Abstract
The present report describes a rare case of spontaneous primary histiocytic sarcoma of the popliteal lymph node in a 19-week-old female Sprague-Dawley (SD) rat. At necropsy, a 10 mm-diameter whitish nodule was found at the site of the femoral muscle in the right hindlimb. Histopathologically, the nodule comprised large pleomorphic histiocyte-like cells with abundant eosinophilic or foamy cytoplasm. Multinucleated giant cells, necrotic foci surrounded by palisading arrays of epithelioid histiocyte-like cells and phagocytosis of cell debris or erythrocytes by the neoplastic cells were occasionally observed. Invasion of the tumor cells into the surrounding adipose tissue was found focally, but there were no distal metastases. Immunohistochemically, the neoplastic cells were positive for vimentin, CD68 (ED1) and lysozyme. We concluded that this tumor occurred in the popliteal lymph node, considering the anatomical location of the lesion and the presence of the remnants of lymphoid tissue involved in the tumor.
Keywords: histiocytic sarcoma, lymph node, rats
Histiocytic sarcoma is the most frequent hematopoietic tumor in aging rats, but rarely occurs before 12 months of age in rats 1 . It has been reported that the spontaneous incidence of this tumor in rats is between 1% and 5% depending on the strain 2 – 4 . The most common localizations of this tumor are different between rat strains and are as follows: the liver and lungs in Sprague-Dawley (SD) rats 4 , liver, bone marrow, lymph nodes, spleen and lungs in Fischer and Donryu rats 3 and subcutis in Wistar rats 5 . In most cases, metastases are observed frequently, and the tumors are generally observed at multiple sites, such as the liver, lymph nodes and lungs 4 .
In this case report, we describe a rare case of spontaneous primary histiocytic sarcoma of the popliteal lymph node in a young female SD rat.
The animal was a female SD rat (Crl:CD(SD)) purchased from Charles River Japan Inc., Japan, and used in a treatment group of a 3-month repeated-dose toxicity study. The animal was housed in a wire mesh cage under routine controlled conditions (temperature, 23 ± 2°C; humidity, 60 ± 20%; lighting, 12 hours) and had free access to a CRF-1 diet (Oriental Yeast Co., Ltd.) and tap water. During the study, no abnormal changes in clinical signs, body weights or blood chemistry were observed in the animal. The treatment and handling of the animal were approved by the Institutional Animal Care and Use Committee of the Toxicology Research Laboratories, Central Pharmaceutical Research Institute, Japan Tobacco Inc.
After 3 months of repeated drug administration in the toxicity study, the animal was submitted for scheduled sacrifice at 19 weeks of age. It was exsanguinated under anesthesia and examined macroscopically. At necropsy, a 10 mm-diameter whitish nodule was found around the femur close to the biceps femoris muscle and the sciatic nerve in the right hindlimb. When sectioned, the nodule was whitish and firm. There were no nodular lesions in the other organs and tissues in this animal. The nodule was considered to be a spontaneous lesion unrelated to any chemically-induced effects because it was found only at the mid dose level of the test article and not at higher doses.
The nodule and other organs from the animal, including the bone marrow, liver, lung, spleen and mesenteric and submandibular lymph nodes, were fixed in 10% neutral buffered formalin. After being embedded in paraffin, the samples were sectioned at 3 μm, stained with hematoxylin and eosin and then subjected to histopathological examination. Immunohistochemistry was also performed on deparaffinized sections of the nodule using antibodies against vimentin (V9; mouse monoclonal, Dako, Denmark, dilution 1:50), CD68 (ED1; mouse monoclonal, BMA Biomedicals, Switzerland, dilution 1:100), lysozyme (rabbit polyclonal, Dako, Denmark, dilution 1:200), alpha1-antitrypsin (rabbit polyclonal, Dako, dilution 1:800) and PCNA (proliferating cell nuclear antigen, PC10; mouse monoclonal, Dako, dilution 1:100); this was followed by Histofine® Simple Stain MAX-PO (MULTI) procedures (Universal Immuno-peroxidase Polymer for rat tissue sections, Nichirei, Tokyo, Japan). The substrate was hydrogen peroxide with the coloring agent, 3,3-diaminobenzidine (DAB). Antigen retrieval for immunohistochemical staining was performed using an autoclave with citrate buffer (pH 6.0).
