Abstract
This review concerns stem cells and their relation to intestinal metaplasia. When gastric regions of mice, Mongolian gerbils or several strains of rats were irradiated with a total dose of 20 Gy of X-rays given in two fractions, intestinal metaplasia was only induced in rats. In addition, it was greatly influenced by rat strain and sex. Alkaline phosphatase (ALP) positive metaplastic foci were increased by administration of ranitidine (H2 receptor antagonist), crude stomach antigens or subtotal resection of the fundus and decreased by cysteamine (gastric acid secretion stimulator), histamine or removal of the submandibular glands. Recent studies have shown that Cdx2 transgenic mice with gastric achlorhydria develop intestinal metaplasia and that in men and animals, Helicobacter pylori (H. pyrlori) infection can cause intestinal metaplasias that are reversible on eradication. Our results combined with findings for H. pylori infection or eradication and transgenic mice suggest that an elevation in the pH of the gastric juice due to disappearance of parietal cells is one of the principal factors for development of reversible intestinal metaplasia. When different organs were transplanted into the stomach or duodenum, they were found to transdifferentiate into gastric or duodenal mucosae, respectively. Organ-specific stem cells in normal non-liver tissues (heart, kidney, brain and skin) also differentiate into hepatocytes when transplanted into an injured liver. Therefore, stem cells have a multipotential ability, transdifferentiating into different organs when transplanted into different environments. Finally, intestinal metaplasia has been found to possibly increase sensitivity to the induction of tumors by colon carcinogens of the 1,2-dimethylhydrazine (DMH), azoxymethane (AOM) or 2-amino-1-methyl-6-phenylimidazo[4.5-b]pyridine (PhIP) type. This carcinogenic process, however, may be relatively minor compared with the main gastric carcinogenesis process induced by N-methy1-N’-nitro-N-nitrosoguanidine (MMNG) or N-methylnitrosourea (MNU), which is not affected by the presence of intestinal metaplasia. The protocol used in these experiments may provide a new approach to help distinguish between developmental events associated with intestinal metaplasia and gastric tumors.
Keywords: X-irradiation, rat, glandular stomach, intestinal metaplasia, gastric cancer
Introduction
Throughout adult life, new developmental commitment of adult stem cells may cause reversible metaplastic conversion to occur in some organs. For example, ectopic bone formation is common in surgical scars, muscle or walls of sclerotic arteries 1 and squamous metaplasia may appear in epithelia of the respiratory tract 2 or urinary bladder. 3 , 4 Barrett’s metaplasia of the esophagus develops as a result of duodenal-esophageal reflex, 5 and gastric metaplasia in the duodenum 6 is observed with mucosal injury related to active duodenitis; both are due to greater acid output.
Intestinal metaplasia results from diverted differentiation of gastric stem cells towards cells of small intestine or colonic phenotypes and is characterized by the presence of intestinal-type, mucin-containing goblet cells, Paneth cells and absorptive cells. 7 , 8 It is more prevalent in men than in women, and an increase with age has been noted. 9 The frequency of intestinal metaplasia varies widely in different countries, areas and races. 10 In the stomach, it has been considered to be a possible precancerous state on the basis of epidemiological surveys. 7 , 10 Several authors have suggested that intestinal metaplasia could play a role in the development of gastric carcinomas, 9 but it is not generally termed precancer because it is common in benign conditions. 10 , 11 Moreover, its pathogenesis remains unclear. The present review describes findings on the induction of intestinal metaplasia for analysis of its relation to neoplasia, with a focus on stem cells having a multipotential ability to transdifferentiate when transplanted into different environments.
Classification of Intestinal Metaplasia
The small intestinal mucosa has been observed in human stomachs since the 19th century, and Sugimura et al. proposed classification into complete and incomplete types. 12 Histochemical and immunochemical stains that identify enzymes or mucosubstances have provided evidence that metaplastic epithelial cells resemble small or large intestinal cells. Teglbaerg and Nielsen 13 therefore subdivided the types into small intestinal and large intestinal types using periodate-borohydride/KOH/PAS and alcian blue pH 2.6-PAS methods. They suggested that intestinal metaplasia of the colonic type should have a certain premalignant potential, whereas intestinal metaplasia of the small intestinal type should merely be of reactive character, without such premalignant potential. They further described incomplete intestinal metaplasia to be a hybrid epithelium, with features of both gastric and intestinal mucosa. In addition, intestinal metaplasia has been classified by Jass and Filipe into three grades, complete or type I in which goblet cells contain sialomucin, incomplete without sulphomucins (type IIA) and incomplete with sulphomucins present in the colon (type IIB), and association with intestinal cancer has been suggested. 14
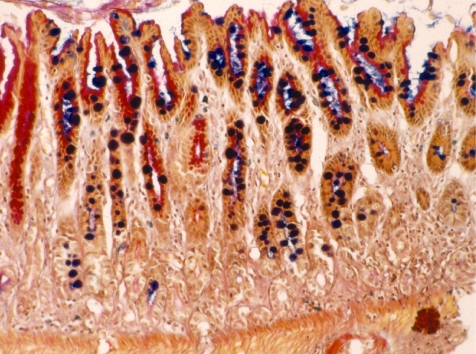
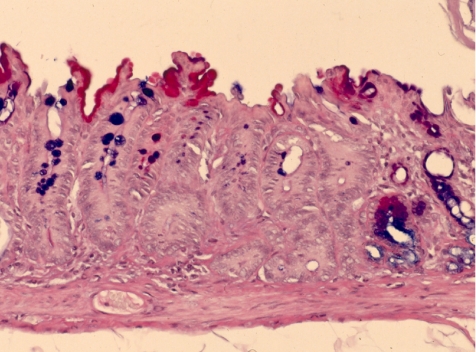
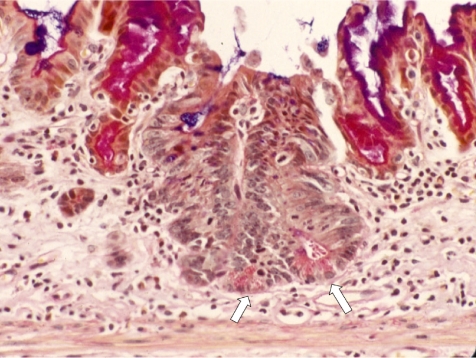
We have proposed three different types of intestinal metaplasia from our experience with animal experiments, 15 one with goblet cells in the gastric mucosa (Type A, Fig. 1); another with intestinal-type crypts without Paneth cells (Type B, Fig. 2); and the last with intestinal-type crypts with Paneth cells (Type C, Fig. 3). Recently, Tsukamoto et al. 16 reported intestinal metaplasia to be divided into two major types, a gastric and intestinal (GI) mixed type and a solely intestinal type (I) type, using gastric and intestinal cell markers.
