Abstract
To illustrate the process of addressing adverse preclinical findings (APFs) as outlined in the first part of this review, a number of cases with unexpected APF in toxicity studies with drug candidates is discussed in this second part. The emphasis is on risk characterization, especially regarding the mode of action (MoA), and risk evaluation regarding relevance for man. While severe APFs such as retinal toxicity may turn out to be of little human relevance, minor findings particularly in early toxicity studies, such as vasculitis, may later pose a real problem. Rodents are imperfect models for endocrine APFs, non-rodents for human cardiac effects. Liver and kidney toxicities are frequent, but they can often be monitored in man and do not necessarily result in early termination of drug candidates. Novel findings such as the unusual lesions in the gastrointestinal tract and the bones presented in this review can be difficult to explain. It will be shown that well known issues such as phospholipidosis and carcinogenicity by agonists of peroxisome proliferator-activated receptors (PPAR) need to be evaluated on a case-by-case basis. The latter is of particular interest because the new PPAR α and dual α/γ agonists resulted in a change of the safety paradigm established with the older PPAR α agonists. General toxicologists and pathologists need some understanding of the principles of genotoxicity and reproductive toxicity testing. Both types of preclinical toxicities are major APF and clinical monitoring is difficult, generally leading to permanent use restrictions.
Keywords: adverse preclinical finding, weight-of-evidence approach, morphologic toxicity, genotoxicity, carcinogenicity, reproductive toxicity
Introduction
In the first part of this review processes for dealing with unexpected adverse preclinical findings (APFs) were discussed and an overview over APFs associated with drug classes and safety issues often encountered in preclinical studies was given. The various steps in dealing with APFs involve:
-
●
Hazard recognition, which also includes the need to verify if the observed effect is indeed a biologically significant APF
-
●
Hazard characterization, which serves to better understand the APF including aspects such as quantitative dose-response, severity, reversibility, and most important, if possible, potential pathogenic pathways and underlying mode of action (MoA) of the drug candidate leading to the APF in question
-
●
Risk evaluation, essentially an intellectual process, including
-
°
MoA aspects, as far as established
-
°
the relevance of the APF for man
-
°
and—particularly if relevance for man can not be excluded with certainty—calculation of safety ratios (The safety ratio is also often called “safety factor” and in some regions “safety margin”. As especially the latter term is partly used in a different way (see first part of the review), it is important to define the exact meaning of such terms.) between exposure at the no observed adverse effect level (NOAEL) 1 in the most sensitive and for man relevant animal species and exposure at the maximal (anticipated) human dose 2 .
To arrive at a full weight of evidence (WoE) evaluation other factors such as therapeutic indication, medical need, and alternative drugs already on the market must also be taken into account
-
°
-
●
Risk management to minimize the risk of humans particularly in early clinical trials
Toxicologic pathologists together with toxicologists play an important role for recognizing potential APFs: They contribute to resolve issues related to such findings and to support the risk management process. They must feel accountable for the health of humans to be exposed to the drug candidate, but must also avoid being overcautious, thus preventing potentially useful drugs to reach the market.
This second part of the review concentrates on a more detailed discussion of selected APFs of drugs as far as possible with reference to their MoA and regarding their relevance for man. Examples will cover morphologic toxicity and tumorigenicity as well as some additional aspects of functional toxicity. Experience regarding APFs in reproductive and genotoxicity studies will be included as far as relevant to the general toxicologist and toxicologic pathologist.
Unsuccessful attempts to resolve preclinical toxicity issues may not be published. However, if an APF did not stop drug development, findings are often mentioned in the package insert. It may be somewhat comforting that most package inserts actually show that APFs were detected during the development of the drug. Successful “troubleshooting” results are sometimes published, as acceptance for publication in a recognized peer-reviewed scientific journal may support registration. Last but not least, personal experience of the authors has played an important role in writing this review.
