Abstract
The antineoplastic platinum complexes cisplatin and its analogues are widely used in the chemotherapy of a variety of human malignancies, and are especially active against several types of cancers. Nedaplatin is a second-generation platinum complex with reduced nephrotoxicity. However, their use commonly causes nephrotoxicity due to a lack of tumor tissue selectivity. Several recent studies have provided significant insights into the molecular and histopathological events associated with nedaplatin nephrotoxicity. In this review, we summarize findings concerning the renal histopathology and molecular pathogenesis induced by antineoplastic platinum complexes, with a particular focus on the comparative nephrotoxicity of cisplatin and nedaplatin in rats.
Keywords: cisplatin, kidney, nedaplatin, nephrotoxicity, renal papilla, toxicogenomics
Introduction
Antineoplastic platinum complexes are widely used to treat several human malignancies. Cisplatin, a first-generation platinum complex, has been successfully used for treatment of several types of cancer, including testicular, head and neck, ovarian, cervical and nonsmall cell lung carcinoma1–4. Although the precise mechanism has not been fully elucidated, the possible mechanisms involved in the anticancer activity of cisplatin are becoming clearer. Cisplatin directly binds to the DNA of tumor cells, forming a cross-link that leads to the arrest of DNA synthesis and replication. In the case of rapidly dividing cells such as cancers, cisplatin can also induce DNA damage, which ultimately leads to irreversible cellular injury and death.
Unfortunately, cisplatin treatment causes several types of side effects due to the drug’s lack of target selectivity. The major limiting factors in the use of cisplatin are neurotoxicity, ototoxicity, nausea, vomiting and nephrotoxicity. Among these factors, the prevalence of nephrotoxicity is extremely high, occurring in about one-third of patients undergoing cisplatin treatment5–7. Nephrotoxicity is therefore regarded as the most common and serious side effect of cisplatin chemotherapy8–10.
Over the last two decades, hundreds of platinum complexes have been synthesized and tested to lessen the adverse effects as well as to improve the effectiveness of these antineoplastic agents11,12. Dose-limiting nephrotoxicity has been a critical concern in the selection of new drug candidates during the early stages of drug development. Several novel cisplatin analogues have been identified that exhibit less severe side effects without sacrificing their beneficial effects. Nedaplatin is one of the second-generation platinum complexes with reduced nephrotoxicity and may serve as a substitute for cisplatin or even surpass cisplatin for use in combination with other drugs. Nedaplatin’s spectrum of activity largely overlaps that of cisplatin, as the drug is active against various solid tumors, including lung cancer13, esophageal cancer14, head and neck cancer15, testicular cancer16 and ovarian cancer17. However, nedaplatin also causes nephrotoxicity at doses that are therapeutic in humans. In this review, we summarize recent findings concerning the renal histopathology and molecular pathogenesis induced by antineoplastic platinum complexes, with a particular focus on the comparative nephrotoxicity of cisplatin and nedaplatin in rats.
Renal Histopathology
Comparative nephrotoxicity of cisplatin and nedaplatin
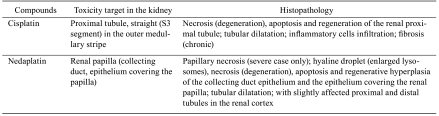
Toxicity target and histopathological characteristics in cisplatin and nedaplatin nephrotoxicity are summarized in Table 1. Renal tubular cell death is a common histopathological feature associated with nephrotoxicity induced by antineoplastic platinum complexes. In in vivo animal models, cisplatin primarily damages the proximal tubule, specifically the S3 segment of the proximal tubule in the outer medullary stripe18–20. The histopathological characteristics of cisplatin nephrotoxicity in rats are massive necrosis and subsequent regeneration of renal proximal tubular cells21–23. In contrast, renal papillary injury is reported to be minor, and the glomerulus undergoes no obvious morphologic changes24,25. Long-term cisplatin treatment causes cyst formation and interstitial fibrosis in the kidney22.
Table 1. Toxicity Target and Histopathological Characteristics in Cisplatin and Nedaplatin-induced Nephrotoxicity in Rats.