Fig. 1.
Goblet cells with gastric mucosa (Type A). Alcian blue-PAS staining, ×200. Round and ultramarine stained goblet cells were found in PAS-positive (pink) gastric glands.
Fig. 2.
Intestinal crypt without Paneth cells (Type B). Alcian blue-PAS staining, ×200. PAS-negative glands have many goblet cells but no Paneth cells at the bottom.
Fig. 3.
Intestinal crypt with Paneth cells (Type C). Alcian blue PAS staining, ×200. Arrows: Paneth cells.
X-ray Induced Intestinal Metaplasia and Its Properties
Dosing of X-ray on induction of intestinal metaplasia 17 – 19
No intestinal metaplasia was induced by four X-ray doses of 1 Gy, but appreciable lesions were noted with six X-ray doses of 5 Gy for a total dose of 30 Gy. An increase in intestinal metaplasia was induced by two X-ray doses of 10 Gy each at a 3-day interval for a total dose of 20 Gy, but no gastric tumors appeared after 12 months. However, gastric tumors were induced after a single X-irradiation dose of 20 Gy, and the incidence was increased with two 20 Gy doses given at an interval of 1 week. In contrast, the incidence of intestinal metaplasia was decreased. Thus, these results provide evidence that the best induction of intestinal metaplasia was two X-ray doses of 10 Gy each at a 3-day interval for a total dose of 20 Gy in 5-week-old male rats.
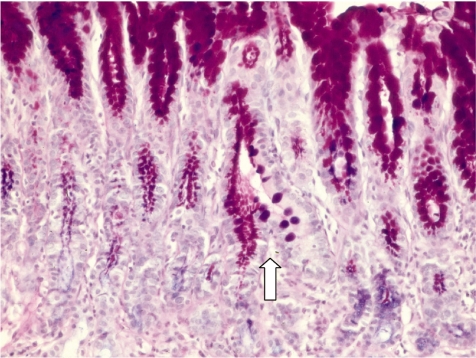
Sequential development of intestinal metaplasia by X-ray exposure in rats 17
Goblet cells in the gastric mucosa (Type A) appeared 1 week after irradiation of two X-ray doses of 10 Gy with a 3-day interval. Intestinal-type crypts without Paneth cells (Type B, Fig. 4) were seen 2 weeks after irradiation, and Paneth cells (Type C) were finally observed at the bottom of intestinal-type crypt with the brush border an upper part of the crypts 8 weeks after irradiation. Crypts that had alkaline phosphatase (ALP) activity (Fig. 5) were also seen around 8 weeks after irradiation.
Fig. 4.
Gastric mucosa differentiating into an intestinal crypt. Alcian blue-PAS staining, ×200. Arrow: One side is PAS-positive gastric gland and in other hand intestinal type crypt.
Fig. 5.
Alkaline phosphatase active stomach.
Strain and spices differences
Strain differences in the susceptibility of rats to induction of intestinal metaplasia by X-irradiation were examined using gastric regions of 5-week-old male rats irradiated with a total dose of 20 Gy of X-rays given in two equal fractions separated by 3 days. Upon sacrifice at 6 months after the last irradiation, the number of intestinal metaplastic crypts positive for ALP was highest in Donryu rats and lowest in Copenhagen rats. 20 Morphologically, the numbers of crypts with intestinal metaplasia in the glandular stomachs of Donryu, Wistar, SD and F344 rats were higher than in ACI (MNNG-sensitive strain), Buffalo and Copenhagen rats. 21 Intestinal metaplasia was more frequently observed in the pyloric glands than in the fundic glands. The results demonstrate that induction of intestinal metaplasia by X-irradiation is greatly influenced by the strain of rat. However, intestinal metaplasia was not induced in mice 17 and Mongolian gerbils 22 using the same irradiation protocol.
Sex differences 23 , 24
The influence of sex hormones on induction of intestinal metaplasia was examined in 5-week-old Crj/CD rats of both sexes. At the age of 4 weeks, animals were gonadectomized, and some groups of rats were given either testosterone or dimethyl estradiol (DES). One week after the operation, they were irradiated with two 10-Gy doses of X-rays to the gastric region at a 3-day interval for a total of 20 Gy. At termination of the experiment at 6 months after X-irradiation, the incidence of intestinal metaplasia with ALP-positive foci in males was significantly higher than in females, the orchidectomized males or the orchidectomized plus DES-treated rats. On the other hand, the incidence of intestinal metaplasia with ALP-positive foci in normal females appeared to be lower than in the ovariectomized females and was increased in rats by treatment with testosterone and decreased by treatment with DES. These results suggested a promoting role for testosterone in the development of intestinal ALP-positive lesions and indicated considerable heterogeneity between intestinal subtypes.
