a normal hepatic triad were observed in the nodule. Thin fibrous septa radiating from the dense central stellate scarring and distended vessels were apparent in one animal. Hepatocytes in the nodule lacked cellular atypia, showed frequent PAS-positive eosinophilic inclusions in the cytoplasm and showed higher positive ratios for PCNA. The present cases resembled focal nodular hyperplasia reported in humans and a chimpanzee.
Keywords: liver, cynomolgus macaque, focal nodular hyperplasia
Nonneoplastic inflammatory lesions such as cellular infiltration and parasitic lesions are frequently observed in the livers of nonhuman primates1,2. On the other hand, neoplastic and nonneoplastic proliferative lesions are rare, and only one case of nonneoplastic proliferative lesion has been reported in the chimpanzee3 (Table 1.) In this report, we describe the histopathology of unusual spontaneous hepato cellular hyperplasia in two young cynomolgus macaques.
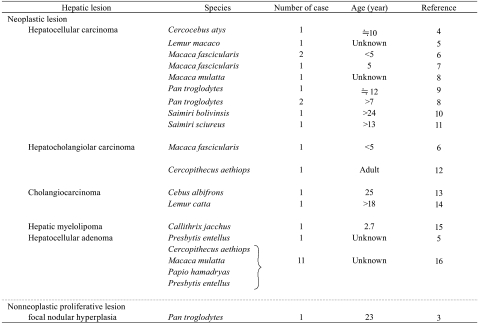
Table 1. Spontaneous Hepatic Neoplastic and Nonneoplastic Proliferative Lesions of Monkeys.
The animals were naïve female cynomolgus macaques (Macaca fascicularis, purpose-bred) born in China. Case 1 was three years old, and Case 2 was four years old. The animal room was maintained at a temperature range of 23 °C to 29 °C and a humidity range of 30% to 70%, with air ven tilation 15 times/hour and artificial illumination for 12 hrs/ day (07:00 to 19:00). The animals were individually housed in stainless steel cages (680 mm deep × 620 mm wide × 770 mm high) and provided with approximately 108 g of chewable animal feed for nonhuman primates (HF Primate 5K91 12G 5K9J, Purina Mills, LLC., St. Louis, MO, USA). Water, certified to meet the water quality standards required by the Japanese Waterworks Law was available ad libitum from an automatic supply system (Edstrom Industries, Inc., Waterford, WI, USA). All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Shin Nippon Biomedical Laboratories, Ltd. and were performed in accordance with standards published by the National Research Council (Guide for the Care and Use of Laboratory Animals, NIH OACU) and the US National Institutes of Health Policy on Humane Care and Use of Laboratory Animals. In accordance with these standards, every effort was made to ensure that the animals were free of pain and discomfort. No abnormal clinical signs were observed before necropsy, and no abnormal findings were observed in the hematology, blood chemistry or urinalysis. The animals were anesthetized by an intravenous injection of sodium pentobarbital and euthanized by exsanguination. After macroscopic observation, the liver was fixed in 10% neutral buffered formalin and processed for routine paraffin embedding. The paraffin sections were stained with hematoxylin and eosin (HE), periodic acid-Schiff stain (PAS), Masson’s trichrome stain (MT) and silver impregnation for reticulin fiber. For immunohistochemistry, a mouse monoclonal antibody against proliferating cell nuclear antigen (PCNA, Dako Japan, Kyoto, Japan) was used. The paraffin sections were deparaffinized and processed for immunohistochemistry (Envision+ Mouse/HRP, Dako Japan, Kyoto, Japan). Small sections of 3% glutaraldehyde-fixed liver tissue were further fixed in 1% osmium tetroxide and processed for transmission electron microscopy.
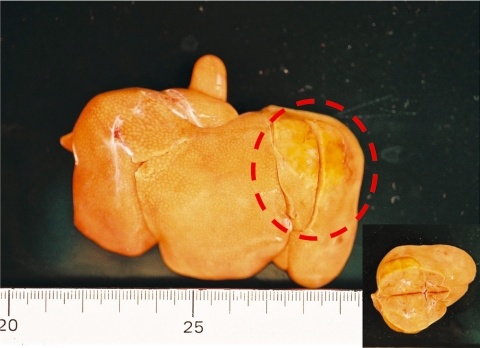
Grossly, single, raised spherical nodules with diameters of 30 mm in Case 1 (Fig. 1) and 5 mm in Case 2 were observed in the left lobe. The nodules were paler than the surrounding tissue. The cut surface of the nodule in Case 1 was swollen. There were no abnormal gross lesions other than in the liver.