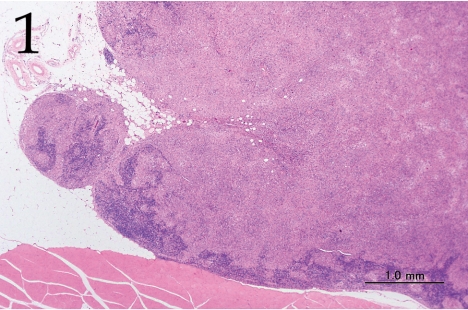
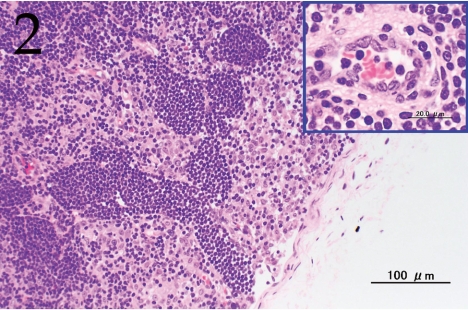
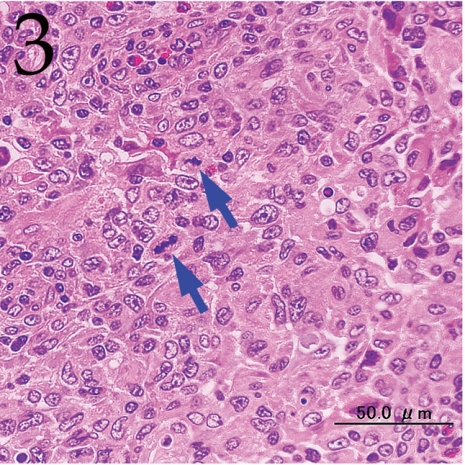
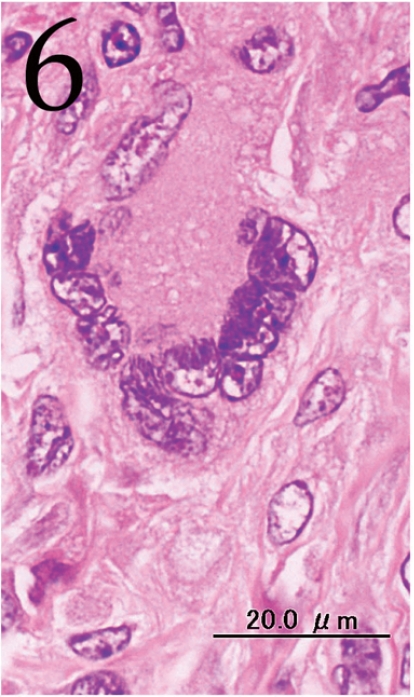
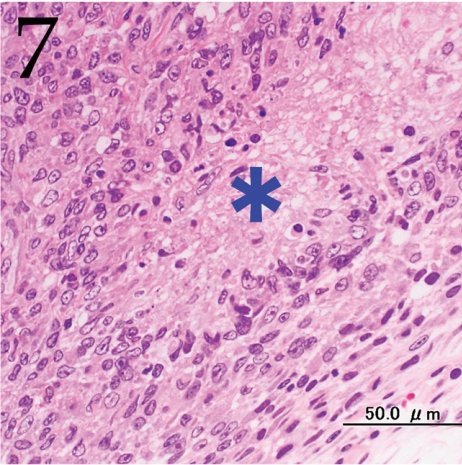
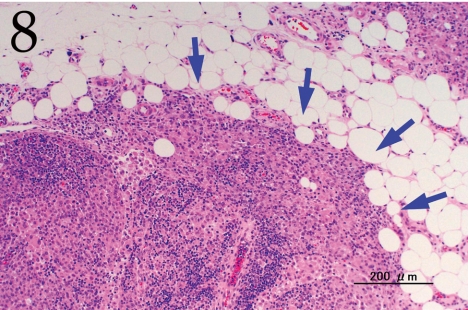
Histopathologically, the nodule was encapsulated with thin fibrous connective tissue and compressed the adjacent muscular and adipose tissues (Fig. 1). Lymph node-like tissue was observed in the subcapsular region of the nodule (Fig. 2). There were small lymphoid cells and blood vessels characterized by simple cuboidal endothelial cells (inset of Fig. 2), which are commonly seen in lymphoid tissues. The tumor cells exhibited various forms, from round to spindle shapes with abundant eosinophilic cytoplasm (Fig. 3), and occasionally contained vacuoles of various sizes and phagocytosed erythrocytes or cell debris (Figs. 4 and 5). The nuclei of the tumor cells were oval to elongated or folded. Bizarre nuclei were often found with moderate anisokaryosis. Multinucleated giant cells (Fig. 6), mitotic figures (Fig. 3) and necrotic foci surrounded by palisading arrays (Fig. 7) of neoplastic epithelioid histiocytic cells were occasionally observed. The tumor partly showed a fascicular pattern consisting of spindle cells with deposition of a fibrous matrix staining blue with Masson’s trichrome stain, indicating the matrix to be collagen fibers (figure not shown). Invasion of the tumor cells into the surrounding adipose tissue was found focally (Fig. 8).
