Abstract
In the present study, modifying effects of diabetes on carcinogenesis induced in type 2 diabetes mellitus model Zucker diabetic fatty (ZDF) rats were investigated using a multiorgan carcinogenesis bioassay. Our re sults demonstrated enhancement of urinary bladder, colon and liver carcinogenesis in ZDF rats treated with five types of carcinogens (DMBDD). Elevated insulin and leptin and decreased adiponectin levels in the serum may be responsible for the high susceptibility of type 2 diabetes mellitus model rats to carcinogenesis in these organs. Possible mechanisms of increased susceptibility of diabetic rats to bladder carcinogenesis could be activation of the PI3K pathway and suppression of p53 in the urothelium in consequence of the above serum protein alterations.
Keywords: type 2 diabetes mellitus, bladder carcinogenesis, PI3K, p53
Introduction
Type 2 diabetes mellitus (T2DM) has been associated with a number of complications, such as cardiovascular disease, diabetic nephropathy and infection1. Recently, with the markedly improved survival of T2DM patients, attributed to the successful treatment of cardiovascular disease and infection control, the relationship between T2DM and cancer has been attracting attention. In epidemiological studies, T2DM was reported to be associated with increased risks of colon, pancreas, mammary, liver and urinary bladder cancers; however, the underlying mechanisms of increased cancer risks in T2DM patients remain unclear2,3.
The influence of diabetes on carcinogenesis induced by chemical carcinogens has been investigated in the colon, stomach and mammary gland in experimental animals4–6. The number of colon tumors initiated by 1,2-dimethylhydrazine dihydrochloride (DMH) was increased in Otsuka Long-Evans Tokushima Fatty (OLETF) rats spontaneously developing T2DM compared with LETO rats, a non-diabetic strain4. Insulin resistance and hyperinsulinemia were suggested as direct risk factors for colon cancer7. Furthermore, db/db diabetic mice were reported to be highly susceptible to stomach carcinogenesis induced by N-methyl-N-nitrosourea (MNU), possibly in association with hyperinsulinemia and hyperleptinemia5. On the other hand, decreased susceptibility to MNU-induced mammary carcinogenesis was reported in streptozotocin-induced diabetic rats, possibly due to significantly lowered plasma levels of insulin and insulin- like growth factor 1 (IGF-1) during the promotion phase of carcinogenesis6. Based on these findings, metabolic abnormalities accompanying diabetes, such as hyperinsulinemia, hyperleptinemia and a high plasma level of IGF-1, have been proposed to play a role in cancer development in DM patients.
Zucker Diabetic Fatty (ZDF) rats closely mimic human adult onset T2DM and its related complications due to the inherited homozygous leptin receptor mutation, which leads to obesity and insulin resistance8. T2DM does not develop in lean littermate (Lean) animals8. ZDF animals show insulin resistance from the time of weaning but maintain normoglycemia until 8–10 weeks of age because of the compensatory hypersecretion of insulin9. ZDF rats develop overt diabetes and have defects in insulin secretion from around 8–10 weeks of age10.
The purpose of the present study was to investigate the modifying effects of T2DM on carcinogenesis in diabetic ZDF rat using a multiorgan carcinogenesis bioassay. The multiorgan carcinogenesis bioassay uses treatment with five types of genotoxic carcinogens, namely N-diethylnitrosamine (DEN), MNU, N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN), diisopropanolnitrosamine (DHPN) and DMH, with target organs including the liver, kidneys, urinary bladder, stomach, small intestine, colon, lungs and thyroid11,12. At the end of the experiment, histopathological analysis was performed to investigate cancer development. We also investigated the serum levels of several biologically active compounds, including insulin, leptin, adiponectin and IGF 1. Furthermore, to elucidate possible mechanisms underlying the modifying effects of T2DM, we analyzed alterations of the gene expression in the bladder urothelium in a 4-week BBN bladder carcinogenicity study.
Materials and methods
Animals
Five-week-old ZDF and control Lean rats were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan) and used after a 1-week acclimation period. They were housed in plastic cages (one rat/cage for ZDF rats, two rats/cage for Lean rats) in an environmentally-controlled room maintained at a temperature of 22 ± 2°C and relative humidity of 44 ± 5%, with a 12-h light/dark cycle and free access to water and food (MF pellet diet; Oriental Yeast Co., Ltd., Tokyo, Japan). Body weight and food intake were measured weekly during the experimental period. Water intake was measured three times a week in the first 4 weeks and once a week thereafter.
Chemicals
DEN, DMH and BBN were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). MNU was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and DHPN was purchased from Nacalai Tesque Inc. (Kyoto, Japan).