In contrast to cisplatin nephrotoxicity, nedaplatin primarily affects the renal papilla in rats23. Papillary injury induced by nedaplatin is morphologically characterized by hyaline droplet changes, apoptosis or regenerative hyperplasia of the collecting duct epithelium and the epithelium covering the renal papilla. These adverse changes in the renal papilla tend to be greater toward the tip. In severe cases, papillary necrosis also occurs. Ultrastructural features in combination with a positive reaction to acid phosphatase confirm that the hyaline droplets associated with nedaplatin nephrotoxicity are hyperplastic lysosomes. In the cortex, only slight necrotic and degenerative changes are observed, such as slight focal tubular necrosis, regeneration and dilatations lined with a flattened epithelium in the proximal and distal tubule. The glomerulus shows no obvious toxic changes.
Time course of the changes of nedaplatin nephrotoxicity
We also characterized the temporal changes in nedaplatin nephrotoxicity in rats26. The early stage of nedaplatininduced nephrotoxicity in rats is characterized by apoptosis, with shrunken cytoplasm and pyknotic/fragmented nuclei in the collecting duct epithelial cells and proximal and distal tubular cells. Four days after nedaplatin dosing, a small number of cortical tubules exhibit focal necrosis, and are filled with debris derived from necrotic and/or apoptotic cells. The cortical tubule also exhibits a degenerative appearance and is characterized by loss of brush borders and abnormally large vacuoles in the cytoplasm. In the period 4 to 7 days following nedaplatin treatment, cystic dilatation and regeneration of the renal tubules occur, with atypical nuclei in the renal cortex. In the collecting duct epithelium, morphological regenerative changes reflecting the tubular injury occur and are characterized by protrusion into the collecting duct lumen of enlarged cells with atypical nuclei. Characteristic papillary necrosis is also observed in the tip of renal papilla 4 or more days after a single nedaplatin dose24,26,27. In the later stage of nedaplatin-induced nephrotoxicity, subsequent cystic dilatation and regeneration occur in the affected tubules, but incomplete tissue repair is still observed 2 weeks after treatment.
Pathogenesis of Nephrotoxicity
Renal uptake and accumulation
To exert cytotoxic activity, the platinum complex must enter a cell. Cisplatin is preferentially taken up by the highly susceptible proximal tubular cells within the S3 segment, the principal site of acute injury in the kidney18–20. Safirstein et al.28 demonstrated renal preference in the accumulation of cisplatin; in renal tissue slices, they found cisplatin at concentrations up to five-fold above the concentration in the medium. Safirstein et al. also demonstrated that the kidney accumulates platinum in part by transport or specific binding to components of the kidney transport system and then biotransforms the platinum intracellularly. Preferential injury to the proximal tubule could therefore be explained by the propensity of cisplatin to accumulate in this tissue.
Although the precise mechanisms involved in the intracellular transport systems of platinum complexes are unclear, several reports have discussed the cellular uptake of platinum complexes under both in vitro and in vivo conditions. In the case of cancer cells, about half of the cisplatin taken up is due to passive diffusion through the plasma membrane, and uptake of the remaining half is mediated by several factors, including transporters29. A high-affinity copper transporter, Ctr1, is believed to play a role in cisplatin uptake in cancer cells. Recent works clearly indicate that Ctr1 protein localizes mainly on the basolateral side of tubular cells in the kidney and contributes to cisplatin uptake by these cells30. Thus, renal Ctr1 expression might be responsible for cisplatin-induced tubular toxicity. In addition, several reports suggest that organic cation transporters (Oct), specifically Oct2, are involved in the uptake of cisplatin in renal tubular cells. Ciarimboli et al.31 confirmed that human Oct2 is responsible for basolateral uptake of cisplatin in the kidney. Yonezawa et al.32 determined that in HEK293 cells stably expressing rat Oct2, renal Oct2 expression is the major determinant of cisplatin-induced tubular toxicity. Filipski et al.33 provided evidence that Oct1/Oct2-deficient mice are protected from renal tubular damage caused by cisplatin. Subsequently, they found that a nonsynonymous single-nucleotide polymorphism in the Oct2 gene is associated with reduced cisplatin-induced nephrotoxicity in humans.
Information regarding the mechanisms involved in the cellular uptake of nedaplatin is relatively limited. However, there is clear evidence that in both humans and rats, nedaplatin, which is less nephrotoxic than cisplatin, does not interact with Oct2 or the apical multidrug and toxin extrusion transporter (MATE) family of proteins34,35. The reduced nephrotoxicity of nedaplatin might therefore be associated with reduced accumulation in the proximal tubules due to its lower affinity for transporters, including Oct2 and the MATE family. Tanaka et al.36 reported that 10 min after a single intravenous dose of radiolabeled nedaplatin, the highest radioactivity is found in the kidney, particularly in the cortex and papilla. After 1 h, the highest radioactivity is observed in the papilla. Differences in the primary site of injury in the kidney may therefore be partially attributed to differences in the renal kinetics of cisplatin and nedaplatin. In addition, histopathological differences may be due to differences in the transporter systems involved, but additional studies are needed to resolve this question.