Fig. 1.
Gross appearance of the liver. The nodule (enclosed by a broken line) was observed in the left lobe. Inset: the nodule was swollen on the cut surface. Case 1.
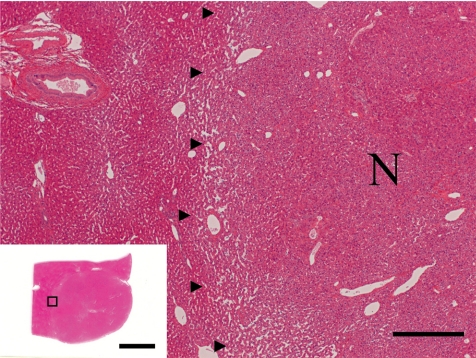
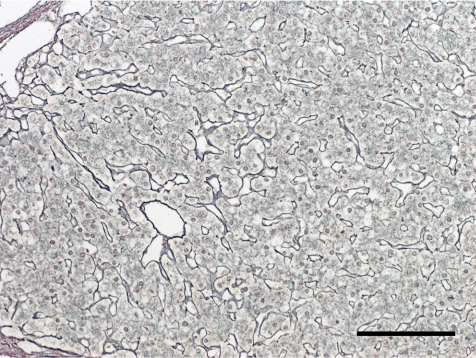
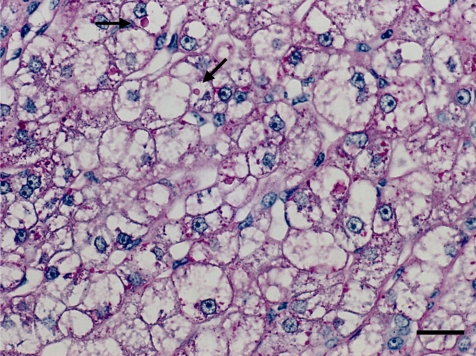
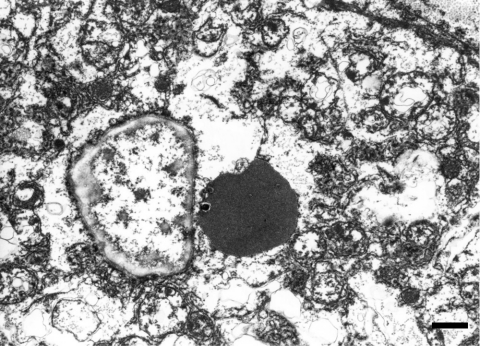
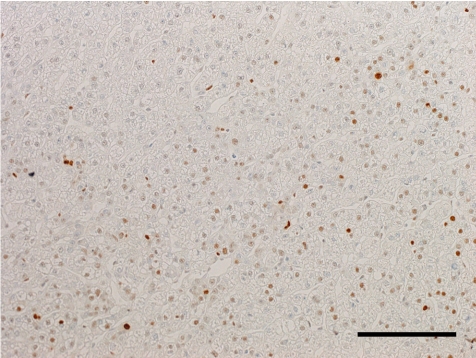
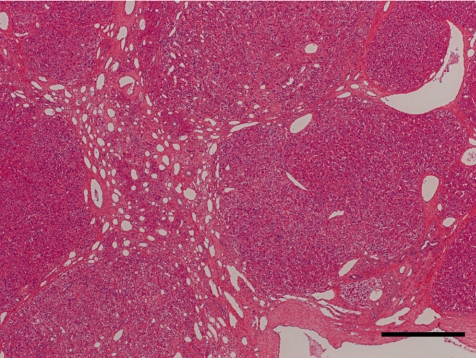
The histopathological features of the nodules in both animals essentially resembled each other. Common findings were as follows: The boundary of the nodules were indistinct, and the hepatic cords in the nodules continued to those in the normal hepatic area. The nodules were discernable by compression of adjacent normal tissue with slightly dilated sinusoid and partial encapsulation (Fig. 2). Extensive fibrosis was prominent in the nodules and divided the hepatic lobules. Bile ducts and blood vessels were observed in the fibrosis; however, the bile ducts were not apparent except in the fibrosis area. Absence of a normal hepatic triad was observed in the nodules. The hepatic cords were partially disordered and composed of two or three cell-layer thick hepatocytes (Fig. 3). Hepatocytes in the nodules lacked cellular atypia, such as pleomorphism, nucleus/cytoplasm ratio and polarity. PAS-positive eosinophilic inclusions, similar in size to erythrocytes, were frequently observed in the hepatocytic cytoplasm (Fig. 4). Electron microscopy showed the inclusion to be membrane-bound homogeneous electron dense material (Fig. 5). Although increased mitosis was not apparent in the HE sections, hepatocytes in the nodules showed a higher positive ratio for PCNA than in normal areas (Fig. 6).
