Abstract
A mass with a diameter of 1.5 cm was found in the thymus of a 4-year and 3-month-old male cynomolgus monkey. Microscopically, the mass consisted of two different patterns of proliferation, dense or fascicular proliferation of elongated spindle cells in a sporadic storiform pattern and dense proliferation of thymic cortex-like lymphoid cells in which the multifocal pale nests resembling the thymic medulla were distributed. In these pale nests, large dendriform cells sometimes forming Hassall’s corpuscles were present. The proliferating spindle cells were positive for cytokeratin. The lymphoid cells in the mass were positive for CD3. We concluded that the mass consisted of the neoplastic thymic epithelium with thymocytes proliferation containing medullary differentiation. The mass was diagnosed as a mixed thymoma according to the WHO classification of thymomas in humans. Mixed thymoma is characterized as a mixture of two types of proliferative lesions, spindle-shaped epithelial proliferation and a lymphocyte predominant lesion with or without medullary differentiation. To the best of our knowledge, this is the first report concerning thymoma in monkeys.
Keywords: thymoma, thymus, monkey, mixed thymoma, medullary differentiation
Thymoma is a neoplasm of the anterior mediastinum composed of a neoplastic thymic epithelium with various degrees of lymphocytes. Although thymomas in humans are well documented and classified histopathologically 1 , 2 , it is an uncommon tumor in animals that has been reported in just a few species. 3 – 5 Human thymomas are classified into several histological types by the WHO classification based on the cell components and malignancy. 2 Medullary thymoma (type A, Spindle cell thymoma) is composed of a neoplastic thymic epithelium assuming a spindle cell appearance with few thymocytes. Predominantly cortical thymoma (type B1) has a polygonal epithelium and abundant immature thymocytes with medulla-like pale areas. These areas are thought to represent medullary differentiation. 2 Cortical thymoma (type B2) is characterized by distinct neoplastic epithelial cells lined among immature thymocytes. Medullary differentiation is absent in this type of thymoma. Mixed thymoma (type AB) has both proliferation areas seen in types A and B. Additionally, well-differentiated thymic carcinoma (type B3) and thymic carcinoma (type C) are also referred to in the WHO classification. In domestic animals, on the other hand, thymomas have not been classified in detail. 5 , 6 These tumors ara categorized as lymphocyte predominant, epithelial predominant, or mixed thymomas 5 or are less well categorized in animals. This categorization is simply based on the populations of the epithelial cells and thymocytes. The morphological characters and proliferative patterns of the neoplastic epithelial cells in each type of thymomas have not been defined or referred to for animals.
The present report describes a thymoma found in a cynomolgus monkey histologically categorized as mixed thymoma in accordance with the classification for humans.
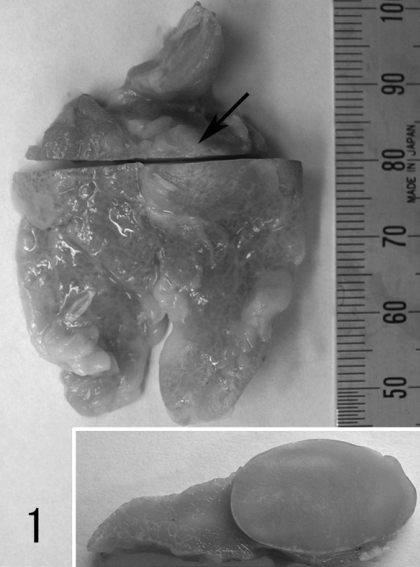
The case was found in a 4-year and 3-month-old male cynomolgus monkey (Macaca fascicularis) provided as an animal in a low dose group of a 4-week oral gavage toxicity study. This monkey was bred and imported from Vietnam. The animal was cared for according to the principles outlined in the guides for the care and use of laboratory animals prepared by the Japanese Association for Laboratory Animal Science and our institution. The examinations of hematology and blood chemistry (containing globulin fraction) revealed no abnormality. At necropsy, a nodule 1.5 cm in diameter was identified in the thymus; the nodule was not adhered to surrounding tissues. The tissue was fixed in a 10% phosphate-buffered formalin solution. Observation after fixation revealed that the mass was an encapsulated 1.5 × 1.0 × 1.0-cm oval that was distinct from the surrounding normal thymic tissues (Fig.1). The cut surface was solid and without normal thymic lobular patterns (Fig.1 inset).