Molecular pathogenesis of cisplatin nephrotoxicity
Oxidative stress is one of the main mechanisms of cisplatin nephrotoxicity37. Various reactive oxygen species (ROS) are produced by cisplatin treatment in cultured renal tubular cells, kidney slices and in vivo in the kidney. Several possible mechanisms have been proposed to account for ROS generation under the pathological conditions of cisplatin nephrotoxicity. After being incorporated into a cell, cisplatin is transformed to a highly reactive form, which then rapidly reacts with antioxidants including glutathione. However, under conditions in which there is excessive generation of the reactive form in a cell, glutathione and related antioxidants are depressed, leading to a change in the redox status and eventually resulting in the accumulation of endogenous ROS. ROS in turn causes oxidative damage within the cells38. In addition, mitochondrial dysfunction might be related to increased ROS production in cisplatin nephrotoxicity due to disruption of the mitochondrial antioxidant defense system39. Although the molecular targets of ROS generated by cisplatin treatment are still largely unknown, ROS may react with multiple molecules in the cell, including mitochondrial lipids and proteins, ultimately leading to cellular injury.
At the molecular level, several genes are known to be deregulated in the kidney by cisplatin treatment. Comprehensive gene expression profiling revealed that oxidative stress response genes are deregulated following 7-day cisplatin treatment in rats. This includes upregulation of the gene encoding metallothionein 1 and downregulation of genes encoding catalase, superoxide dismutase and γ-GT40. Aleksunes et al.41 demonstrated the induction of cytoprotective genes/proteins following cisplatin treatment, including NAD(P)H dehydrogenase, quinone 1, heme oxygenase 1 (Hmox1) and glutamate cysteine ligase catalytic subunit, all of which are known to be induced by activation of the transcription factor Nrf2 (nuclear factor E2-related factor 2). These changes in gene expression could be responsible for downstream cell death associated with oxidative stress-mediated tubular injury in cisplatin nephrotoxicity. There is also some evidence suggesting that cisplatin treatment induces glutathione depletion in the kidney42 and that glutathione supplementation attenuates cisplatin nephrotoxicity43. Taken together, the available evidence indicates that oxidative stress plays an important role in the mechanism of cisplatin nephrotoxicity.
Excessive oxidative stress leads to the death of renal tubular cells as a result of cisplatin-induced cellular injury, which leads to tubular cell necrosis and apoptosis. Several apoptotic pathways have been implicated in this process, including the extrinsic pathway mediated by death receptors, the endoplasmic reticulum-stress pathway and the mitochondrial-associated intrinsic apoptotic pathway12,44,45. In addition to the involvement of these apoptotic pathways, p21 is also known to play an important role in directing the switching of signals in renal tubular cells between survival cell death46,47. Both necrosis and apoptosis are reported to be induced in renal tubular cells in vivo48,49. This difference of phenotype in cisplatin-induced cellular injury can be explained by evidence demonstrating that exposure to high concentrations of cisplatin lead to necrotic cell death, while exposure to much lower concentrations result in apoptosis50. Furthermore, it has also been determined that ROS play a role in mediating apoptosis but not necrosis following cisplatin exposure50. Thus, the mechanism of cisplatin-induced renal tubular necrosis is thought to be concentration-dependent.