Fig. 1.
Lower magnification of the lesion. The nodule is adjacent to muscle and adipose tissue and compressed remnants of lymph node tissue are present in the lesion. H&E. Bar=1.0 mm.
Fig. 2.
Lymph node-like tissue was observed in the subcapsular region of the nodule. Inset: high endothelial venule (HEV). H&E. Bar=100 μm.
Fig. 3.
Pleomorphism and frequent mitotic figures (arrows) in the tumor cells. H&E. Bar=50 μm.
Fig. 4.
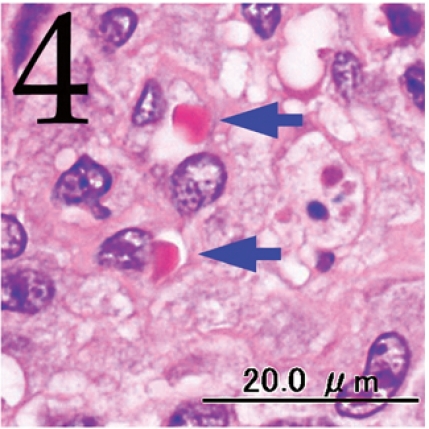
Phagocytosis of erythrocytes by tumor cells (arrows). H&E. Bar=20 μm.
Fig. 5.
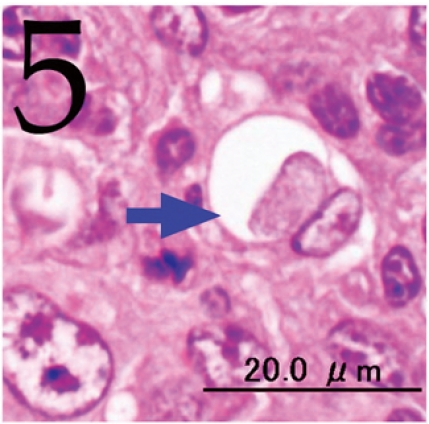
Phagocytosis of cellular debris by a tumor cell (arrow). H&E. Bar=20 μm.
Fig. 6.
Multinucleated giant cell. H&E. Bar=20 μm.
Fig. 7.
Necrosis (asterisk) surrounded by palisading arrays of tumor cells with elongated, irregular nuclei are sometimes observed. H&E. Bar=50 μm.
Fig. 8.
Tumor cells invading the adjacent adipose tissue (arrows). H&E. Bar=200 μm.
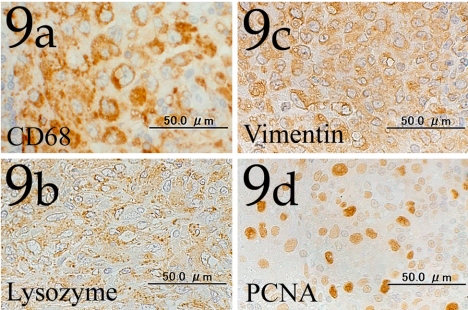
Immunohistochemically, the cytoplasm of the tumor cells was positive for CD68, lysozyme and vimentin (Figs. 9a , 9b and 9c), and the nuclei of the cells were also frequently stained with PCNA (Fig. 9d).