Fig. 1.
Thymus fixed in 10% phosphate-buffered formalin solution. A discolored nodule is observed in the thymus (arrow). The cut surface has no distinct lobular pattern (inset).
The mass was subjected to a routine histological examination with hematoxylin and eosin staining. Additionally, reticulin silver impregnation staining was applied to the section. Immunohistochemical staining with cytokeratin (WSS), vimentin (V9), S100, lysozyme, CD3, CD20 and proliferating cell nuclear antigen (PCNA) was also performed. Table 1 represents the source of the antibodies and conditions for the immunohistochemical staining. All antibodies were purchased from Dako Cytomation (Glostrup, Denmark) except for the lysozyme, wthich was purchased from Nichirei Biosciences (Tokyo, Japan).
Table 1. Reagents for Immunohistochemistry.
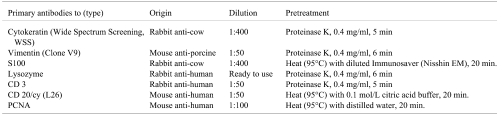
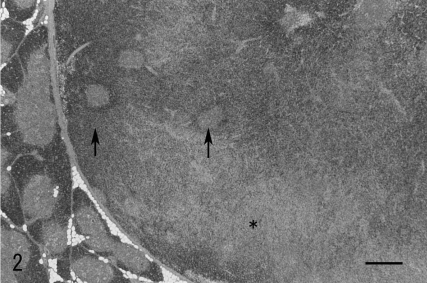
Histologically, the mass was capsulated with the fibrous membrane and distinct from the surrounding normal thymic tissues (Fig. 2). There were no thymic lobular patterns inside the mass. The mass had a pale area and dark area stained strongly with hematoxylin. In the dark area, multifocal pale nests were scattered. In the pale area, fascicular or close proliferations of large spindle or elongated cells were observed in a sporadic storiform pattern (Fig. 3a). The nuclei were oval or elliptic and palely basophilic with few atypia. Mitotic figures were rarely observed. Among the proliferating cells, small lymphoid cells were distributed. Reticular fibers were almost unrecognizable but surrounded the vessel wall or sometimes enclosed a few groups of tumor cells. The individual proliferating cells were not surrounded by the reticulin fibers (Fig. 3a inset). In the dark area, small lymphoid cells like in the thymic cortex were densely present (Fig. 3b). Large cells with dendriform cytoplasm were scattered among the lymphoid cells. The nests scattered in this area were similar to the normal thymic medulla. The nests consisted of large dendriform cells resembling thymic epithelial cells and sparse lymphoid cells (Fig. 3b). The dendriform cells were sometimes enlarged and keratinized and seemed to form Hassall’s body (Fig. 3b inset).
Fig. 2.
The nodule is composed of a pale area (*) and dark area with multi-focal pale nests (arrow). HE. Bar=500 μm.
Fig. 3.
a: Pale area of Fig. 2. Dense proliferation of large spindle or elongated cells with the small lymphoid cells distributed. HE. Bar=100 μm. The individual proliferating cells are not surrounded by reticulin fibers. Reticulin silver impregnation (inset). b: Dark area of Fig. 2. Small lymphoid cells are closely observed. The pale nests resemble the thymic medulla and seemed to form Hassall’s body (Inset). HE. Bar=100 μm.