Experimental protocol
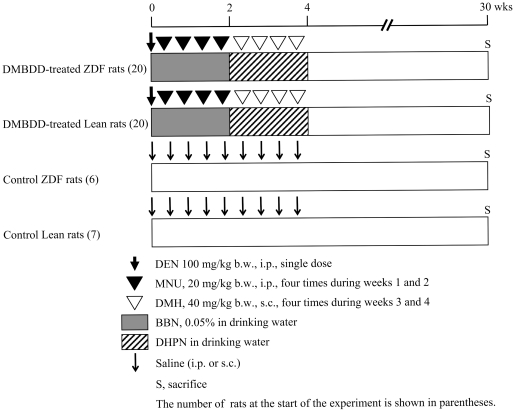
Experimental protocols were approved by the Institution Animal Care and Use Committee of Osaka City University Medical School. The experimental protocol of the rat multiorgan carcinogenesis bioassay used in experiment 1 is shown in Fig. 111,12. Twenty-six male ZDF rats at 6 weeks of age were divided randomly into two groups. Twenty ZDF rats were treated with five carcinogens, DEN, MNU, BBN, DMH and DHPN (DMBDD), as follows: a single i.p. injection of DEN (100 mg/kg b.w.) at the beginning of the experiment, followed by four i.p. injections of MNU (20 mg/kg b.w.) from week 1 to 2, and four s.c. injections of DMH (40 mg/kg b.w.) from week 3 to 4. Also, 0.05% BBN in drinking water was administered to rats for two weeks from commencement of the experiment, and then DHPN in drinking water was administered to the rats for the following 2 weeks. Six control ZDF rats were administered tap water and received vehicle injections (0.9% saline) in the same manner. Twenty-seven male Lean rats at 6 weeks of age were divided randomly into two groups and treated with DMBDD (20 rats) and vehicle (7 rats), respectively, as described above. DHPN was administered to Lean rats at a concentration of 0.1% in drinking water. As ZDF rats were drinking more water than Lean rats from week 3 to 4, the concentrations of DHPN administered to the ZDF rats were diluted to give the same amount of DHPN per b.w. as the Lean rats based on their body weight and water intake. After the initiation treatments, all rats were maintained without any treatment until the end of the study. Blood glucose was examined weekly from week 3 to 7 and once every two weeks from week 8 to 30 via the tail vein using a blood glucose test meter (Glutest Ace R; Sanwa Kagaku Kenkyusho Co., Ltd., Nagoya, Japan). At the end of week 30, all surviving animals were euthanized under deep anesthesia, target organs were taken out and the liver, kidney and spleen weights were measured. All target organs were fixed in 10% phosphate-buffered formalin and embedded in paraffin for histopathological examination. Blood serum was collected for biochemical analyses. Part of the liver tissues was snap frozen in liquid nitrogen and stored at −80°C.
Fig. 1.
Experimental protocol of experiment 1 (DMBDD model).
In experiment 2, 12, 6-week-old male ZDF or Lean rats were divided randomly into two groups each. BBN in drinking water was administered for 4 weeks from commencement of the experiment to ZDF (6) or Lean (6) rats. The remaining 6 ZDF and 6 Lean rats were administered tap water. Given the differences in water intake and body weight between the ZDF and Leans rats, water intake and body weights were measured three times a week, and the BBN concentration was adjusted to give the same amount of BBN per b.w. to both strains of rats based on the water intake per body weight per day from commencement of the experiment. Briefly, 0.05% BBN was administered to the strain of rats drinking less water (g/kg, b.w.), and the concentration of BBN was diluted and administered to the strain of rats drinking more water. Blood glucose was examined weekly from week 1 to 4. At week 4, all rats were euthanized under deep anesthesia, and blood serum was collected for biochemical analyses. The bladder mucosa was harvested using the method described previously13. Part of the liver tissues was snap frozen in liquid nitrogen and stored at −80°C.
Blood biochemistry analysis
Blood biochemistry was analyzed at the end of the experiments (week 30 in experiment 1 and week 4 in experiment 2) by Mitsubishi Chemical Medience Corporation (Tokyo, Japan). The serum levels of insulin, leptin, adiponectin, IGF-1, tumor necrosis factor α (TNFα) and inter leukin-6 (IL-6) of all surviving rats were measured using rat ELISA kits (insulin, Shibayagi Co., Ltd., Gunma, Japan; leptin, Morinaga Institute of Biological Science, Inc., Kanagawa, Japan; adiponectin, Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan; IGF-1, TNFα and IL-6, R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturers’ instructions.
RNA preparation and real-time quantitative PCR
RNA from the bladder mucosa and liver was isolated using TRIZOL Reagent (Life Technologies Japan Ltd., Tokyo, Japan) according to the manufacturer’s instructions. Synthesis of cDNA was performed with 600 ng RNA using an Advantage RT-for-PCR kit (Takara Bio, Inc., Shiga, Japan). Real-time quantitative PCRs for phosphatidylinositol 3-kinase (PI3K), p53, PCNA and β-actin as an internal control for the bladder mucosa and IGF-1 and rsp18 as an internal control for the liver were performed using an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Inc., Tokyo, Japan) as described previously13,14. Briefly, 20 µl containing 1 µl of the respective TaqMan Gene Expression Assays (Applied Biosystems, Inc., Tokyo, Japan), 10 µl TaqMan Fast Universal PCR Master Mix (Applied Biosystems, Inc., Tokyo, Japan) and 5 µl diluted cDNA were applied to a Fast 96-well Reaction Plate.