Fig. 2.
The nodule (N) was discernable by compression of adjacent normal tissue (border marked with arrowheads). Bar = 500 µm. Inset: low power view of the nodule (N). Bar = 10 mm, Case 1, HE.
Fig. 3.
Hepatic cords were partially disordered and composed of two or three cell-layer thick hepatocytes. Case 1, silver impregnation. Bar = 100 µm.
Fig. 4.
PAS-positive intracytoplasmic inclusions (arrows) in the hepatocytes. Case 1, Periodic acid-Schiff. Bar = 20 µm.
Fig. 5.
The inclusion was membrane-bound homogeneous electron dense material. Case 1, transmission electron micrograph. Bar = 1 µm.
Fig. 6.
Numerous PCNA-positive hepatocytes. Case 1, anti-PCNA immunohistochemistry. Bar = 100 µm.
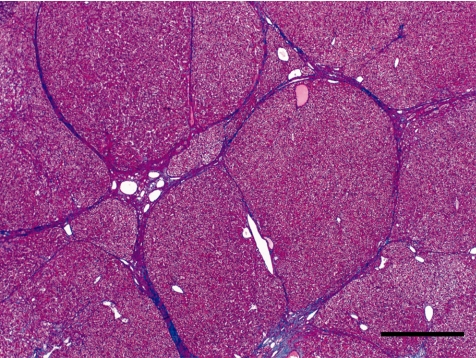
In Case 1, thin fibrous septa radiated from the central dense stellate scar. (Fig. 7). The fibrous septa irregularly separated the hepatic lobules and partially formed a pseu dolobule-like structure (Fig. 8). Bile ducts and distended blood vessels were abundant in the central dense stellate scar. In some areas, hepatocytes showed large and clear cytoplasms (Fig. 4). Nucleoli were prominent, and binucleated hepatocytes were frequently observed in the area.
Fig. 7.
Fibrous septa radiated from a central stellate scar, known as a “wagon-wheel” appearance. Case 1, HE Bar = 500 µm.
Fig. 8.
The fibrous septa irregularly separated the hepatic lobules forming a pseudolobule-like structure. Case 1, Masson’s trichrome. Bar = 500 µm.
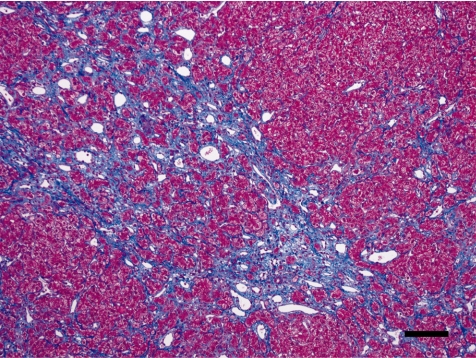
In Case 2, fibrosis was much less, and the stellate scar was obscure (Fig. 9). Fibrous tissue partially entered into the hepatic lobules and irregularly separated the hepatic cords, where the basic lobular structures, such as the central vein and portal triad, were lost. Hepatocytes labeled with anti-PCNA antibody were more numerous than in Case 1.
Fig. 9.
Fibrous tissue partially entered into the hepatic lobules and irregularly separated the hepatic cords. Case 2, Masson’s trichrome. Bar = 100 µm.
It is unlikely that the lesions in the present cases were responses to inflammatory change, such as hepatitis or cholangitis, because they were focal, lacked apparent inflammatory cell infiltration and showed expansive proliferation. The high PCNA-positive rate of the hepatocytes showed high proliferative activity in the nodules. However, the lesions were considered not to be neoplasia but to be hyperplasia because apparent cellular atypia was lacking in the hepatocytes, the basic lobular structure was maintained and the hepatic cords in the nodules continued to those in normal hepatic areas. Therefore, the nodules were considered to be nonneoplastic hyperplastic lesions.
Tumor-like lesions in the livers of domestic animals are classified as nodular hepatocellular hyperplasia (similar lesions are also known as nodular hyperplasia or hyperplastic nodule) and regenerative hepatocellular hyperplasia17–21 and are classified in humans as focal nodular hyperplasia and nodular regenerative hyperplasia22,23.