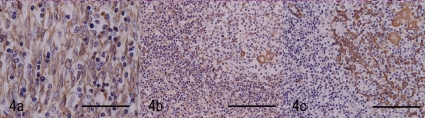
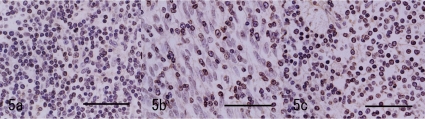
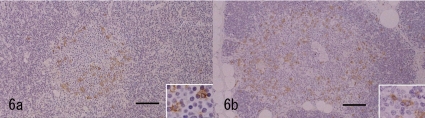
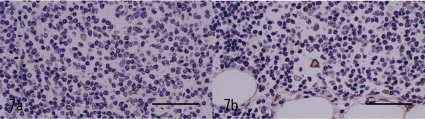
Immunohistochemical analysis was performed according to the conditions presented in Table 1. The closely-proliferating large spindle cells in the pale area were positive for cytokeratin (CK; Fig. 4a). The large cells in the dark area were also positively stained (Fig. 4b). The number of CK positive cells in the dark area increased compared with the normal thymic cortex in this animal (Fig. 4b, c). In the nests, large CK positive cells were distributed reticulately, similar to those of the thymic medulla, but loosely and weakly compared with the cells of the normal thymic medulla in this animal (Fig. 4b, c). The large cells were negative for vimentin (V9), S100 and lysozyme. Many lymphoid cells in the dark area were positive for CD3, but a few of them were negative (Fig. 5a). The CD3 positive reactive lymphoid cells were more prominent in the pale area than in the dark area (Fig. 5b). In the nests, most lymphoid cells were positive for CD3 (Fig. 5c). The CD20 expressed in the margin of the nests seemed to surround the nests (Fig 6a). This labeling pattern was the same as that of the medulla of the normal thymic tissue in this animal (Fig. 6b). In the pale nests, S100 positive cells were absent (Fig. 7a), although S100 positive cells were sparsely distributed (Fig 7b) in the normal medulla in this animal, suggesting the cells were dendritic cells (DC) presenting in the medulla only. The proliferating spindle cells were rarely positive for PCNA (Fig. 8a). Remarkable positive labeling of the lymphoid cells for PCNA was observed throughout the mass except for in the pale nests, in which the positive lymphoid cells were sparse (Fig. 8b). In the non-neoplastic area, PCNA was expressed mainly in the cortical lymphocytes and sparsely in the medulla (Fig. 8c).
Fig. 4.
Immunohistochemical staining for cytokeratin (CK). a: Pale area of Fig. 2. The proliferating spindle cells are strongly positive for CK. Bar=50 μm. b: Dark area of Fig. 2. The positively labeled spindle cells among the lymphoid cells are prominent compared with the normal thymic cortex of this animal. The nest is stained similarly to the normal thymic medulla of this animal. Bar=100 μm. c: The normal thymic cortex and medulla of this animal. Bar=100 μm.
Fig. 5.
Immunohistochemistry for CD3. a: Dark area of Fig. 2. Many CD3-Positive lymphoid cells are seen, but a few of them was negative. Bar=50 μm. b: The pale area of Fig. 2. Many lymphoid cells are positive for CD3. Bar=50 μm. c: Pale nests of Fig. 2. CD3-positive lymphoid cells are predominant. Bar=50 μm.
Fig. 6.
Immunohistochemistry for CD20. a: CD20 positive cells surround the pale nest. The labeling pattern is similar to the normal thymic medulla of this animal. The cytoplasm is labeled positively (Inset). Bar=100 μm. b: Normal thymic medulla of this animal. The positive cells are distributed in the margin of the medulla. The cytoplasm is labeled positively (Inset). Bar=100 μm.
Fig. 7.
Immunohistochemistry for S100. a: No positive cells for S100 in the pale nest. Bar=50 μm. b: Normal thymic medulla of this animal. S100 positive cells are observed. Bar=50 μm.
Fig. 8.
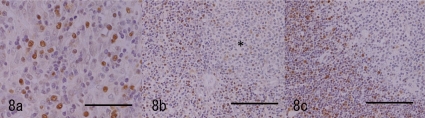
Immunohistochemistry for PCNA. a: The pale area of Fig. 2. The proliferating spindle cells are rarely positive for PCNA, while many lymphoid cells are positive. Bar=50 μm. b: The dark area of Fig. 2. The lymphoid cells are positive for PCNA except for the nest (*) in which positive labeling is sparse. The labeling pattern is similar to the normal thymic cortex and medulla of this animal. Bar=100 μm. c: The normal thymic cortex and medulla of this animal. Bar=100 μm.