Serially diluted standard cDNA was included in each TaqMan PCR reaction to create standard curves. The amounts of gene products in the test samples were estimated relative to the respective standard curves. Values for target genes were normalized to those for β-actin or rsp18.
Statistical analysis
All mean values are reported as means ± SD. Statistical analyses were performed using the StatLight program (Yukms Co., Ltd., Tokyo, Japan). Homogeneity of variance analysis was performed by the F test. Differences in mean values between the control and carcinogen-treated groups were evaluated by Student’s t-test when the variance was homogeneous and Welch’s t-test when the variance was heterogeneous. Incidence was assessed by Fisher’s exact probability test. P values less than 0.05 were considered significant.
Results
General observations
Experiment 1: Five DMBDD-treated ZDF rats were found dead or moribund at weeks 17, 21, 22, 27 and 29. Furthermore, two control ZDF rats were found dead or moribund without any discernible cause at weeks 21 and 26. All DMBDD-treated and control Lean rats were alive at the end of the study. As bladder, small intestine and liver tumors were found in the DMBDD-treated ZDF rat that died at week 17, all rats were included in the effective animals for histopathological analysis.
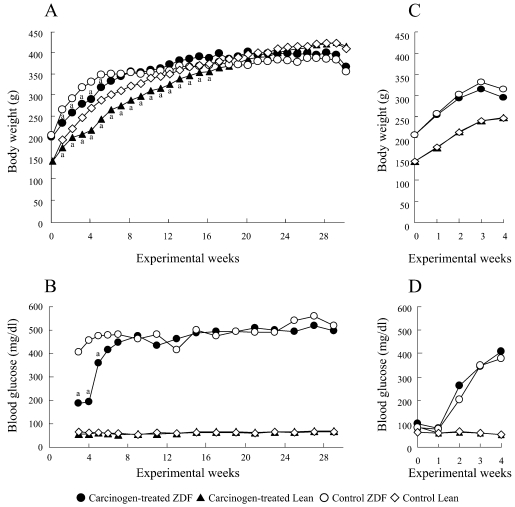
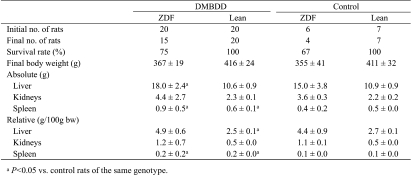
Body weight curves, final body weight and absolute and relative organ weights of the rats in experiment 1 are shown in Fig. 2A and Table 1. The body weights of both the DMBDD-treated ZDF and Lean rats were significantly decreased during administration of DMBDD compared with the control rats of the same genotype; however, the final body weights of both the ZDF and Lean rats administered DMBDD were not significantly changed as compared with the control rats of the same genotype. The body weights of the ZDF rats were significantly higher during the DMBDD treatment period but were significantly lower than those of the Lean rats at the end of the study irrespective of whether or not they received carcinogen treatment.
Fig. 2.
Time course of body weight and blood glucose level in experiment 1 (A, B) and experiment 2 (C, D). a P<0.05 vs. control rats of the same genotype.
Table 1. Final Body and Organ Weights (Experiment 1).
During BBN treatment (weeks 1 and 2), the total BBN intake of the ZDF rats (0.54 ± 0.07 mg/kg b.w.) was significantly lower than that of the Lean rats (0.63 ± 0.04 mg/kg b.w.). During DHPN treatment (weeks 3 and 4), almost the same total amount of DHPN was given to both strain rats (1.3 ± 0.4 mg/kg b.w. for the ZDF rats and 1.3 ± 0.1 mg/ kg b.w. for the Lean rats) by adjusting the concentration of DHPN based on the water intake and body weight, as described in the Methods.
DMBDD administration inhibited food intake of both the ZDF and Lean rats compared with the control rats of the same genotype. Food intake was significantly increased in the DMBDD-treated and non-treated ZDF rats when compared with the Lean rats receiving the same treatment throughout the experiment (data not shown).
The absolute liver and spleen weights and relative spleen weight of the DMBDD-treated ZDF rats were significantly higher than those of the control ZDF rats. Furthermore, the absolute and relative spleen weights in the DMBDD-treated Lean group were significantly elevated compared with those of the Lean control animals.