Nodular hepatocellular hyperplasia occurs frequently in aged dogs17–19 and has been also reported in swine at a low incidence17,20. There are many morphological differences between the present cases and canine nodular hepatocellular hyperplasia. Canine nodular hepatocellular hyperplasia is characterized by randomly distributed multiple distinct nodules and occurs throughout. Although compression of surrounding normal tissue by nodules is common, increased fibrosis in the nodules and encapsulation never occurs. Swine nodular hepatocellular hyperplasia is classi fied into two types, nonfibrous and fibrous20. The fibrous type possesses some resemblance to the present cases. Spe cifically, hepatic cords were disordered by fibrous tissue and composed of two or more cells layer. However, stellate scar fibrosis has not been reported in swine. Another type of nodular hyperplastic lesion in domestic animals, designated as regenerative hepatocellular hyperplasia, has been reported in dogs17,21. Regenerative hepatocellular hyperplasia occurs as a result of chronic hepatic injury, which is hepatic disruption of the normal hepatic parenchymal architecture, and significant fibrosis. In the present cases, fibrosis was found only in the nodules, which were not completely sur rounded by fibrous tissue.
Focal nodular hyperplasia occurs in any age group in humans, but many more cases have been reported in women than in men23,25. A histopathological feature of focal nodular hyperplasia is central stellate scar with distended vessels22,23,26. Bile ducts are usually absent, but a periseptal ductal proliferation may be prominent. Furthermore the hepatocytes are arranged in two-cell-thick plates. The morphological characteristics of the present cases, such as the stellate scar with distended vessels and bile ducts, and the absence of a normal hepatic triad in particular, closely resembled focal nodular hyperplasia in humans22,23,26, and only one case has been reported in the chimpanzee3. It was considered inappropriate to classify the present cases as regenerative hepatocellular hyperplasia; therefore, they were diagnosed as focal nodular hyperplasia.
The eosinophilic intracytoplasmic inclusions in the hepatocytes, which have not previously been reported in nonhuman primates, were not shown to be viral inclusions by electron microscopical examination. However, the pathogenesis was not identified. Similar homogeneous globular eosinophilic inclusions have been described in the hepatic cytoplasm of untreated laboratory beagle dogs24.
The pathogenesis of focal nodular hyperplasia in humans is considered to occur by a reactive process following a localized insult to the liver parenchyma; however, this has been controversial23. Information on the occurrence of focal nodular hyperplasias that morphologically resemble human cases in readily available experimental animals is valuable.
Acknowledgments
We would like to thank Dr. Shinichiro Nakamura (Shiga University of Medical Science) for his advice and are grateful to Shuichiro Iwashige for his excellent technical assistance.
References
- 1.Foster JR. Spontaneous and drug-induced hepatic pathol ogy of the laboratory beagle dog, the cynomolgus macaque and the marmoset. Toxicol Pathol. 33: 63–74 2005 [DOI] [PubMed] [Google Scholar]
- 2.Ito T, Chatani F, Sasaki S, Ando T, Miyajima H. Spontaneous lesions in cynomolgus monkeys used in toxicity studies. Exp Anim. 41: 455–469 1992 [DOI] [PubMed] [Google Scholar]
- 3.Porter BF, Goens SD, Brasky KM, Hubbard GB. A case report of hepatocellular carcinoma and focal nodular hyperplasia with a myelolipoma in two chimpanzees and a review of spontaneous hepatobiliary tumors in non-human primates. J Med Primatol. 33: 38–47 2004 [DOI] [PubMed] [Google Scholar]
- 4.Clark JD, Olsen RE. Hepatoma in mangabey (cercocebus artys). Vet Pathol. 10: 89–93 1973 [DOI] [PubMed] [Google Scholar]
- 5.O’Gara RW, Adamson RH. Spontaneous and induced neoplasms in nonhuman primate. In: Pathology of Simian Primates, part I, TW Fiennes (ed). Karger, Basel. 190–238. 1972 [Google Scholar]