The mass of the present case was located in the thymus and surrounded by a distinct fibrous capsule. In the mass, the CK positive spindle cells were proliferating with lymphoid cells. The proliferating cells contained almost no nuclear atypia or infiltrative lesions. Thus, we diagnosed the present case as thymoma. Thymic lymphoma was excluded because of proliferation of CK positive epithelial cells, thymic medullary-like differentiation, no malignant features and lack of regional lymph node involvement.
Thymomas in humans are classified histologically based on the resemblance to the cortical or medullary thymic regions and the composition of the epithelial cells and lymphocytes. Medullary thymoma (type A) is characterized as proliferation of spindled epithelial cells arranged in a whorled storiform pattern. Predominantly cortical thymoma (type B1) has abundant lymphocytes and inconspicuous neoplastic epithelial cells among the lymphocytes. Focal medullary differentiation characterized by small pale areas of epithelial cells within a background of mature thymocytes is particularly observed in this type. Cortical thymoma (type B2) has more and larger epithelial cells than type B1; however, the areas of medullary differentiation are absent or rare in this type of thymoma. Mixed thymoma (type AB) has both elements of type A and type B. The cell figures are similar to those of type A and type B1 but are less commonly similar to type B2.
In our case, the neoplastic spindle cells originating from the thymic epithelium proliferated closely, sometimes arranged in a storiform pattern. The mass also consisted of lymphoid cell-predominant areas in which the epithelial cells were sparse but more abundant than those in the normal cortex of this animal. In this area, the pale nests seemed as the medullary differentiation were distributed. In consideration of these components, this thymoma was classified as mixed thymoma (type AB), particularly combining type A with B1, because of the presence of the medulla-like nests, as defined in the human thymoma classification.
Immunohistochemically, CD3, a mature T cell marker, was expressed most in the nests resembling the thymic medulla and least in the dark area resembling the thymic cortex. The CD3 positive cells were distributed similarly in the normal cortex and medulla. Comparison of the patterns of expression of CD20 , a B cell marker, between the nest and normal medulla revealed that the patterns were the same; however, those of S100, a dendritic cell marker, were different. Dendritic cells are components of the thymic medulla and work as antigen-presenting cells in the same manner as in the lymph follicles. Thus, the thymic medulla has some similarity to the lymph follicles. The nests in the neoplastic mass are probably represent differentiation toward a thymic medulla based on the distributions of epithelial cells and expressions of CD3 and CD20. However, the nests may be incomplete medullas because they lack dendritic cells, one of the components of the thymic medulla, and the reticular epithelial cells in the nests were loose and weak compared with the normal medulla. Previous reports concerning thymomas in animals have referred to differentiation towards a thymic medulla in a dog and rats. 7 – 10 In these cases, including our case, the tumor tissues seemed to imitate or try to construct thymic tissues, though they were incomplete. Expressions of CD4 and CD8, the important markers of thymocyte differentiation in the thymic cortex and medulla, were not detected, probably because the tissues were fixed in formalin and embedded in paraffin.
The association of thymoma with myasthenia gravis (MG) is well known in both humans and animals. 1 , 5 , 11 In the present case, the animal had no clinical signs or abnormality in the blood examination.
Thymomas occur more frequently with aging in both in humans and animals. 1 , 5 In dogs, the median age of affected animals is about 10 years 5 , 12 or 8.2 years. 13 In cows, thymomas occur mostly in animals over 3 years old. 4 , 14 , 15 Furthermore, reports regarding some strains of rats, which are used as experimental animals, have described that thymomas are not observed in any animals younger than 2 years old. 8 – 10 Our case was relatively young in consideration of the other reports, even though the tumor was found coincidentally without any clinical symptoms at the scheduled necropsy in a toxicological study. In addition, thymomas in young animals or children are sometimes invasive and aggressive clinically. 1 , 16 In contrast, our case was found in a young animal but was benign.
In conclusion, our report is the first to describe a thymoma in a cynomolgus monkey. It was diagnosed as a mixed thymoma in accordance with the classification of human thymomas of the WHO. This case will be valuable data for both toxicological studies and veterinary pathology.