Genetic Alteration
Maintenance of intestinal differentiation appears to depend on the presence of Cdx2, an intestine-specific transcription factor, and loss Cdx2 expression leads to focal gastric differentiation in the colon. 25 In contrast, aberrant expression of Cdx2 in the upper gastrointestinal tract is a key event in the pathogenesis of Barrett’s esophagus 26 and intestinal metaplasia in the stomach. 27 Cdx2 expression correlates with development of intestinal metaplasia, 28 and the levels in the corpus lesser curvature significantly decrease after eradication of Helicobacter pylori (H. pylori). 29 Cdx1 30 and Cdx2 31 are major transcription factors in the development of intestinal metaplasia, which is supported by transgenic mouse studies, which have shown that ectopic expression of either Cdx1 or Cdx2 in the gastric epithelium is sufficient to induce a metaplastic conversion. 32 , 37 It is considered that Cdx2 is a master regulator of the intestinal differentiation program.
Judd et al. 33 reported that N/K-ATPase-deficient (Atp4a(–/–)) transgenic mice with gastric achlorhydria and hypergastrinemia develop incomplete intestinal metaplasia. Silberg et al. 27 found that ectopic expression of Cdx2 in the gastric epithelium is sufficient to cause transdifferentiation of the gastric mucosa into intestinal-type cells. They also found that sucrose isomaltase (SI) was not activated in the transgenic mouse stomach. Mutoh et al. 32 reported that all of the gastric mucosal cells except enterochromaffin-like cells were completely replaced by intestinal-type cells, including goblet cells and absorptive cells, in another transgenic mouse strain in which Cdx2 was expressed in parietal cells under the control of the H+/K+-ATPase promoter. In this case, parietal cells disappeared after approximately 6 weeks, and the pH in the stomach increased from 2 to more than 7. Differentiation of intestinal-type cells may be induced not only by the expression of Cdx2, but also by the loss of parietal cells in the transgenic stomach, as reported by Mutoh et al.. 32 Li et al. 34 found that GIF-11 Runx3+p53 + cells expressed SI when cultured and showed that some Runx3 mouse gastric epithelial cells differentiated into intestinal-type cells that expressed Cdx2. Thus, Fukamachi et al. 35 suggested that gastric epithelium cells can differentiate into intestinal-type cells, probably due to expression of Cdx2 when the function of Runx3 is impaired. In contrast, Yuasa et al. reported that X-irradiation-induced intestinal metaplasia is not associated with alterations of the H-ras, K-ras and p53 genes. 36
Helicobacter pylori Infection
The discovery of H. pylori in adult patients by Marshall and Warren 37 was a major event in modern gastroenterology and was honored with the Nobel Prize in 2005. The WHO has classified H. pylori as a group I carcinogen for gastric carcinomas, and infected individuals have a two to eight times higher risk of stomach tumor development than the general population. Correa 38 , 39 suggested that chronic gastritis, gastric atrophy, intestinal metaplasia, dysplasia and gastric cancer develop stepwise. Eradication of H. pylori infection produces a marked increase in the regression rate of precancerous lesions and the relative risk of gastric atrophy and intestinal metaplasia. 39 Ito et al. followed up 22 patients in whom H. pylori had been eradicated 5 years previously and confirmed that glandular atrophy is reversible in both the gastric corpus and antrum. 40 They also demonstrated increased gastric acidity accompanied by an improvement of gastric atrophy 1 year after eradication. 41 Kashiwagi reported that the grade of reflux esophagitis improved in a 3-year follow-up group and that reflux esophagitis that develops after H. pylori eradication therapy rarely becomes a long-term clinical problem in patients who complete the treatment successfully. 42
Wyatt et al. found that foci of gastric metaplasia in the duodenal epithelium were an acquired change and were more common in men, perhaps because of their greater acid output, and suggested that mucosal injury is related to active duodenitis. 43 Ford et al. provided evidence that H. pylori eradication with acid suppression improves healing of duodenal ulcers compared with acid suppression alone. 44 However, Hobsley et al. reported that duodenal ulcers could recur after eradication of H. pylori infection. 45 , 46 Thus, in human beings, H. pylori infection can cause reflux esophagitis, intestinal metaplasia in the glandular stomach and duodenal ulcers, but after eradication, all these lesions can recur.
In 1996, Hirayama et al. described a Mongolian gerbil model of human H. pylori infection with the bacteria detectable throughout a 12-month period and the resultant chronic active gastritis, peptic ulcers and intestinal metaplasia resembling lesions apparent in humans. 47 H. pylori infection in itself does not induce gastric tumors in Mongolian gerbils 48 , 49 . Heterotopic proliferative glands, which finally included Paneth cells induced by H. pylori infection in the stomachs of Mongolian gerbils, were obviously reduced, with few remnants after eradication of H. pylori. 50 , 51 The researchers considered that metaplastic and heterotopic prolifetice glands are reversible on eradication. Mizoshita et al. suggested that intestinal metaplasia induced by H. pylori infection in Mongolian gerbils is a paracancerous phenomenon rather than a premalignant condition and that its infection may trigger intestinalization of both stomach cancers and non-neoplastic mucosa. 52
Therefore, there are data suggesting that cancer and intestinal metaplasia arise from different cell lineages, such that intestinal metaplasia may not be a precursor lesion but rather a marker of increased risk. 53
Mechanisms of Induction of Intestinal Metaplasia and Roles of Stem Cells
The esophagus epithelium can undergo metaplastic change to become the gastric or duodenal epithelium, 1 , 5 , 54 , 55 the gastric epithelium can become the intestinal epithelium, 56 , 57 and vice versa 1 , 6 , 58 and the large intestinal epithelium can change to become the small intestinal epithelium 59 – 61 under the influence of different gastrointestinal tract diseases. Thus, tissue differentiation in the gastrointestinal tract appears to be malleable. Wyatt et al. 43 found that foci of gastric metaplasia in the duodenal epithelium were an acquired change and again were more common in men, suggesting a relation for mucosal injury with active duodenitis.