Experiment 2: All rats survived to the end of the study. Body weight curves are shown in Fig. 2C. The average body weights of the ZDF rats were significantly higher than those of the Lean rats throughout the experiment. BBN treatment had no effect on the body weights of the ZDF and Lean rats. There was no significant difference in the total intake of BBN between ZDF (1.4 ± 0.5 mg/kg b.w.) and Lean rats (1.1 ± 0.0 mg/kg b.w.).
Time course changes of blood glucose level
Time course changes of the blood glucose level in experiments 1 and 2 are shown in Fig. 2B and D, respectively.
In experiment 1, the blood glucose levels were significantly higher in the DMBDD-treated and control ZDF rats throughout the experimental period compared with the Lean rats receiving the same treatment, respectively. The level of blood glucose was significantly suppressed only in the DMBDD-treated ZDF rats compared with the control ZDF rats at weeks 3–5, possibly due to the significant decrease of food intake during weeks 1–5 caused by DMBDD treatment.
In experiment 2, the blood glucose levels of the BBN- initiated and control ZDF rats were significantly elevated compared with the Lean rats receiving the same treatment from week 2 to 4; however, BBN treatment had no effect on the blood glucose level in the ZDF and Lean rats.
Blood biochemistry
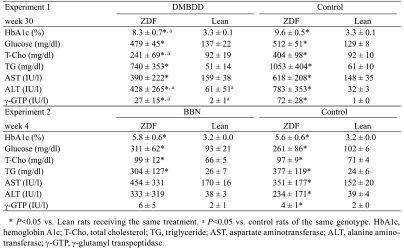
The results of the blood biochemistry analysis in experiments 1 and 2 are shown in Table 2.
Table 2. Blood Biochemistry.
In experiment 1, the level of hemoglobin A1c (HbA1c), one of the characteristics of diabetes, was significantly higher in both the DMBDD-treated and control ZDF animals than in the Lean rats receiving the same treatment. Furthermore, the total cholesterol (T-Cho) and triglyceride (TG) levels were also significantly increased in the DMBDD- administered and control ZDF rats but not in the Lean rats receiving the same treatment.
In experiment 2, the levels of HbA1c, T-Cho and TG were significantly higher in both the BBN-treated and control ZDF rats than in the Lean rats receiving the same treatment. Moreover, no influence of BBN treatment was apparent on HbA1c, T-Cho and TG in both the ZDF and Lean rats.
Histopathological findings in experiment 1
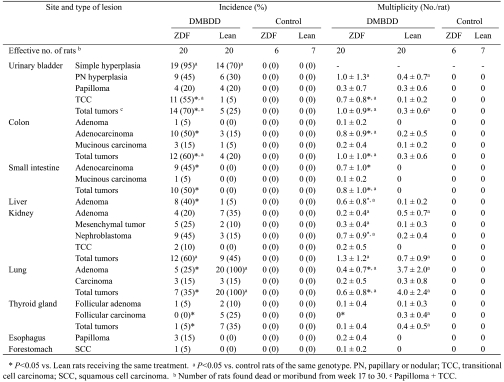
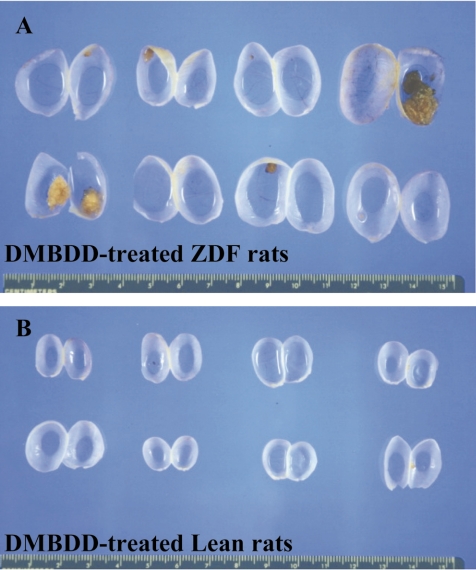
Table 3 summarizes the data on the incidence and multiplicity of preneoplastic and neoplastic lesions induced by DMBDD administration. No tumors were found in the control ZDF and Lean rats. Macroscopically, several very large tumors occupying the whole urinary bladder were found in the DMBDD-treated ZDF rats but not in the Lean rats (Fig. 3). Histological examination demonstrated significant elevation of the incidences and multiplicities of transitional cell carcinomas (TCC: 55%, 0.7 ± 0.8/rat) and total tumors (papilloma +TCC: 70%, 1.0 ± 0.9/rat) in the DMBDD-administered ZDF animals as compared with the DMBDD-initiated Lean rats (TCC, 5% and 0.1 ± 0.2/rat; total tumors, 25% and 0.3 ± 0.6/rat).
Table 3. Histopathological Findings (Experiment 1).
Fig. 3.
Macroscopic view of the urinary bladder in experiment 1. A: DMBDD-treated ZDF rats. B: DMBDD-treated Lean rats.