Acknowledgments
We thank Mr. Steve Yamakami for language editing.
References
- 1. Rosai J, Levine GD.Tumors of the thymus. In: Atlas of Tumor Pathology Second Series Fascicle 13. Armed Forces Institute of Pathology, Washington, D.C. 34–161.1976.
- 2.Hasserjian RP, Ströbel P, Marx A. Pathology of thymic tumors. Semin Thorac Cardiovasc Surg. 2005;17:2–11. doi: 10.1053/j.semtcvs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Parker GA, Casey HW. Thymomas in domestic animals. Vet Pathol. 1976;13:353–364. doi: 10.1177/030098587601300505. [DOI] [PubMed] [Google Scholar]
- 4.Sandison AT, Anderson LJ. Tumors of the thymus in cattle, sheep, and pigs. Cancer Res. 1969;29:1146–1150. [PubMed] [Google Scholar]
- 5. Jacobs RM, Messick JB, Valli VE.Thymoma. In: Tumors in Domestic Animals, 4th ed. DJ Meuten (ed). Iowa State Press, Iowa. 165–166.2002.
- 6. Valli VE, Jacobs RM, Parodi AL, Vernau W, Moore PF.Histological classification of hematopoietic tumors of domestic animals. In: World Health Organization International Histological Classification of Tumors of Domestic Animals Second Series. FY Schulman (ed). Armed Forces Institute of Pathology and American Registry of Pathology, Washington, D.C. 47–48.2002.
- 7.Simpson RM, Waters DJ, Gebhard DH, Casey HW. Massive thymoma with medullary differentiation in a dog. Vet Pathol. 1992;29:416–419. doi: 10.1177/030098589202900507. [DOI] [PubMed] [Google Scholar]
- 8. Kuper CF, Beems RB, Bruijntjes JP, Schuurman HJ, Vos JG.Thymoma. In: Pathology of the Aging Rat volume 1, U Mohr, DL Dungworth and CC Capen (eds). ILSI Press, Washington, D.C. 39–41.1992.
- 9.Hinsull SM, Bellamy D. Spontaneous thymoma in an inbred strain of rat. J Natl Cancer Inst. 1977;58:1609–1614. doi: 10.1093/jnci/58.6.1609. [DOI] [PubMed] [Google Scholar]
- 10.Kuper CF, Beems RB, Hollanders VMH. Spontaneous pathology of the thymus in aging wistar (Cpb:WU) rats. Vet Pathol. 1986;23:270–277. doi: 10.1177/030098588602300307. [DOI] [PubMed] [Google Scholar]
- 11.Okumura M, Fujii Y, Shiono H, Inoue M, Minami M, Utsumi T, Kadota Y, Sawa Y. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen Thorac Cardiovasc Surg. 2008;56:143–150. doi: 10.1007/s11748-007-0185-8. [DOI] [PubMed] [Google Scholar]
- 12.Atwater SW, Powers BE, Park RD, Straw RC, Ogilvie GK, Withrow SJ. Thymoma in dogs: 23 cases (1980–1991) J Am Vet Med Assoc. 1994;205:1007–1013. [PubMed] [Google Scholar]
- 13.Aronsohn MG, Schunk KL, Carpenter JL, King NW. Clinical and pathologic features of thymoma in 15 dogs. J Am Vet Med Assoc. 1984;184:1355–1362. [PubMed] [Google Scholar]
- 14.Momotani E, Nakamura N, Shoya S. Morphologic evidence of the histogenesis of epithelial thymoma in a cow. Am J Vet Rec. 1981;42:114–121. [PubMed] [Google Scholar]
- 15.Ecco R, Langohr IM, Túry E, Santos HL Júnior, Jacobina GC. Mixed thymoma in a cow. J Vet Diagn Invest. 2006;18:503–507. doi: 10.1177/104063870601800518. [DOI] [PubMed] [Google Scholar]
- 16.Day MJ. Reviews of thymic pathology in 30 cats and 36 dogs. J Small Anim Pract. 1997;38:393–403. doi: 10.1111/j.1748-5827.1997.tb03492.x. [DOI] [PubMed] [Google Scholar]