The development of intestinal metaplasia with ALP-positive foci has been shown to be increased by administration of ranitidine, an H2 receptor antagonist, 24 or a crude stomach extract 19 and by pyloroplasty or pyloroplasty plus vagotomy. 62 On the other hand, intestinal metaplasia is decreased by cysteamine, 24 which stimulates gastric acid secretion, and histamine or removal of the submandibular glands. 63 A close relationship between the fundic pH and ALP-positive foci exists, and subtotal resection of the fundus increases the development of intestinal metaplasia induced by X-irradiation as assessed in terms of ALP-positive foci and total intestinal metaplasia. 64 The fact that goblet cells are observed in the pylorus until 7 days of age and then disappear by 14 days of age is in line with the concurrent decrease in the pH value with the increase in the number of parietal cells. 65
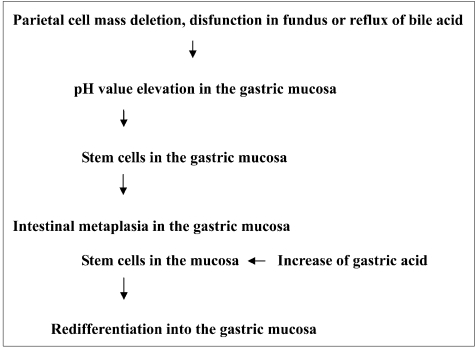
On the other hand, as described above, metaplasia may disappear with H. pylori eradication and appears in Cdx2 transgenic mice due to decrease in acid output. Therefore, taking all of the available findings into account, our working hypothesis is as follows: elevation of the gastric juice pH due to disappearance of parietal cells is one of the principal factors responsible for the development of intestinal metaplasia from gastric stem cells, and this process is reversible (Fig. 6). In other words, it is considered that stem cells in intestinal metaplasia may newly differentiate into the gastric mucosa under acidic conditions.
Fig. 6.
Working hypothesis for induction of intestinal metaplasia.
Stem Cells
When gastric tissue was transplanted into the duodenum, 66 pepsinogen-positive chimeric glands with goblet cells appeared in the grafts. Esophageal grafts transplanted into the glandular stomach or duodenum similarly transdifferentiate into the mucosa of these respective sites. Moreover, we also founded that pieces of ear (skin), bladder, trachea, diaphragm, pyloric gland and forestomach from 8-week-old male GFP-F344 rats, when transplanted into the duodenums of F344 strain rats demonstrated goblet cells with alcian-blue PAS-positive mucin and brush borders with ALP. 67 A GFP-positive duodenal mucosa was observed in all cases by immunohistochemical staining. Moreover, the GFP-positive cells were found to have the GFP transgene by PCR analysis. In the duodenum, the microenvironment might thus be conducive to the development of metaplasia if it is associated with an increase in proliferation. As a result, the bladder, trachea, ear (skin), diaphragm, pyloric gland and forestomach tissue of the F344 rat contained stem cells that have multipotential ability for differentiation when transplanted into different environments.
Adult stem cells have been reported in several tissue sources, including the central nervous system, 68 bone marrow, 69 retina, 70 brain, 71 hair follicle, 72 inner ear, 73 adipose tissues, 74 oral mucosal epithelium, 75 liver, 76 skeletal muscle 77 and skin. 78 Heart, kidney, brain and skin pieces from male F344 transgenic rats carrying the GFP gene, when transplanted into F344 rats one day after intraperitoneal injection of carbon tetrachloride, transdifferentiate into hepatocytes. 79 Thus, tissue stem cells have multipotential ability. Other examples of extensive plasticity include the in vivo differentiation of a bone marrow population enriched for hematopoietic stem cells into mature hepatocytes in the livers of rodents 80 , 81 and derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. 82 Such differentiation of bone marrow cells into mature cells of the liver has also been reported to occur in humans. 83 , 84 Together with the data presented here, the available findings indicate that mammalian stem cells persist in various organs and that such cells can be induced to undergo other organ differentiation with an appropriate microenvironment. Our experimental system with its unique feature of the GFP marker has clear advantages compared with previous animal models.
Correlation Between Intestinal Metaplasias and Gastric Tumors
The colonic mucosa transplanted into the fundic gland lacks susceptibility to typical gastric carcinogens, MNNG 85 or MNU given orally, 54 but is sensitive to a colonic carcinogen, DMH. 86
The incidences of gastric tumors against the frequency of intestinal metaplasia with or without Paneth cells per rat yielded a significant inverse relationship, 87 , 88 suggesting that the development of intestinal metaplasia and gastric tumors might be independent responses to treatment with MNNG or MNU. However, induction of an intestinal metaplastic mucosa in the glandular stomach by X-rays was associated with a greater tendency for tumorigenesis in response to DMH or AOM, 89 – 91 in contrast to the non-susceptible normal gastric mucosa. Transplant experiments such as those reported here can be of assistance in clarifying the role of the microenvironment in determining the risk of tumorigenesis.
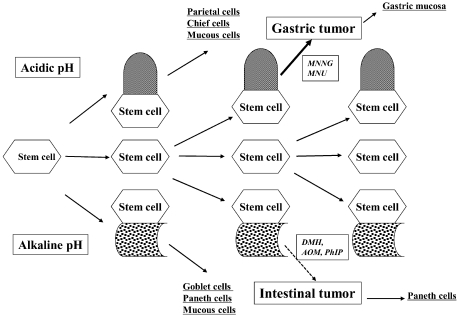
The number of intestinal metaplasias with ALP-positive foci induced by X-rays in the Donryu rats was decreased by the treatment with AOM, but aberrant crypt-like foci appeared within some of the affected areas, with the appearance of cystic structures with pyknotic nuclei exhibiting binding of anti-8-hydroxyguanosine antibodies. 91 Thus, it would appear that areas of intestinal metaplasia with or without Paneth cells induced by X-irradiation might be susceptible to colonic carcinogen damage. When male F344 rats were X-irradiated and AOM was injected and PhIP given by intragastric intubation 16 weeks after the first dose, 92 tumors in the pylorus of the glandular stomach were observed in 4 of 29 animals in the X-rays+AOM group and 4 of 25 animals receiving X-rays+PhIP after 12 months. No such lesions were found in the chemical or X-ray alone groups. Intestinal metaplasia and induced tumors were found to be positive for Cdx2 by histochemistry. In summary, the presence of intestinal metaplasia, with or without Paneth cells, may increase the sensitivity of the stomach to the induction of tumors by carcinogens like DMH, AOM and PhIP, but not MNNG or MNU. The results are compatible with the conclusion that intestinal metaplasias are targets of DMH-type carcinogens in the normal gastric mucosa (Fig. 7). The protocol used in our studies may provide a new approach to distinguish between developmental events associated with intestinal metaplasia and gastric tumors.