Examples of Addressing APFs
General Toxicity
Neural toxicity
Nerve cells are special, as after birth they can not multiply and therefore do not regenerate with partial exception of severed nerve processes. CNS toxicity in preclinical safety studies is a severe APF and relatively rare with drug candidates, but a number of industrial chemicals are known to be neurotoxic 3 – 5 . The developing brain appears to be particularly vulnerable 4 . CNS toxicity may also be secondary e.g. to seizures 6 , disturbance of circulation or exhaustion of nerve cells by excitatory amino acids 7 – 9 . For functional CNS toxicity see first part of this review.
Peripheral neuropathy, also not frequently seen with drug candidates, may occur e.g. because of prolonged hypoglycemia with antidiabetic drugs at high doses 10 , 11 . Retinal toxicity is a special type of neural toxicity and needs to be distinguished in particular from light-induced retinopathy of albino rodents 12 . Some drugs which induce retinotoxicity in laboratory animals are discussed in the following paragraph.
Retinal toxicity : The eye is one of those organs where toxicity is not acceptable, particularly if potentially leading to permanent visual impairment. Early retinal toxicity can manifest itself already in a two week study, initially by a subtle decrease in the number of nuclei e.g. of the outer nuclear photoreceptor layer and retinal thinning. With longer treatment, nuclear layers may disappear, and the pigment epithelial layer may become disrupted.
Do such findings mean that development of the drug candidate needs to be abandoned? Not necessarily, as illustrated by a number of drugs on the market, which produce toxicity in laboratory animal eyes—though often only in one animal species or strain, mainly in albino rats— but are deemed safe for humans 13 . The retina of rats and mice is damaged e.g. by ribavarin used in case of hepatitis C and by various lipid lowering drugs. A number of animal species is adversely affect by nalidixic acid 14 , a drug against urinary tract infection, and tranexamic acid 15 against angioneurotic edema. However, some drugs such as hydroxychloroquine, are toxic also for the human eye and may lead to irreversible retinopathy 16 , 17 .
Besides being hepatotoxic, adversely affecting the endocrine and cardiovascular system, and inducing phospholipidosis, some CNS drugs are also known to be retinotoxic in animals, partly at relatively low doses. Such animal retinotoxicity was seen e.g. with pregabalin (neuropathic pain) in albino rats; pramipexole (Parkinson’s disease) in albino rats, but not in pigmented rats, albino mice, monkeys, and minipigs; aripiprazole (psychosis) in albino rats, but not in albino mice and monkeys; and with citalopram in albino rats 18 . However, no retinal changes were detected in humans treated with these drugs by careful eye monitoring.
If retinal toxicity is observed, it is not sufficient to refer to literature or other sources of information related to the APF. It is necessary to characterize the hazard, that is to
-
●
Show that e.g. only one species or strain is affected, or —if two rodent species (rats and mice) are affected—non-rodents (e.g. dog and monkey) are without eye lesions
-
●
Define when the lesion starts to appear
-
●
Determine the exact NOAEL, e.g. by using more sensitive investigations such as morphometry or, if deemed necessary, electron microscopy (EM).
In addition to ophthalmoscopy, electroretinogram (ERG) investigations in animals may have to be considered. However, recording and interpreting ERGs is a demanding expert task. Species-related differences regarding the function of the retina limit the predictive value of these tools 19 , 20 . At least initially it is important to monitor humans by ophthalmoscopy, vision tests, ERG, etc. in clinical studies with drugs which, based on preclinical findings, may be oculotoxic. Binding of drugs to eye melanin of laboratory animals is not predictive of ocular toxicity 21 .
Endocrine system including effector organs
Increased incidences of endocrine tumors are seen with many drug candidates in chronic toxicity and lifetime bioassay studies. These tumors generally result from disturbance of the hormonal balance. They are often due to the specific endocrine physiology of rodents (for more details see the first part of this review) and therefore without relevance to man 22 . A few examples are discussed below.
Uterine tumors: Dopaminergic drugs have endocrinological and neurological clinical indications. Among them are ergot alkaloids which, based on their structure-activity relationship, can be divided into three classes: Lysergic acid amines (e.g. bromocriptine), clavines (e.g. pergolide), and 8-α-aminoergolines (e.g. lisuride and mesulergine). Dopaminergic ergot alkaloids have significant endocrine effects in rodents, particularly in rats, through their inhibitory effect on the secretion of prolactin (PRL) from the anterior pituitary 23 . The elucidation of the MoA of dopaminergic drug candidates in laboratory animals also illustrates the importance of carefully planning additional experiments, as an explanation for the uterine findings is only possible taking into account the age-specific sex hormone climate in aging rats.