The incidences and multiplicities of colon adenocarcinoma and total colon tumors (adenocarcinoma, 50% and 0.8 ± 0.9/rat; total tumors, 60% and 1.0 ± 1.0/rat) were significantly increased in the DMBDD-treated ZDF rats as compared with the DMBDD-treated Lean rats (adenocarcinoma, 15% and 0.2 ± 0.5/rat; total tumors, 20% and 0.3 ± 0.6/rat). Furthermore, the incidences and multiplicities of small intestine adenocarcinoma and total tumors were significantly higher in the DMBDD-administered ZDF rats (adenocarcinoma, 45% and 0.7 ± 1.0/rat; total tumors, 50% and 0.8 ± 1.0/rat) than in the DMBDD-treated Lean animals (no tumor was found).
The incidence and multiplicity of hepatocellular ad enoma were significantly increased in the DMBDD-treated ZDF rats (40%, 0.6 ± 0.8/rat) compared with the DMBDDtreated Lean group (5%, 0.1 ± 0.2/rat). No hepatocellular carcinomas were found in the DMBDD-initiated ZDF and Lean rats.
No renal cell carcinoma was observed in any groups. There were no significant differences in renal cell adenoma between the DMBDD-treated ZDF and Lean rats. The multiplicity of nephroblastoma in the DMBDD-treated ZDF rats (0.7 ± 0.9/rat) was significantly increased compared with the DMBDD-administered Lean animals (0.2 ± 0.4/rat).
On the other hand, the incidences and multiplicities of adenoma and total tumors in lung and thyroid follicular carcinoma were significantly decreased in the DMBDD-treated ZDF rats compared with the DMBDD-initiated Lean group.
Serum levels of insulin, leptin, adiponectin and IGF-1
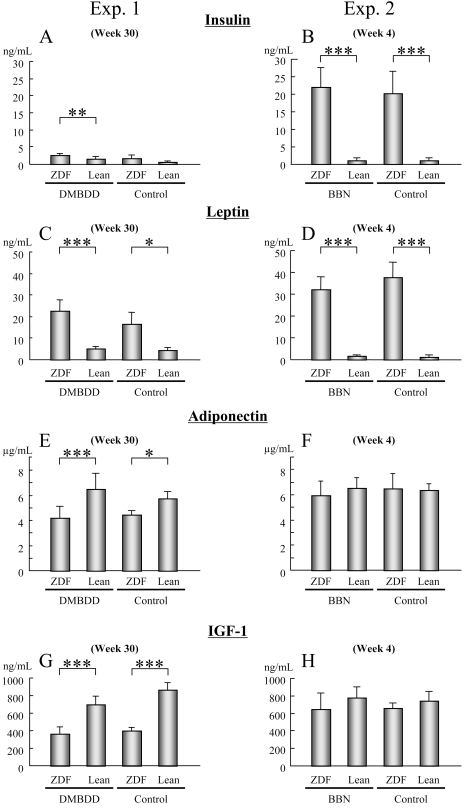
In experiment 1, the serum insulin level in the DMBDD-treated ZDF rats was significantly higher than in the treated Lean groups (Fig. 4A, P<0.001). Furthermore, a significant increase in serum leptin level was found in the ZDF rats compared with the Lean rats receiving the same treatment (Fig 4C; DMBDD-treated, P<0.0001; control, P<0.05). Moreover, there was a significant decrease in the serum adiponectin level in the ZDF rats compared with the Lean rats receiving the same treatment (Fig. 4E; DMBDD-treated, P<0.0001; control, P<0.05). In contrast to our expectation, the serum levels of IGF-1 of the DMBDD-treated and control ZDF rats were significantly decreased compared with the Lean rats receiving the same treatment (Fig. 4G; DMBDD-treated, P<0.0001; control, P<0.0001).
Fig. 4.
Serum levels of insulin, leptin, adiponectin and IGF-1 in ZDF and Lean rats. Data from experiment 1 (A, C, E and G) and experiment 2 (B, D, F and H). Serum concentrations of insulin (A and B), leptin (C and D), adiponectin (E and F) and IGF-1 (G and H) were measured by ELISA. *, ** and ***: P<0.05, P<0.001 and P<0.0001, respectively, vs. Lean rats receiving the same treatment.
In experiment 2, the serum insulin level was significantly increased in the BBN-treated and control ZDF rats compared with the Lean rats receiving the same treatment (Fig. 4B; BBN-treated, P<0.0001; control, P<0.0001). Similarly, the serum leptin level in the ZDF rats was significantly increased compared with the Lean rats receiving the same treatment (Fig. 4D; BBN-treated, P<0.0001; control, P<0.0001); however, no significant differences were apparent in the serum level of adiponectin and IGF-1 (Fig. 4F and H).
The TNFα and IL-6 serum levels were under the limit of detection.