Fig. 7.
Flow chart for stem cells in the stomach.
Acknowledgments
I am grateful to Dr M.A. Moore for critical reading of this review.
References
- 1.Slack JM. Homoeotic transformations in man: implications for the mechanism of embryonic development and for the organization of epithelia. J Theor Biol. 1985;114:463–490. doi: 10.1016/s0022-5193(85)80179-x. [DOI] [PubMed] [Google Scholar]
- 2.Leube RE, Rustad TJ. Squamous cell metaplasia in the human lung: molecular characteristics of epithelial stratification. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61:227–253. doi: 10.1007/BF02890425. [DOI] [PubMed] [Google Scholar]
- 3.Kvist E, Sjølin KE, Laursen H, Orntoft TF, Sturmer MA. Squamous cell metaplasia of the bladder urothelium. A retrospective study of 36 patients. APMIS. 1992;100:650–654. doi: 10.1111/j.1699-0463.1992.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 4.Nepomnyashchikh GI, Aidagulova SV, Nepomnyashchikh DL, Boboev MM, Isaenko VI, Abdullaev NA, Ivaninskii OI, Kunin IS. 67 stereotypes of structural modification of the urothelium in various diseases of the urinary bladder and prostate. Bull Exp Biol Med. 2008;146:415–419. doi: 10.1007/s10517-009-0308-6. [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ. Intestinal metaplasia at the gastroesophageal junction. Gastroenterology. 2004;126:567–575. doi: 10.1053/j.gastro.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 6.Mann NS, Mann SK, Rachut E. Heterotopic gastric tissue in the duodenal bulb. J Clin Gastroenterol. 2000;30:303–306. doi: 10.1097/00004836-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Morson BC. Intestinal metaplasia of gastric mucosa. Brit J Cancer. 1955;9:365–376. doi: 10.1038/bjc.1955.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarvi H, Lauren P.On the role of heterotopias of the intestinal epithelium in the pathogenesis of gastric cancer. Acta Pathol Microbiol Scand Supp1. 29: 26–43. l95l. [PubMed]
- 9. Nakamura K, Sugano H, Takagi K.Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 59: 251–258. l968. [PubMed]
- 10.Rubin W, Ross LL, Jeffries GH, Slessenger MH. Intestinal metaplasia and heterotopia: a fine structural study. Lab Invest. 1966;15:1024–1049. [PubMed] [Google Scholar]
- 11. Ming SC, Goldman H, Freiman DC.Intestinal metaplasia and histogenesis of carcinoma in human stomach: light and electron microscopic study. Cancer. 20: 1418–1429. l967. [DOI] [PubMed]
- 12. Sugimura T, Kawachi T, Kogure K, Tokunaga A, Tanaka N, Sasajima K, Koyama Y, Hirota T, Sano R.Enzymologic changes in abnormal differentiation—Intestinal metaplasia in human gastric mucosa—A possible precancerous changes. In: Differentiation and Control of Malignancy of Tumor Cells. W Nakahara, T Ono, T Sugimura, and H Sugano (eds). Univ. Park Press, Baltimore, MD. 251–263.1971.
- 13.Teglbaerg PS, Nielsen HO. “Small intestinal type” and “colonic type” intestinal metaplasia of the human stomach. Acta Path Microbiol Scand Sect A. 1978;86:351–355. doi: 10.1111/j.1699-0463.1978.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 14.Jass JR, Filipe MI. The mucin profiles of normal gastric mucosa, intestinal metaplasia and its variants gastric carcinoma. Histochem J. 1981;23:931–939. doi: 10.1007/BF01002633. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H, Naito M, Ito A. The effect of sex difference on induction of intestinal metaplasia in rats. Acta Pathol Jpn. 1984;34:305–312. doi: 10.1111/j.1440-1827.1984.tb07558.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsukamoto T, Mizoshita T, Tatematsu M. Gastric-and-intestinal mixed-type intestinal metaplasia: aberrant expression of transcription factors and stem cell intestinalization. Gastric Cancer. 2006;9:156–166. doi: 10.1007/s10120-006-0375-6. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe H.Pathological studies on intestinal metaplasia– in particular references to acid of gastric mucosa and gastric tumorigenesis. Proc RINMB. 29: 193–216.1988.