Molecular pathogenesis of nedaplatin nephrotoxicity
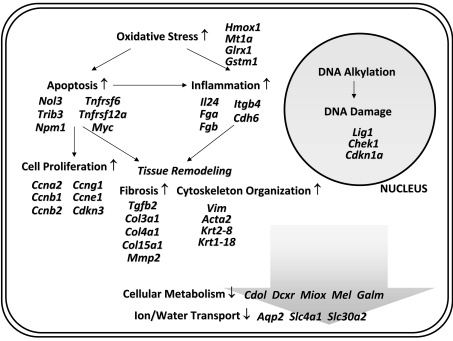
Application of toxicogenomics is a promising strategy to determine the molecular mechanisms underlying chemical toxicity and to aid in the early detection of toxic effects51–54. Recently, our laboratory conducted a toxicogenomic study to broaden understanding of the cellular and molecular mechanisms involved in nedaplatin nephrotoxicity50. Using DNA microarrays, gene expression profiles were analyzed in two renal regions, the cortex and papilla, following administration of a single dose of nedaplatin in rats. We found striking similarities in the gene expression profiles between the renal cortex and papilla. More specifically, we found that several genes belonging to various functional categories are deregulated in both renal regions in accordance with histopathological changes associated with nedaplatin nephrotoxicity (Fig. 1). Upregulated genes include those involved in apoptosis, cell cycle regulation, DNA metabolism, cell migration/adhesion, cytoskeleton organization and oxidative stress, while genes reflecting renal malfunction, including calcium homeostasis, are downregulated. One of the major histopathological changes associated with nedaplatin nephrotoxicity is apoptosis in renal tubules. Upregulation of myelocytomatosis viral oncogene homolog, death-associated protein, tribbles homolog 3 and TNF receptor superfamily member 6 in nedaplatin nephrotoxicity is thought to be related to apoptotic cell death. In addition, we also observed that the expression of various oxidative stress responsive genes, including Hmox1, metallothionein 1a, glutaredoxin 1 and glutathione S-transferase mu 1, is enhanced, as is the case in cisplatin nephrotoxicity. The Hmox1 gene encodes the rate-limiting enzyme in the degradation of heme55, and protects against oxidative stress56,57. Metallothioneins play a role in the detoxification of toxic metals and in protection against oxidative stress58–60. Glutaredoxins are thiol oxidoreductases that use reduced glutathione as a hydrogen donor to reduce protein disulfides or glutathione–protein-mixed disulfides in a coupled system with glutathione reductase and NADPH61,62. Glutathione S-transferase, a member of the phase II group of xenobiotic metabolizing enzymes, is known to conjugate reactive compounds to glutathione prior to their excretion from the body63, and also acts as a glutathione peroxidase, converting lipid peroxides to the hydroxyl form and oxidizing reduced glutathione63. On the basis of these observations, we concluded that nedaplatin treatment affects both the renal papilla and cortex through oxidative stress-related mechanisms.
Fig. 1.
Putative cellular pathways of nedaplatin nephrotoxicity. This schematic pathway was assembled using gene expression data obtained from nedaplatin-treated rat kidneys 6 days after a single dosing. Upregulation and downregulation are represented by ↑ or ↓, respectively.
Our research also provided novel evidence indicating that cytokeratin subtypes 14 and 19 are overexpressed in the papilla following nedaplatin treatment50. Distinct positive expression of cytokeratins 14 and 19 was specifically localized to the epithelium covering the papilla and/or the collecting duct epithelium. Cytokeratins, which are a diverse group of intermediate filament proteins, are known to play a critical role in differentiation and tissue specialization and function in the maintenance of epithelial cell structural integrity. The wide acceptance of cytokeratins as excellent epithelial cell markers is largely related to the differentiation-specific expression patterns shown by individual isotypes64–66. Cytokeratin 14 is expressed in the basal and first suprabasal layers of various stratified squamous epithelia, but not in simple epithelial cells67. Cytokeratin 19 is a major component of simple epithelia in a wide range of epithelial tissues64. In nedaplatin-induced renal papillary toxicity, increased expression of cytokeratins 14 and 19 might reflect abnormal squamous differentiation of the epithelium covering the renal papilla and regeneration related to the repair process of the injured epithelial cells in the collecting duct.
Amelioration of Nedaplatin Nephrotoxicity
As described above, nedaplatin has the potential to affect the entire nephron, with characteristic papillary injury occurring where the drug concentration reaches maximum levels46,48. Hydration is thought of as a promising approach for ameliorating cisplatin nephrotoxicity because it promotes excretion of the drug into the urine and dilutes the drug concentration in the urine by causing polyuria7,11,68. We evaluated whether hydration before dosing does in fact ameliorate nedaplatin nephrotoxicity. We found that hydration dramatically reduces nedaplatin nephrotoxicity, but has no clear effect on myelotoxicity. Measurement of urinary platinum excretion revealed that the total amount of platinum excreted is significantly higher in hydrated rats than in nonhydrated rats. In terms of the urinary nedaplatin concentration, hydrated rats have a lower concentration compared with nonhydrated rats. Hydration at the time of nedaplatin dosing could therefore be an effective strategy for minimizing nephrotoxicity.