Fig. 9.
Tumor cells showing positive immunohistochemical reactions for CD68 (Fig. 9a), lysozyme (Fig. 9b) and vimentin (Fig. 9c). The tumor cells frequently show a high intensity immunohistochemical reaction for PCNA (Fig. 9d). Immunohistochemistry. Bar=50 μm.
The morphological and immunohistochemical characteristics of the present proliferative lesion were consistent with a histiocytic proliferative disorder 1 , 3 – 5 . The lesion showed both sarcomatous and granulomatous appearances consisting of large pleomorphic histiocytic cells with abundant cytoplasm. The tumor showed malignant properties including cell atypia, mitotic figures, necrotic foci and invasion into the adjacent adipose tissue, whereas no similar proliferative lesions were found in the other organs or tissues. We diagnosed the tumor as a histiocytic sarcoma based on the typical histopathological findings.
In SD rats, previous studies have reported histiocytic sarcomas in 105 out of 2227 animals (incidence rate: 4.7% 4 ) and in 40 out of 3530 animals (1.1% 2 ). There were no clear sex differences in the incidence of this tumor. Histiocytic sarcomas rarely occur in young rats. In Squire’s study of SD rats, over 80% of the histiocytic sarcomas were observed in rats that were more than 18 months of age at the time of death, while relatively few (<5%) sarcomas were observed in rats less than one year old 4 . First onset of this tumor has been reported to be at around 40 weeks of age in SD rats 7 and 75 weeks of age in F344 and Donryu rats 3 . In our case, histiocytic sarcoma was found in a 19-week-old rat, and this is much earlier than previous reports.
There has been considerable confusion in the usage of the terms histiocytic sarcoma and malignant fibrous histiocytoma (MFH) in rats. Rat MFH cells originally possess histiocyte/macrophage-like features 8 . MFH cells show positive immunoreactivity against CD68 (ED1) and have cytoplasmic lysosomal granules that are similar to those of histiocytic sarcoma cells 8 . However, histiocytic sarcomas differ morphologically from MFHs. Histiocytic sarcoma consists of sheets of round to fusiform cells with granulomatous areas 3 , 9 . On the other hand, MFH consists of histiocytic and fibroblastic cells arranged predominantly in a storiform-pleomorphic growth pattern 10 , 11 . No typical morphological features of MFH were observed in the present case, although the tumor was partly accompanied by a fibrous matrix.
The blood vessels with simple cuboidal endothelial cells were considered to be high endothelial venules (HEVs), which are specific venules commonly seen in the paracortical zone of the lymphoid tissues 12 . Small lymphoid cells were found around the blood vessels at varying degrees in the present lesion. The presence of HEVs and lymphoid cells around the blood vessels indicated that this tumor occurred in the lymphoid tissues. The tumor was located close to the biceps femoris muscle in the right hindlimb. We concluded that it arose from the popliteal lymph node, based on the results of the histopathological examinations and the anatomical location of the tumor.
The localization of histiocytic sarcomas differs between rat strains. In SD rats, the most common localizations are the liver and lungs 4 . In Donryu and F344 rats, the bone marrow, liver, lymph nodes, spleen and lungs are very common sites 3 . Ogasawara et al. suggested that histiocytic sarcomas arise from the hematopoietic organs or liver in Donryu and F344 rats 3 . Most histiocytic sarcomas metastasize readily to adjacent tissues and the lungs, and they are commonly observed at multiple sites in rats 1 , 4 . Squire et al. reported that among 105 cases of histiocytic sarcomas in SD rats, only 14 animals had the lesion in a single site, and the most common localization was the liver 4 . In their case, only one animal had a solitary histiocytic sarcoma in a lymph node, indicating that the liver is the most likely primary site of histiocytic sarcoma in SD rats 4 . In our case, the tumor cells were limited to the popliteal lymph node, and no metastases were observed. Therefore, histiocytic sarcoma in this case was considered to arise from the popliteal lymph node. Most histiocytic sarcomas found in the lymph nodes in SD rats are probably metastases from the liver. A solitary histiocytic sarcoma in the lymph node is very rare in SD rats.