- 18.Watanabe H. Experimentally induced intestinal metaplasia in Wistar rats by X-ray irradiation. Gastroenteology. 1978;75:796–799. [PubMed] [Google Scholar]
- 19.Watanabe H, Fujii I, Tedara Y. Induction of intestinal metaplasia in the rat gastric mucosa by local X-irradiation. Pathol Res Pract. 1980;170:104–114. doi: 10.1016/S0344-0338(80)80159-2. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H, Naito M, Kawashima K, Ito A. Intestinal metaplasia induced by X-irradiation in different strains of rats. Acta Pathol Jpn. 1985;35:841–847. doi: 10.1111/j.1440-1827.1985.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H, Fujimoto N, Masaoka Y, Ohtaki M, Ito A. Strain differences in the induction of intestinal metaplasia by X-irradiation in rats. J Gastroenterol. 1997;32:295–299. doi: 10.1007/BF02934483. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Shiraki K, Ishimura Y, Ohara M, Uesaka T, Katoh O, Watanabe H. The morphological changes of gastric mucosa in Mongolian gerbils by X-irradiation. Nagasaki Med J. 2000;75:210–212. (in Japanese) [Google Scholar]
- 23. Watanabe H, Okamoto T, Matsuda M, Takahashi T, Ogundigie PO, Ito A.Effects of sex hormones on induction of intestinal metaplasia by X-irradiation in rats. Acta Patho1 Jpn. 43: 456–463.1993. [DOI] [PubMed]
- 24.Watanabe H, Kamikawa M, Nakagawa Y, Takahashi T, Ito A. The effects of ranitidine and cysteamine on intestinal metaplasia induced by X-irradiation in rats. Acta Pathol Jpn. 1988;38:1285–1296. doi: 10.1111/j.1440-1827.1988.tb02279.x. [DOI] [PubMed] [Google Scholar]
- 25.Beck F, Chawengsaksophak K, Waring P, Playford RJ, Furness JB. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci USA. 1999;96:7318–7323. doi: 10.1073/pnas.96.13.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips RW, Frierson HF, Jr, Moskaluk CA. Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol. 2003;27:1442–1447. doi: 10.1097/00000478-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 28.Tsukamoto T, Inada K, Tanaka H, Mizoshita T, Mihara M, Ushijima T, Yamamura Y, Nakamura S, Tatematsu M. Down-regulation of a gastric transcription factor, Sox2, and ectopic expression of intestinal homeobox genes, Cdx1 and Cdx2: inverse correlation during progression from gastric/intestinal-mixed to complete intestinal metaplasia. J Cancer Res Clin Oncol. 2004;130:135–145. doi: 10.1007/s00432-003-0519-6. [DOI] [PubMed] [Google Scholar]
- 29.Shiotani A, Uedo N, Iishi H, Tatsuta M, Ishiguro S, Nakae Y, Kamada T, Haruma K, Merchant JL. Re-expression of sonic hedgehog and reduction of CDX2 after Helicobacter pylori eradication prior to incomplete intestinal metaplasia. Int J Cancer. 2007;121:1182–1189. doi: 10.1002/ijc.22835. [DOI] [PubMed] [Google Scholar]
- 30.Silberg DG, Furth EE, Taylor JK, Schuck T, Chiou T, Traber PG. CDX1 protein expression in normal, metaplastic, and neoplastic human alimentary tract epithelium. Gastroenterology. 1997;113:478–486. doi: 10.1053/gast.1997.v113.pm9247467. [DOI] [PubMed] [Google Scholar]
- 31.Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, David L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36–40. doi: 10.1002/path.1246. [DOI] [PubMed] [Google Scholar]
- 32.Mutoh H, Sakurai S, Satoh K, Osawa H, Hakamata Y, Takeuchi T, Sugano K. Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice. Gut. 2004;53:1416–1423. doi: 10.1136/gut.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judd LM, Andringa A, Rubio CA, Spicer Z, Shull GE, Miller ML. Gastric achlorhydria in H/K-ATPase-deficient (Atp4a(–/–)) mice causes severe hyperplasia, mucocystic metaplasia and upregulation of growth factors. J Gastroenterol Hepatol. 2005;20:1266–1278. doi: 10.1111/j.1440-1746.2005.03867.x. [DOI] [PubMed] [Google Scholar]
- 34.Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, Kim HM, Kim WJ, Yamamoto H, Yamashita N, Yano T, Ikeda T, Itohara S, Inazawa J, Abe T, Hagiwara A, Yamagishi H, Ooe A, Kaneda A, Sugimura T, Ushijima T, Bae SC, Ito Y. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 35.Fukamachi H, Mimata A, Tanaka I, Ito K, Ito Y, Yuasa Y. In vitro differentiation of Runx3−/− p53−/− gastric epithelial cells into intestinal type cells. Cancer Sci. 2008;99:671–676. doi: 10.1111/j.1349-7006.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuasa H, Inada K, Watanabe H, Tatematsu M. A phenotypic shift from gastric-intestinal to solely intestinal cells types in intestinal metaplasia in rat stomach following treatment with X-rays. J Toxicol Pathol. 2002;15:85–93. [Google Scholar]
- 37.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 38.Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, Tannenbaum S, Collazos T, Ruiz B. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–4740. [PubMed] [Google Scholar]
- 39.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, Mera R. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 40.Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, Sumii M, Tanaka S, Yoshihara M, Chayama K. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002;16:1449–1456. doi: 10.1046/j.1365-2036.2002.01311.x. [DOI] [PubMed] [Google Scholar]
- 41.Haruma K, Mihara M, Okamoto E, Kusumoki H, Hananoki M, Tanaka S, Yoshihara M, Sumi K, Kajiyama G. Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus. Evaluation of 24-h pH monitoring. Aliment Pharmacol Ther. 1999;13:155–162. doi: 10.1046/j.1365-2036.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 42.Kashiwagi H. Ulcers and gastritis. Endoscopy. 2005;37:110–115. doi: 10.1055/s-2004-826147. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt JI, Rathbone BJ, Sobala GM, Shallcross T, Heatley RV, Axon AT, Dixon MF. Gastric epithelium in the duodenum: its association with Helicobacter pylori and inflammation. J Clin Pathol. 1990;43:981–986. doi: 10.1136/jcp.43.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833–1855. doi: 10.1111/j.1572-0241.2004.40014.x. [DOI] [PubMed] [Google Scholar]