We also examined the usefulness of prolonging the infusion time for reducing nephrotoxicity resulting from nedaplatin treatment69. This study provided evidence that prolonging the infusion time effectively minimizes the nephrotoxicity associated with nedaplatin. This effect may be related to a reduction in the maximal drug concentration in circulating blood, which consequently reduces the concentration of drug in the nephron. Because nedaplatin and cisplatin have similar nephrotoxic mechanisms, nedaplatin nephrotoxicity may be limited by cotreatment with antioxidants, although the capacity of antioxidants to ameliorate nedaplatin nephrotoxicity has not been studied.
Usefulness of Animal Models of Renal Failure Using Differences Between Cisplatin- and Nedaplatin-Induced Lesions
Because cisplatin specifically injures the proximal tubule, several studies have used this drug to elucidate the pathophysiological mechanisms involved in the development of nephrotoxicity. The chronic cisplatin nephrotoxicity experimental model provides particularly useful insights into the detailed mechanisms underlying chronic renal failure and exhibits pathologic features similar to those seen with human lesions. Interestingly, the rat cisplatin-induced chronic nephrotoxicity model showed that interactions between different macrophage populations play an important role in the development of renal interstitial fibrosis70–73. Recently, it was reported that epithelial-mesenchymal transition of tubular epithelial cells contributes significantly to the development of renal fibrosis. The cisplatin nephrotoxicity model was used to confirm that prostaglandin E2, derived from activation of cyclooxygenase-1, may regulate renal epithelial regeneration via the prostaglandin E2 receptor EP4 through inhibition of apoptosis and epithelial-mesenchymal transition74. Comparatively little research has focused on the pathophysiology of chronic nephrotoxicity using the nedaplatin nephrotoxicity model. Further studies using this model may enhance understanding of the mechanisms leading to chronic renal failure and progressive fibrosis in humans, especially with respect to renal papillary injury with metaplastic changes in the papillary epithelium. These efforts may lead to the development of a novel therapeutic strategy for chronic renal failure in humans.
Conclusions
Although reduced nephrotoxicity compared with cisplatin makes nedaplatin a promising second-generation platinum complex, this drug may also cause nephrotoxicity at doses that are therapeutic in humans, especially in the case of patients with deteriorated renal function. Histopathologically, nedaplatin nephrotoxicity in rats is characterized by apoptosis and/or necrosis, subsequent regeneration and cystic dilatation, not only in the proximal tubule but also in the distal tubule and the collecting duct. In severe cases, papillary necrosis also occurs. Comprehensive gene expression profiling revealed that nedaplatin nephrotoxicity might be induced by oxidative stress-mediated mechanisms. Furthermore, several genes have been identified as being specifically deregulated. Deregulation of the genes encoding cytokeratins 14 and 19 reflects the characteristic renal papillary injury associated with nedaplatin. Overall, recent research has provided significant insights into the molecular and histopathological events that are responsible for nedaplatin nephrotoxicity. These insights could lead to the establishment of an effective strategy for the safe use of nedaplatin in humans.
References
- 1.Rozencweig M, von Hoff DD, Slavik M, Muggia FM. Cis-diamminedichloroplatinum (II). A new anticancer drug. Ann Intern Med. 86: 803–812 1977 [DOI] [PubMed] [Google Scholar]
- 2.Prestayko AW, D’Aoust JC, Issell BF, Crooke ST. Cisplatin (cis-diamminedichloroplatinum II). Cancer Treat Rev. 6: 17–39 1979 [DOI] [PubMed] [Google Scholar]
- 3.Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann Intern Med. 100: 704–713 1984 [DOI] [PubMed] [Google Scholar]
- 4.Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 34: 1522–1534 1998 [DOI] [PubMed] [Google Scholar]
- 5.Meyer KB, Madias NE. Cisplatin nephrotoxicity. Miner Electrolyte Metab. 20: 201–213 1994 [PubMed] [Google Scholar]