- 6.Reindel JF, Walsh KM, Toy KA, Borowski WF. Spontaneously occurring hepatocellular neoplasia in adolescent cynomolgus monkeys (Macaca fascicularis). Vet Pathol. 37: 656–662 2000 [DOI] [PubMed] [Google Scholar]
- 7.Yoshizawa K, Oishi Y, Tsubura A, Sano K, Tsubota K, Ikeda K, Fukuhara Y, Senzaki H, Tsubura A. Hepatocellular carcinoma with PIVKA-II production in a young laboratory monkey. J Toxicol Pathol. 15: 61–68 2002 [Google Scholar]
- 8.Tabor E. Nonhuman primate models for non-A, non-B hepatitis. Cancer Detect Prev. 14: 221–225 1989 [PubMed] [Google Scholar]
- 9.Abe K, Kagei N, Teramura Y, Ejima H. Hepatocellular carcinoma associated with chronic Schistosoma mansoni infection in a chimpanzee. J Med Primatol. 22: 237–239 1993 [PubMed] [Google Scholar]
- 10.Borda JT, Ruiz JC, Sanchez-Negrette M. Spontaneous hepatocellular carcinoma in Saimiri boliviensis. Vet Pathol. 33: 724–726 1996 [DOI] [PubMed] [Google Scholar]
- 11.Moriss TH, Abdi MM. Hepatocellular carcinoma in a squirrel monkey (Saimiri sciureus). J Med Primatol. 25: 137–139 1996 [DOI] [PubMed] [Google Scholar]
- 12.Seibold HR, Wolf RH. Neoplasms and proliferative lesions in 1065 nonhuman primate necropsies. Lab Anim Sci. 23: 533–539 1973 [PubMed] [Google Scholar]
- 13.Brown RJ, O’Neill TP, Kessler MJ, Andress D. Cholangiocarcinoma in a capuchin monkey (cebus Albifrons). Vet Pathol. 17: 626–629 1980 [DOI] [PubMed] [Google Scholar]
- 14.Chang J, Wagner JL, Kornegay RW. Spontaneous cholangiocarcinoma in a ring-tailed lemur (Lemur catta). Lab Anim Sci. 29: 374–376 1979 [PubMed] [Google Scholar]
- 15.Kakinuma C, Harada T, Watanabe M, Shibutani Y. Spontaneous adrenal and hepatic myelolipomas in the common marmoset. Toxicol Pathol. 22: 440–445 1994 [DOI] [PubMed] [Google Scholar]
- 16.Beniashvili DS. Tumors of liver, bile duct, and gallbladder. In: Experimental Tumors in Monkeys, Dzhemali Sh. Beniashvili (ed). CRC Press Inc., Boca Raton. 26–42, 1994 [Google Scholar]
- 17.Tumors of the Liver. In: Histological of the Alimentary System of Domestic Animals. KW Head, JM Cullen, RR Dubielzig, RW Else, W Misdorp, AK Patnaik, S Tateyama, and I van der Gaag (eds), Second Series, Volume X, Published by the Armed Forces Institute of Pathology in cooperation with the American Registry of Pathology and The World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology, Washington, DC. 121–133. 2003 [Google Scholar]
- 18.Bergman JR. Nodular hyperplasia in the liver of the dog: An association with changes in Ito cell population. Vet Pathol. 22: 427–438 1985 [DOI] [PubMed] [Google Scholar]
- 19.Fabry A, Benjamin SA, Angelton GM. Nodular hyperplasia of the liver in the beagle dog. Vet Pathol. 19: 109–119 1982 [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M, Tsuda H, Ito N. Histopathological classification of spontaneous hyperplastic liver nodule in slaughtered swine. J Comp Pathol. 93: 603–612 1983 [DOI] [PubMed] [Google Scholar]
- 21.Blue JT, French TW, Meyer DJ. The liver. In: Diagnostic Cytology and Hematology of the Dog and Cat, Second Edition, RL Cowell, RD Tyler, and JH Meinkoth (eds). Mosby, St. Louis. 183–194. 1999 [Google Scholar]
- 22.Ishak GK, Markin SR. Tuomorlike conditions. In: Anderson’s Pathology, 10th ed. I Damjanov and J Linder (eds). Mosby, St. Louis. 1832–1839. 1996 [Google Scholar]
- 23.Rosai J. Tumors and tumorlike conditions, Liver cell tumors and tumorlike conditions, Focal nodular hyperplasia. In: ROSAI AND ACKERMAN’S SURGICAL PATHOLOGY. 9th ed. J Rosai (ed). Mosby. 992–993. 2004 [Google Scholar]
- 24.Greaves P. Liver and Pancreas. In: Histopathology of Pre-clinical Toxicity Studies, 3rd ed. P Greaves (ed). Elsevier Ltd. 457–569, 2007 [Google Scholar]
- 25.Nguyen BN, Fléjou JF, Belghiti TB, Degott C. Focal nodular hyperplasia of the liver: acomprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 23: 1441–1454 1999 [DOI] [PubMed] [Google Scholar]
- 26.Wanless IR. Vascular disorders. In: Pathology of the Liver. 4th ed. RNM Macsween, AD Burt, BC Portmann, KG Ishak, PJ Scheuer, and PP Anthony (eds). Churchill Living-stone. 573–593. 2002 [Google Scholar]