- 45.Hobsley M, Tovey FI, Holton J. Controversies in the Helicobacter pylori/duodenal ulcer story. Trans R Soc Trop Med Hyg. 2008;102:1171–1175. doi: 10.1016/j.trstmh.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 46.Hobsley M, Tovey FI, Holton J. Precise role of H pylori in duodenal ulceration. World J Gastroenterol. 2006;12:6413–6419. doi: 10.3748/wjg.v12.i40.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996;31(Suppl 9):24–28. [PubMed] [Google Scholar]
- 48.Elfvin A, Bölin I, Von Bothmer C, Stolte M, Watanabe H, Fändriks L, Vieth M. Helicobacter pylori induces gastritis and intestinal metaplasia but no gastric adenocarcinoma in Mongolian gerbils. Scand J Gastroenterol. 2005;40:1313–1320. doi: 10.1080/00365520510023611. [DOI] [PubMed] [Google Scholar]
- 49.Sun YQ, Petersson F, Monstein HJ, Söderholm JD, Rehfeld JF, Borch K. Long-term morpho-functional development of Helicobacter pylori-induced gastritis in Mongolian gerbils. Scand J Gastroenterol. 2005;40:1157–1167. doi: 10.1080/00365520510023378. [DOI] [PubMed] [Google Scholar]
- 50.Nozaki K, Shimizu N, Tsukamoto T, Inada K, Cao X, Ikehara Y, Kaminishi M, Sugiyama A, Tatematsu M. Reversibility of heterotopic proliferative glands in glandular stomach of Helicobacter pylori-infected Mongolian gerbils on eradication. Jpn J Cancer Res. 2002;93:374–381. doi: 10.1111/j.1349-7006.2002.tb01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tatematsu M, Tsukamoto T, Mizoshita T. Role of Helicobacter pylori in gastric carcinogenesis: the origin of gastric cancers and heterotopic proliferative glands in Mongolian gerbils. Helicobacter. 2005;10:97–106. doi: 10.1111/j.1523-5378.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 52.Mizoshita T, Tsukamoto T, Takenaka Y, Cao X, Kato S, Kaminishi M, Tatematsu M. Gastric and intestinal phenotypes and histogenesis of advanced glandular stomach cancers in carcinogen-treated, Helicobacter pylori-infected Mongolian gerbils. Cancer Sci. 2006;97:38–44. doi: 10.1111/j.1349-7006.2006.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito A, Shimoda T, Nakanishi Y, Ochiai A, Toda G. Histologic heterogeneity and mucin phenotypic expression in early gastric cancer. Pathol Inter. 2001;51:165–171. doi: 10.1046/j.1440-1827.2001.01179.x. [DOI] [PubMed] [Google Scholar]
- 54.Ando Y, Uesaka T, Kido S, Fujimoto N, Tatematsu M, Ishimura Y, Shiraki K, Hirata S, Kuramoto K, Shoji S, Katoh O, Watanabe H. No susceptibility of colon tissue implanted into the glandular stomach of rats to N-Nitroso-N-methylurea (MNU) carcinogenesis. Oncol Rep. 1998;5:1373–1376. doi: 10.3892/or.5.6.1373. [DOI] [PubMed] [Google Scholar]
- 55.Dayal Y, Wolfe HJ. Gastrin-producing cells in ectopic gastric mucosa of developmental and metaplastic origins. Gastroenterology. 1978;75:655–660. [PubMed] [Google Scholar]
- 56.Ming SC. Cellular and molecular pathology of gastric carcinoma and precursor lesions: A critical review. Gastric Cancer. 1998;1:31–50. doi: 10.1007/s101200050053. [DOI] [PubMed] [Google Scholar]
- 57.Jovanovic I, Tzardi M, Mouzas IA, Micev M, Pesko P, Milosavijevic T, Zois M, Sganzos M, Delides G, Kanavaros P. Changing pattern of cytokeratin 7 and 20 expression from normal epithelium to intestinal metaplasia of the gastric mucosa and gastroesophageal junction. Histol Histopathol. 2002;17:445–454. doi: 10.14670/HH-17.445. [DOI] [PubMed] [Google Scholar]
- 58.Ciancio G, Nuti M, Orsini B, Iovi F, Ortolani M, Palomba A, Amorosi A, Surrenti E, Ilani SM, Surrenti C. Regression of duodenal gastric metaplasia in Helicobacter pylori positive patients with duodenal ulcer disease. Dig Liver Dis. 2002;34:16–21. doi: 10.1016/s1590-8658(02)80054-8. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka M, Saito H, Kusumi T, Fukuda S, Shimoyama T, Sasaki Y, Suto K, Munakata A, Kudo H. Observer variation of diagnoses based on simple biopsy criteria differentiating among Crohn’s disease, ulcerative colitis, and other forms of colitis. J Gastroenterol Hepatol. 2001;16:1353–1359. doi: 10.1046/j.1440-1746.2001.02638.x. [DOI] [PubMed] [Google Scholar]
- 60.Cunliffe RN, Rose FR, Keyte J, Abberley L, Chan WC, Mahida YR. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut. 2001;48:176–185. doi: 10.1136/gut.48.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada Y, Yoshimi N, Hirose Y, Matsunaga K, Katayama M, Sakata K, Shimizu M, Kuno T, Mori H. Sequential analysis of morphological and biological properties of beta-catenin-accumulated crypts, provable premalignant lesions independent of aberrant crypt foci in rat colon carcinogenesis. Cancer Res. 2001;61:1874–1878. [PubMed] [Google Scholar]
- 62.Fujii I, Watanabe H, Naito M, Kawashima K, Ito A. The induction of intestinal metaplasia in rats by pyloroplasty or pyloroplasty plus vagotomy. Pathol Res Pract. 1985;180:502–505. doi: 10.1016/S0344-0338(85)80012-1. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe H, Okamoto T, Fudaba Y, Ogundigie PO, Ito A. Influence of gastric pH modifiers on development of intestinal metaplasia induced by X-irradiation in rats. Jpn J Cancer Res. 1993;84:1037–1042. doi: 10.1111/j.1349-7006.1993.tb02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kinoshita K, Watanabe H, Ando Y, Katayama M, Yamamoto H, Hirano N, Yoshikuni S, Yamamoto T. Effects of subtotal resection of the fundus on development of intestinal metaplasia induced by X-ray irradiation in Donryu rats. Pathol Int. 2000;50:879–883. doi: 10.1046/j.1440-1827.2000.01128.x. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe H, Naito M, Kawashima K, Ito A. PH-related differentiation in the epithelia of the gastric mucosa of postnatal rats. Acta Pathol Jpn. 1985;35:569–576. doi: 10.1111/j.1440-1827.1985.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe H, Ando Y, Uesaka T, Ishimura Y, Shiraki K, Lu H, Ohara M, Shoji S, Katoh O. Grafting of stomach tissue into the duodenum in F344 rats results in chimeric crypts and tumor development. J Clin Exp Cancer Res. 2000;19:207–210. [PubMed] [Google Scholar]