- 6.McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug Saf. 13: 228–244 1995; . [DOI] [PubMed] [Google Scholar]
- 7.Santoso JT, Lucci JA, 3rd, Coleman RL, Schafer I, Hannigan EV. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: a randomized trial. Cancer Chemother Pharmacol. 52: 13–18 2003; . [DOI] [PubMed] [Google Scholar]
- 8.Goldstein RS, Mayor GH. Minireview. The nephrotoxicity of cisplatin. Life Sci. 32: 685–690 1983 [DOI] [PubMed] [Google Scholar]
- 9.Safirstein R, Winston J, Goldstein M, Moel D, Dikman S, Guttenplan J. Cisplatin nephrotoxicity. Am J Kidney Dis. 8: 356–367 1986 [DOI] [PubMed] [Google Scholar]
- 10.Menczer J, Ben-Baruch G, Rizel S, Brenner H. Acute renal failure associated with intraperitoneal cisplatin chemotherapy with systemic thiosulfate protection in ovarian cancer patients. Eur J Gynaecol Oncol. 12: 403–406 1991 [PubMed] [Google Scholar]
- 11.Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 44: 1173–1183 2006 [DOI] [PubMed] [Google Scholar]
- 12.Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. Oncol Rep. 10: 1663–1682 2003 [PubMed] [Google Scholar]
- 13.Fukuda M, Shinkai T, Eguchi K, Sasaki Y, Tamura T, Ohe Y, Kojima A, Oshita F, Hara K, Saijo N. Phase II study of (glycolato-O,O) diammineplatinum(II), a novel platinum complex, in the treatment of non-small-cell lung cancer. Cancer Chemother Pharmacol. 26: 393–396 1990 [DOI] [PubMed] [Google Scholar]
- 14.Taguchi T, Wakui A, Nabeya K, Kurihara M, Isono K, Kakegawa T, Ota K. A phase II clinical study of cisdiammine glycolato platinum, 254-S, for gastrointestinal cancers. Gan To Kagaku Ryoho. 19: 483–488 1992; . [PubMed] [Google Scholar]
- 15.Inuyama Y, Miyake H, Horiuchi M, Hayasaki K, Komiyama S, Ota K. A late phase II clinical study of cis-diammine glycolato platinum, 254-S, for head and neck cancers. Gan To Kagaku Ryoho. 19: 871–877 1992; . [PubMed] [Google Scholar]
- 16.Akaza H, Togashi M, Nishio Y, Miki T, Kotake T, Matsumura Y, Yoshida O, Aso Y. Phase II study of cis-diammine(glycolato)platinum, 254-S, in patients with advanced germ-cell testicular cancer, prostatic cancer, and transitional-cell carcinoma of the urinary tract. Cancer Chemother Pharmacol. 31: 187–192 1992 [DOI] [PubMed] [Google Scholar]
- 17.Kato T, Nishimura H, Yakushiji M, Noda K, Terashima Y, Takeuchi S, Takamizawa H, Suzuki M, Arai M, Ota M. Phase II study of 254-S (cis-diammine glycolato platinum) for gynecological cancer. Gan To Kagaku Ryoho. 19: 695–701 1992; . [PubMed] [Google Scholar]
- 18.Dobyan DC. Long-term consequences of cis-platinuminduced renal injury: a structural and functional study. Anat Rec. 212: 239–245 1985 [DOI] [PubMed] [Google Scholar]
- 19.Leibbrandt ME, Wolfgang GH, Metz AL, Ozobia AA, Haskins JR. Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int. 48: 761–770 1995 [DOI] [PubMed] [Google Scholar]
- 20.Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH. Metabolism of cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol. 14: 1–10 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner MW. Mechanism of cis-platinum nephrotoxicity: II. Morpholog ic observations. J Pharmacol Exp Ther. 213: 551–556 1980 [PubMed] [Google Scholar]
- 22.Dobyan DC, Hill D, Lewis T, Bulger RE. Cyst formation in rat kidney induced by cis-platinum administration. Lab Invest. 45: 260–268 1981 [PubMed] [Google Scholar]
- 23.Chopra S, Kaufman JS, Jones TW, Hong WK, Gehr MK, Hamburger RJ, Flamenbaum W, Trump BF. Cis-diamminedichlorplatinum-induced acute renal failure in the rat. Kidney Int. 21: 54–64 1982 [DOI] [PubMed] [Google Scholar]
- 24.Uehara T, Watanabe H, Itoh F, Inoue S, Koshida H, Nakamura M, Yamate J, Maruyama T. Nephrotoxicity of a novel antineoplastic platinum complex, nedaplatin: a comparative study with cisplatin in rats. Arch Toxicol. 79: 451–460 2005 [DOI] [PubMed] [Google Scholar]
- 25.Nagothu KK, Bhatt R, Kaushal GP, Portilla D. Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int. 68: 2680–2693 2005 [DOI] [PubMed] [Google Scholar]
- 26.Uehara T, Tsuchiya N, Masuda A, Torii M, Nakamura M, Yamate J, Maruyama T. Time course of the change and amelioration of nedaplatin-induced nephrotoxicity in rats. J Appl Toxicol. 28: 388–398 2008 [DOI] [PubMed] [Google Scholar]