IGF-1 mRNA expression in the liver
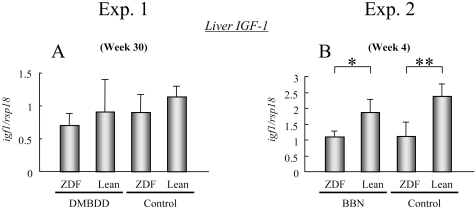
Since there was no increase of the IGF-1 level in the serum of ZDF rats at weeks 4 and 30, its mRNA expression level was examined in the liver, which is the main organ in which IGF-1 is produced. IGF-1 mRNA expression was inhibited in the liver of the DMBDD-treated and control ZDF rats compared with the Lean rats receiving the same treatment at week 30 in experiment 1 but without significance (Fig. 5A). Furthermore, there was a significant decrease of IGF-1 mRNA expression in the BBN-treated and control ZDF rats compared with the Lean rats receiving the same treatment at week 4 in experiment 2 (Fig. 5B; BBN-treated, P<0.05; control, P<0.001).
Fig. 5.
IGF-1 mRNA expression in the liver. * and **: P<0.05 and P<0.001, respectively, vs. Lean rats receiving the same treatment.
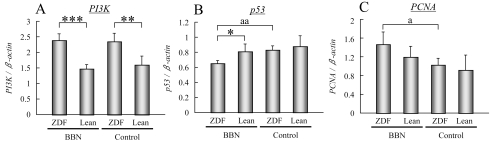
PI3K, p53 and PCNA mRNA expression in the bladder epithelium of BBN-treated rats (experiment 2)
Since alterations of the serum levels of insulin, leptin and adiponectin were observed, we further investigated the downstream effector molecules of these receptors.
Significant increases of PI3K mRNA expression in the bladder epithelium of the BBN-initiated and control ZDF rats were found compared with the Lean rats receiving the same treatment (Fig. 6A; BBN-treated, P<0.0001; control, P<0.001).
Fig. 6.
PI3K, p53 and PCNA mRNA expression in the bladder epithelium of BBN-treated rats (experiment 2). *, ** and ***: P<0.05, P<0.001 and P<0.0001, respectively, vs. Lean rats receiving the same treatment. a and aa: P<0.05 and P<0.001, respectively, vs. control rats of the same genotype.
Four-week treatment with BBN significantly decreased the p53 mRNA expression level in the urothelium of the ZDF rats but had no effect on the Lean rats (Fig. 6B, P<0.001). Furthermore, p53 mRNA expression was significantly inhibited in the BBN-treated ZDF rats compared with the treated Lean animals (Fig. 6B, P<0.05).
The expression of mRNA of PCNA, which is a mark er for cell proliferation, was significantly increased in the BBN-treated ZDF rats compared with the control ZDF animals (Fig. 6C, P<0.05) and showed a tendency to increase as compared with the BBN-treated Lean rats.
Discussion
The present study demonstrated enhancement effects of T2DM on urinary bladder, colon and liver carcinogenesis in ZDF type 2 diabetes rats, indicating the high multiorgan carcinogenic susceptibility of this strain of rats. In epidemiological studies, T2DM was reported to be associated with elevation of colon, pancreas, mammary, liver and urinary bladder cancers2,3. To the best of our knowledge, this study provides the first experimental evidence for a relationship between DM and urinary bladder cancer.
Several serum changes in T2DM patients have been suggested to be responsible for the increased cancer risks, including increases in insulin, IGF-1, leptin, TNFα and IL-6, as well as decreased adiponectin15. In the present study, the serum insulin level was significantly higher in the DMBDD-treated ZDF rats than in the DMBDD-treated Lean rats at week 30. It has been reported that hyperinsulinemia by injection of insulin enhanced the tumorigenesis of azoxymethane-induced colon carcinogenesis in rats16,17. Furthermore, hyperinsulinemia is considered to promote carcinogenesis not only directly but also indirectly by increasing the synthesis of IGF-118. Unexpectedly, the serum level of IGF-1 was significantly lower in the ZDF rats than in the Lean rats irrespective of whether or not they received carcinogen treatment at week 30, and there was no significant difference at week 4 between the two strains of rats. In contrast, the IGF-1 mRNA expression level in the liver, the major site of IGF-1 synthesis, was significantly lower in the ZDF rats than in the Lean rats receiving the same treatment at week 4 but was not significant at week 30 between the two strains of rats. Further study examining the protein expression level of IGF-1 in the liver to explain this discrepancy is necessary. Nevertheless, the changes of serum IGF-1 were not related to the increased cancers of the urinary bladder, colon and liver under the present conditions.