- 67.Watanabe H, Ochiya T, Ueda S, Kominami Y, Gon R, Hayashi M, Nishiki M, Sasaki A, Shiraishi M, Kamiya K. A rat model of heterototrophi transplantation into duodenum and liver. Surg Front. 2008;15:454–458. (in Japanese) [Google Scholar]
- 68.Yadirgi G, Marino S. Adult neural stem cells and their role in brain pathology. J Pathol. 2009;217:242–253. doi: 10.1002/path.2480. [DOI] [PubMed] [Google Scholar]
- 69.Ratajczak MZ, Zuba-Surma EK, Machalinski KM. Bone-marrow-derived stem cells—our key to longevity? J Appl Genet. 2007;48:307–319. doi: 10.1007/BF03195227. [DOI] [PubMed] [Google Scholar]
- 70.Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int J Dev Biol. 2004;48:1003–1014. doi: 10.1387/ijdb.041870am. [DOI] [PubMed] [Google Scholar]
- 71.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiede S, Kloepper JE, Bodò E, Tiwari S, Kruse C, Paus R. Hair follicle stem cells: walking the maze. Eur J Cell Biol. 2007;86:276–355. doi: 10.1016/j.ejcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Lou X, Zhang Y, Yuan C. Multipotent stem cells from the young rat inner ear. Neurosci Lett. 2007;416:28–33. doi: 10.1016/j.neulet.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 74.Matsubara Y, Saito E, Suzuki H, Watanabe N, Murata M, Ikeda Y. Generation of megakaryocytes and platelets from human subcutaneous adipose tissues. Biochem Biophys Res Commun. 2009;378:716–720. doi: 10.1016/j.bbrc.2008.11.117. [DOI] [PubMed] [Google Scholar]
- 75.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. New Eng J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 76.Walkup MH, Gerber DA. Hepatic stem cells: in search of. Stem Cells. 2006;24:1833–1840. doi: 10.1634/stemcells.2006-0063. [DOI] [PubMed] [Google Scholar]
- 77.Cao B, Huard J. Muscle-derived stem cells. Cell Cycle. 2004;3:104–107. [PubMed] [Google Scholar]
- 78.Fernandes KJ, Miller FD. Isolation, expansion, and differentiation of mouse skin-derived precursors. Methods Mol Biol. 2009;482:159–170. doi: 10.1007/978-1-59745-060-7_10. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe H, Ochiya T, Ueda S, Kominami Y, Gon R, Nishiki M, Hayashi M, Sasaki A, Shiraishi M, Kashimoto N, Myojin Y, Kamiya K. Differentiation of a hepatic phenotype after heterotropic transplantation of heart, kidney, brain, and skin tissues into liver in F344 rats. Biochem Biophys Res Commun. 2007;354:841–845. doi: 10.1016/j.bbrc.2006.12.236. [DOI] [PubMed] [Google Scholar]
- 80.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature Med. 2000;5:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 81.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 82.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 83.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 84.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 85. Nakagawa Y, Ando Y, Fujimoto N, Masaoka Y, Tanizaki M, Shoji S, Katoh O, Watanabe H.Colon tissue implanted into the glandular stomach in rats lacks susceptibility to N-methyl-N’-nitro-N-nitrosoguanidine MNNG) carcinogenesis. Oncol Rep. 4: 517–519. l997. [DOI] [PubMed]
- 86.Nakagawa Y, Watanabe H, Takahashi T, Ito A, Dohi K. Carcinogenicity of 1,2-dimethylhydrazine in colorectal tissue heterotopically transplanted into the glandular stomach of rat. Jpn J Cancer Res. 1992;83:24–30. doi: 10.1111/j.1349-7006.1992.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Watanabe H, Ito A.Relationship between gastric tumorigenesis and intestinal metaplasia in rat given X-radiation and or N-methy1-N’-nitro-N-nitrosoguanidine. J Natl Cancer Inst. 76: 865–870. l986. [PubMed]
- 88.Watanabe H, Ando Y, Yamada K, Okamoato T, Ito A. Lack of any positive effects of intestinal metaplasia on induction of gastric tumors in Wistar rats treated with N-methy1-N-nitrosourea in their drinking water. Jpn J Cancer Res. 1994;85:892–896. doi: 10.1111/j.1349-7006.1994.tb02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ando Y, Watanabe H, Tatematsu M, Hirano K, Furihata C, Fujimoto N, Toge T, Ito A. Gastric tumorigenicity of 1,2-dimethylhydrazine on the background of gastric intestinal metaplasia induced by X-irradiation in CD(SD) rats. Jpn J Cancer Res. 1996;87:433–436. doi: 10.1111/j.1349-7006.1996.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watanabe H, Uesaka T, Kido S, Ishimura Y, Shiraki K, Kuramoto K, Hirata S, Shoji S, Katoh O, Fujimoto N. Gastric tumor induction by 1,2-dimethylhydrazine in Wistar rats with intestinal metaplasia caused by X-irradiation. Jpn J Cancer Res. 1999;90:1207–1211. doi: 10.1111/j.1349-7006.1999.tb00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe H, Fujimoto N, Masaoka Y, Kurosumi M, Oguri T, Takahashi T, Kido S, Hirata S, Kuramoto K, Shoji S, Katoh O. Effects of azoxymethane on X-ray induced intestinal metaplasia in Donryu rats. Oncol Rep. 1998;5:837–840. doi: 10.3892/or.5.4.837. [DOI] [PubMed] [Google Scholar]
- 92.Kashiwabara S, Kashimoto N, Uesaka T, Wakabayashi K, Kamiya K, Watanabe H. Tumor induction by azoxymethane (AOM) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in F344 rat gastric mucosa featuring intestinal metaplasia caused by X-irradiation. J Exp Clin Cancer Res. 2005;24:305–312. [PubMed] [Google Scholar]