- 27.Uehara T, Miyoshi T, Tsuchiya N, Masuno K, Okada M, Inoue S, Torii M, Yamate J, Maruyama T. Comparative analysis of gene expression between renal cortex and papilla in nedaplatin-induced nephrotoxicity in rats. Hum Exp Toxicol. 26: 767–780 2007 [DOI] [PubMed] [Google Scholar]
- 28.Safirstein R, Miller P, Guttenplan JB. Uptake and metabolism of cisplatin by rat kidney. Kidney Int. 25: 753–758 1984 [DOI] [PubMed] [Google Scholar]
- 29.Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 67: 1171–1176 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 296: F505–F511 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 167: 1477–1484 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui K. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 70: 1823–1831 2005 [DOI] [PubMed] [Google Scholar]
- 33.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 86: 396–402 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1–3 and multidrug and toxin extrusion family). J Pharmacol Exp Ther. 319: 879–886 2006 [DOI] [PubMed] [Google Scholar]
- 35.Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 74: 477–487 2007 [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Nagasaki T, Okabe H, Uchida N, Norikura R, Inazawa K, Yamauchi A, Katsuyama Y, Segawa K, Murakami T, Adachi Y, Mizojiri K, Matsubara T, Kawai K, Akaboshi M. Studies on the disposition of a new anti-neoplastic agent, nedaplatin. Whole-body autoradiography, blood concentration and excretion of [195mPt]nedaplatin following intravenous administration in rats. Iyakuhin Kenkyu. 26: 887–895 1995; . [Google Scholar]
- 37.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 31: 971–997 1999 [DOI] [PubMed] [Google Scholar]
- 38.Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 73: 994–1007 2008 [DOI] [PubMed] [Google Scholar]
- 39.Santos NA, Catão CS, Martins NM, Curti C, Bianchi ML, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 81: 495–504 2007 [DOI] [PubMed] [Google Scholar]
- 40.Huang Q, Dunn RT, 2nd, Jayadev S, DiSorbo O, Pack FD, Farr SB, Stoll RE, Blanchard KT. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol Sci. 63: 196–207 2001 [DOI] [PubMed] [Google Scholar]
- 41.Aleksunes LM, Goedken MJ, Rockwell CE, Thomale J, Manautou JE, Klaassen CD. Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther. 335: 2–12 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerrero-Beltrán CE, Calderón-Oliver M, Tapia E, Medina-Campos ON, Sánchez-González DJ, Martínez-Martínez CM, Ortiz-Vega KM, Franco M, Pedraza-Chaverri J. Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicol Lett. 192: 278–285 2010; . [DOI] [PubMed] [Google Scholar]
- 43.Okuda M, Masaki K, Fukatsu S, Hashimoto Y, Inui K. Role of apoptosis in cisplatin-induced toxicity in the renal epithelial cell line LLC-PK1. Implication of the functions of apical membranes. Biochem Pharmacol. 59: 195–201 2000 [DOI] [PubMed] [Google Scholar]
- 44.Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 72: 53–62 2007 [DOI] [PubMed] [Google Scholar]
- 45.Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol. 13: 858–865 2002 [DOI] [PubMed] [Google Scholar]
- 46.Megyesi J, Udvarhelyi N, Safirstein RL, Price PM. The p53-independent activation of transcription of p21 WAF1/ CIP1/SDI1 after acute renal failure. Am J Physiol. 271: F1211–F1216 1996 [DOI] [PubMed] [Google Scholar]
- 47.Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol. 289: F514–F520 2005 [DOI] [PubMed] [Google Scholar]
- 48.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 285: F610–F618 2003 [DOI] [PubMed] [Google Scholar]
- 49.Faubel S, Ljubanovic D, Reznikov L, Somerset H, Dinarello CA, Edelstein CL. Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int. 66: 2202–2213 2004 [DOI] [PubMed] [Google Scholar]
- 50.Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol. 270: F700–F708 1996 [DOI] [PubMed] [Google Scholar]
- 51.Battershill JM. Toxicogenomics: regulatory perspective on current position. Hum Exp Toxicol. 24: 35–40 2005 [DOI] [PubMed] [Google Scholar]