In recent studies, leptin, a hormone secreted by adipocytes, was indicated to act as a mitogen and angiogenic factor in addition to its neuroendocrine function19. Furthermore, epidemiological studies have shown that an elevated level of serum leptin was associated with a high risk of colorectal cancer in men20 and breast cancer21. Meanwhile, adiponectin, which is also an adipocyte-secreted hormone, was reported to have an antiproliferative effect22; thus, low adiponectin concentrations were associated with malignancies in the colon23 or mammary gland24. In the present study, neither DMBDD initiation nor BBN treatment affected the serum levels of leptin or adiponectin in the ZDF and Lean rats; however, the serum level of leptin was significantly higher and the serum adiponectin level was significantly lower in the ZDF rats than in the Lean rats receiving the same treatment at week 30. Therefore, the elevation of leptin and decrease of adiponectin may represent a highly susceptible risk of carcinogenesis of the urinary bladder, colon and liver.
Recently, epidemiological studies have clearly indicated that individuals with diabetes have an increased risk of bladder cancer3; however, the mechanisms by which diabetes mellitus contributes to bladder carcinoma are not clear. In this study, both the incidence and multiplicity of bladder cancer were significantly increased in the DMBDD-treated ZDF rats compared with the treated Lean rats in the 30-week multiorgan carcinogenicity study. In the 4-week BBN bladder carcinogenicity study, the serum insulin level at week 4 was significantly increased in the treated and control ZDF rats compared with the Lean rats receiving the same treatment. Similarly, the serum leptin level in the ZDF rats was significantly increased compared with the Lean rats receiving the same treatment at week 4. On the other hand, no significant differences were apparent in the serum adiponectin level at week 4. Thus, changes of insulin and leptin, but not adiponectin levels, may be related to the high susceptibility of ZDF rats to bladder carcinogenesis from an early stage of carcinogenesis. Insulin and leptin have been reported to activate the PI3K signaling pathway15, which is known to be associated with malignant behavior, including cell growth, proliferation and cell survival25. Furthermore, its activation of PI3K has been demonstrated to be responsible for carcinogenesis in regard to bladder, colon, liver and other cancers26–29. High mRNA expression levels of PI3K in the ZDF rats suggested that the PI3K pathway may be responsible for the high susceptibility to bladder carcinogenesis of rats with T2DM.
In addition, the decrease of p53 in obesity has been demonstrated to play an important role in obesity-associated cancers, such as breast and prostate cancer30,31. The low mRNA expression level of p53 in the BBN-treated ZDF rats compared with the BBN-treated Lean rats may be responsible, at least in part, for their high susceptibility to bladder carcinogenesis.
No increased risk of lung cancer in diabetes was found in a recent epidemiological study32. With regard to the relationship between diabetes and thyroid cancer risk, there is no report available in the published literature. The findings of the present study that the incidences and multiplicities of lung tumors and thyroid follicular carcinoma were significantly decreased in the DMBDD-treated ZDF rats compared with the DMBDD-initiated Lean group indicate a low susceptibility of ZDF rats to lung and thyroid carcinogenesis in this model. However, the mechanism by which T2DM affects lung and thyroid carcinogenesis and whether these findings are relevant to human lung and thyroid cancers are not currently clear.
In conclusion, our results demonstrated that urinary bladder, colon and liver carcinogenesis are enhanced in ZDF type 2 diabetes rats. The possible mechanisms are related to increased serum levels of insulin and leptin and a decreased serum level of adiponectin. Furthermore, the high susceptibility to bladder carcinogenesis in T2DM might be a consequence of PI3K pathway activation and decreased p53 expression. Further studies to analyze the mechanisms underlying the high susceptibility of T2DM rats to colon and liver carcinogenesis are necessary.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Kaori Toma, Rie Onodera and Azusa Inagaki and the assistance of Yukiko Iura in preparation of this manuscript. This research was supported in part by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan.