To our knowledge, there are very few available reports on histiocytic sarcoma of the lymph node as a single site, especially in young rats. This is a rare case of a primary histiocytic sarcoma occurring spontaneously in the lymph node in a young SD rat.
References
- 1.Frith CH, Ward JM, Chandra M. The morphology, immunohistochemistry, and incidence of hematopoietic neoplasms in mice and rats. Toxicol Pathol. 1993;21:206–218. doi: 10.1177/019262339302100213. [DOI] [PubMed] [Google Scholar]
- 2.Frith CH. Morphologic classification and incidence of hematopoietic neoplasms in the Sprague-Dawley rat. Toxicol Pathol. 1988;16:451–457. doi: 10.1177/019262338801600405. [DOI] [PubMed] [Google Scholar]
- 3.Ogasawara H, Mitsumori K, Onodera H, Imazawa T, Shibutani M, Takahashi M. Spontaneous histiocytic sarcoma with possible origin from the bone marrow and lymph node in Donryu and F-344 rats. Toxicol Pathol. 1993;21:63–70. doi: 10.1177/019262339302100108. [DOI] [PubMed] [Google Scholar]
- 4.Squire RA, Brinkhous KM, Peiper SC, Firminger HI, Mann RB, Strandberg JD. Histiocytic sarcoma with a granuloma-like component occurring in a large colony of Sprague-Dawley rats. Am J Pathol. 1981;105:21–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Barsoum NJ, Hanna W, Gough AW, Smith GS, Sturgess JM, de la Iglesia FA. Histiocytic sarcoma in Wistar rats. A light microscopic, immunohistochemical, and ultrastructural study. Arch Pathol Lab Med. 1984;108:802–807. [PubMed] [Google Scholar]
- 6.Wright JA, Goonetilleke UR, Waghe M, Horne M, Stewart MG. An immunohistochemical study of spontaneous histiocytic tumours in the rat. J Comp Pathol. 1991;104:223–232. doi: 10.1016/s0021-9975(08)80105-3. [DOI] [PubMed] [Google Scholar]
- 7.Son WC, Gopinath C. Early occurrence of spontaneous tumors in CD-1 mice and Sprague-Dawley rats. Toxicol Pathol. 2004;32:371–374. doi: 10.1080/01926230490440871. [DOI] [PubMed] [Google Scholar]
- 8.Yamate J, Fumimoto S, Kuwamura M, Kotani T, Lamarre J. Characterization of a rat subcutaneous malignant fibrous histiocytoma and its tumor lines, with reference to histiocytic features. Vet Pathol. 2007;44:151–160. doi: 10.1354/vp.44-2-151. [DOI] [PubMed] [Google Scholar]
- 9.Yamate J, Maeda M, Tsukamoto Y, Benn SJ, Laithwaite JE, Allan A, Kannan Y, Ide M, Kuwamura M, Kotani T, Sakuma S, LaMarre J. Macrophage-like cell line (HS-P) from a rat histiocytic sarcoma. J Comp Pathol. 2001;124:183–191. doi: 10.1053/jcpa.2000.0452. [DOI] [PubMed] [Google Scholar]
- 10.Maekawa A, Ogiu T, Onodera H, Furuta K, Matsuoka C, Ohno Y, Tanigawa H, Salmo GS, Matsuyama M, Hayashi Y. Foreign-body tumorigenesis in rats by various kinds of plastics-induction of malignant fibrous histiocytomas. J Toxicol Sci. 1984;9:263–272. doi: 10.2131/jts.9.263. [DOI] [PubMed] [Google Scholar]
- 11.Ward JM, Kulwich BA, Reznik G, Berman JJ. Malignant fibrous histiocytoma. An unusual neoplasm of soft-tissue origin in the rat that is different from the human counterpart. Arch Pathol Lab Med. 1981;105:313–316. [PubMed] [Google Scholar]
- 12.Ohtani O, Ohtani Y. Structure and function of rat lymph nodes. Arch Histol Cytol. 2008;71:69–76. doi: 10.1679/aohc.71.69. [DOI] [PubMed] [Google Scholar]