- 52.Heinloth AN, Irwin RD, Boorman GA, Nettesheim P, Fannin RD, Sieber SO, Snell ML, Tucker CJ, Li L, Travlos GS, Vansant G, Blackshear PE, Tennant RW, Cunningham ML, Paules RS. Gene expression profiling of rat livers reveals indicators of potential adverse effects. Toxicol Sci. 80: 193–202 2004 [DOI] [PubMed] [Google Scholar]
- 53.Irwin RD, Boorman GA, Cunningham ML, Heinloth AN, Malarkey DE, Paules RS. Application of toxicogenomics to toxicology: basic concepts in the analysis of microarray data. Toxicol Pathol. 32: 72–83 2004; . [DOI] [PubMed] [Google Scholar]
- 54.Searfoss GH, Ryan TP, Jolly RA. The role of transcriptome analysis in pre-clinical toxicology. Curr Mol Med. 5: 53–64 2005 [DOI] [PubMed] [Google Scholar]
- 55.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 61: 748–755 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauer M, Bauer I. Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal. 4: 749–758 2002 [DOI] [PubMed] [Google Scholar]
- 57.Takahashi T, Morita K, Akagi R, Sassa S. Protective role of heme oxygenase-1 in renal ischemia. Antioxid. Redox Signal. 6: 867–877 2004 [DOI] [PubMed] [Google Scholar]
- 58.Chubatsu LS, Meneghini R. Metallothionein protects DNA from oxidative damage. Biochem J. 291: 193–198 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miles AT, Hawksworth GM, Beattie JH, Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol. 35: 35–70 2000 [DOI] [PubMed] [Google Scholar]
- 60.Hagrman D, Goodisman J, Dabrowiak JC, Souid AK. Kinetic study on the reaction of cisplatin with metallothionein. Drug Metab Dispos. 31: 916–923 2003 [DOI] [PubMed] [Google Scholar]
- 61.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 264: 13963–13966 1989 [PubMed] [Google Scholar]
- 62.Holmgren A, Aslund F. Glutaredoxin. Meth Enzymol. 252: 283–292 1995 [DOI] [PubMed] [Google Scholar]
- 63.Hayes JD, Strange RC. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic Res. 22: 193–207 1995 [DOI] [PubMed] [Google Scholar]
- 64.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 31: 11–24 1982 [DOI] [PubMed] [Google Scholar]
- 65.Moll R. Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell Biochem. 31: 205–262 1998 [PubMed] [Google Scholar]
- 66.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 40: 403–439 2002 [DOI] [PubMed] [Google Scholar]
- 67.Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal anti bodies to keratins: strategies, applications, and limitations. Lab Invest. 52: 243–256 1985 [PubMed] [Google Scholar]
- 68.Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol. 50: 147–158 1993 [DOI] [PubMed] [Google Scholar]
- 69.Uehara T, Tsuchiya N, Torii M, Yamate J, Maruyama T. Amelioration of nedaplatin-induced nephrotoxicity by continuous infusion in rats. J Toxicol Pathol. 20: 141–147 2007 [Google Scholar]
- 70.Yamate J, Tatsumi M, Nakatsuji S, Kuwamura M, Kotani T, Sakuma S. Immunohistochemical observations on the kinetics of macrophages and myofibroblasts in rat renal interstitial fibrosis induced by cis-diamminedichloroplatinum. J Comp Pathol. 112: 27–39 1995; . [DOI] [PubMed] [Google Scholar]
- 71.Yamate J, Ishida A, Tsujino K, Tatsumi M, Nakatsuji S, Kuwamura M, Kotani T, Sakuma S. Immunohistochemical study of rat renal interstitial fibrosis induced by repeated injection of cisplatin, with special reference to the kinet ics of macrophages and myofibroblasts. Toxicol Pathol. 24: 199–206 1996 [DOI] [PubMed] [Google Scholar]
- 72.Yamate J, Machida Y, Ide M, Kuwamura M, Sawamoto O, LaMarre J. Effects of lipopolysaccharide on the appearance of macrophage populations and fibrogenesis in cisplatin-induced rat renal injury. Exp Toxicol Pathol. 56: 13–24 2004 [DOI] [PubMed] [Google Scholar]
- 73.Yamate J, Machida Y, Ide M, Kuwamura M, Kotani T, Sawamoto O, LaMarre J. Cisplatin-induced renal interstitial fibrosis in neonatal rats, developing as solitary nephron unit lesions. Toxicol Pathol. 33: 207–217 2005 [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto E, Izawa T, Juniantito V, Kuwamura M, Sugiura K, Takeuchi T, Yamate J. Involvement of endogenous prostaglandin E2 in tubular epithelial regeneration through inhibition of apoptosis and epithelial-mesenchymal transition in cisplatin-induced rat renal lesions. Histol Histopathol. 25: 995–1007 2010 [DOI] [PubMed] [Google Scholar]