References
- 1.Kasper DL, Brouwaid E, Fauci AS, Hauser SL, Longo DL, Jameson JL. HARRISON’S PRINCIPLES OF Internal Medicine, Vol. 2005 [Google Scholar]
- 2.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 47: 87–95 2010 [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 49: 2819–2823 2006 [DOI] [PubMed] [Google Scholar]
- 4.Terai K, Sakamoto K, Goto M, Matsuda M, Kasamaki S, Shinmura K, Takita N, Kamano T. Greater development of 1,2-dimethylhydrazine-induced colon cancer in a rat model of type 2 diabetes mellitus. J Int Med Res. 34: 385–389 2006 [DOI] [PubMed] [Google Scholar]
- 5.Yoshizawa N, Yamaguchi H, Yamamoto M, Shimizu N, Furihata C, Tatematsu M, Seto Y, Kaminishi M. Gastric carcinogenesis by N-Methyl-N-nitrosourea is enhanced in db/db diabetic mice. Cancer Sci. 100: 1180–1185 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocca C, Gutierrez A, Nunez M, Croci M, Martin G, Cricco G, Rivera E, Bergoc R. Suppression of mammary gland tumorigenesis in diabetic rats. Cancer Detect Prev. 27: 37–46 2003 [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 6: 164–179 1995 [DOI] [PubMed] [Google Scholar]
- 8.Clark JB, Palmer CJ, Shaw WN. The diabetic Zucker fatty rat. Proc Soc Exp Biol Med. 173: 68–75 1983 [DOI] [PubMed] [Google Scholar]
- 9.Friedman JE, de Vente JE, Peterson RG, Dohm GL. Altered expression of muscle glucose transporter GLUT-4 in diabetic fatty Zucker rats (ZDF/Drt-fa). Am J Physiol. 261: E782–E788 1991 [DOI] [PubMed] [Google Scholar]
- 10.Sturis J, Pugh WL, Tang J, Ostrega DM, Polonsky JS, Polonsky KS. Alterations in pulsatile insulin secretion in the Zucker diabetic fatty rat. Am J Physiol. 267: E250–E259 1994 [DOI] [PubMed] [Google Scholar]
- 11.Fukushima S, Hagiwara A, Hirose M, Yamaguchi S, Tiwawech D, Ito N. Modifying effects of various chemicals on preneoplastic and neoplastic lesion development in a wide-spectrum organ carcinogenesis model using F344 rats. Jpn J Cancer Res. 82: 642–649 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi K, Wanibuchi H, Salim EI, Shen J, Wei M, Mitsuhashi M, Kudoh S, Hirata K, Fukushima S. Revised rat multi-organ carcinogenesis bioassay for whole-body detection of chemopreventive agents: modifying potential of S-methylcysteine. Cancer Lett. 206: 15–26 2004 [DOI] [PubMed] [Google Scholar]
- 13.Wei M, Arnold L, Cano M, Cohen SM. Effects of co-administration of antioxidants and arsenicals on the rat urinary bladder epithelium. Toxicol Sci. 83: 237–245 2005 [DOI] [PubMed] [Google Scholar]
- 14.Wei M, Hamoud AS, Yamaguchi T, Kakehashi A, Morimura K, Doi K, Kushida M, Kitano M, Wanibuchi H, Fukushima S. Potassium bromate enhances N-ethyl-Nhydroxyethylnitrosamine-induced kidney carcinogenesis only at high doses in Wistar rats: indication of the existence of an enhancement threshold. Toxicol Pathol. 37: 983–991 2009 [DOI] [PubMed] [Google Scholar]
- 15.Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 10: 610–616 2009 [DOI] [PubMed] [Google Scholar]
- 16.Corpet DE, Jacquinet C, Peiffer G, Tache S. Insulin injections promote the growth of aberrant crypt foci in the colon of rats. Nutr Cancer. 27: 316–320 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev. 5: 1013–1015 1996 [PubMed] [Google Scholar]
- 18.Boni-Schnetzler M, Schmid C, Meier PJ, Froesch ER. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol. 260: E846–E851 1991 [DOI] [PubMed] [Google Scholar]
- 19.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 207: 12–22 2006 [DOI] [PubMed] [Google Scholar]
- 20.Stattin P, Palmqvist R, Soderberg S, Biessy C, Ardnor B, Hallmans G, Kaaks R, Olsson T. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep. 10: 2015–2021 2003 [PubMed] [Google Scholar]
- 21.Han C, Zhang HT, Du L, Liu X, Jing J, Zhao X, Yang X, Tian B. Serum levels of leptin, insulin, and lipids in relation to breast cancer in china. Endocrine. 26: 19–24 2005 [DOI] [PubMed] [Google Scholar]
- 22.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 86: s858–s866 2007 [DOI] [PubMed] [Google Scholar]
- 23.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 97: 1688–1694 2005 [DOI] [PubMed] [Google Scholar]
- 24.Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, Hankinson SE. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 92: 1510–1516 2007 [DOI] [PubMed] [Google Scholar]
- 25.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 296: 1655–1657 2002 [DOI] [PubMed] [Google Scholar]
- 26.Knowles MA, Platt FM, Ross RL, Hurst CD. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 28: 305–316 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3’-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 61: 7426–7429 2001 [PubMed] [Google Scholar]
- 28.Yeh KT, Chang JG, Chen YJ, Chen ST, Yu SY, Shih MC, Perng LI, Wang JC, Tsai M, Chang CP. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in hepatocellular carcinoma. Cancer Invest. 18: 123–129 2000 [DOI] [PubMed] [Google Scholar]
- 29.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2: 489–501 2002 [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Chang YC, Liu CL, Chang KJ, Guo IC. Leptininduced growth of human ZR-75–1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/ CIP1. Breast Cancer Res Treat. 98: 121–132 2006 [DOI] [PubMed] [Google Scholar]
- 31.Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int. 101: 1317–1322 2008 [DOI] [PubMed] [Google Scholar]
- 32.Hall GC, Roberts CM, Boulis M, Mo J, MacRae KD. Diabetes and the risk of lung cancer. Diabetes Care. 28: 590–594 2005 [DOI] [PubMed] [Google Scholar]