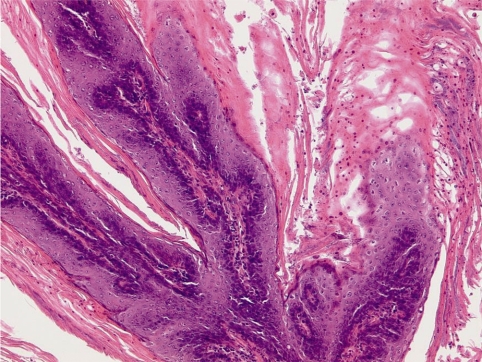
Bromocriptine was found to induce squamous cell metaplasia of the uterine endometrium in a chronic (53 week) rat study at the high dose of 82 mg/kg bw/day in feed admixture. The typical appearance with squamous cell metaplasia, polypoid structures, and some stromal inflammation is shown in Fig. 1.
Fig. 1.
Squamous cell metaplasia of the endometrium of a female OFA rat treated in feed for 53 weeks with 82 mg/kg bw/day of bromocriptine. H&E, lens 10×.
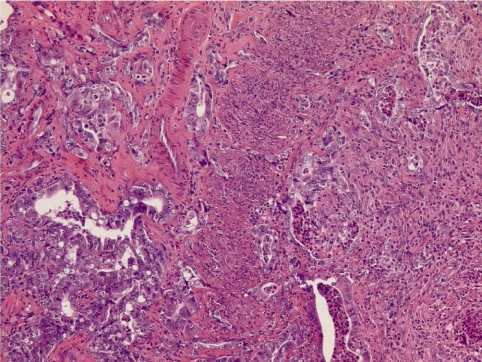
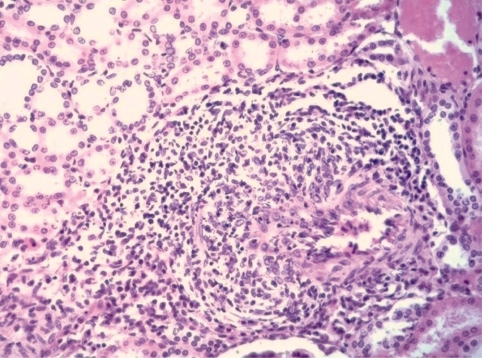
In the lifetime bioassay with lower doses (1.8, 9.9, and 44.5 mg/kg bw/day in feed) these lesions had progressed in the mid and high dose to uterine adenocarcinomas as shown in Fig. 2 with mostly tubular structures of various sizes, which are partly multilayered and show cellular and nuclear polymorphism, as well as diffuse infiltration into the myometrium.
Fig. 2.
Uterine adenocarcinomas of an OFA rat treated in feed for 100 weeks with 44.5 mg/kg bw/day of bromocriptine. H&E, lens 10× .
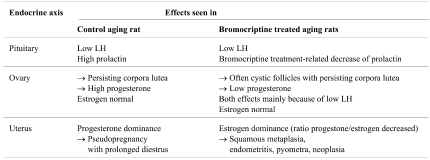
The MoA of bromocriptine leading to these uterine changes is summarized in Table 1 .
Table 1. Endocrine Reproductive Climate in Aging Female Rats and Mode of Action of Bromocriptine Leading to Uterine Tumors (modified after 23 ).
Because of high PRL and low luteinizing hormone (LH), untreated older rats stop cycling and remain in diestrus (pseudopregnancy) with relatively high progesterone production. By lowering PRL bromocriptine treatment initiates regular cyclical activity, but this is short-lived, as the insufficient preovulatory LH surge can not trigger ovulation thus leading to cystic follicles. As a consequence of the lack of estrous cycles, also corpora lutea tend to persist. However, because of the low PRL levels these corpora lutea do not produce significant amounts of progesterone. Therefore, the estrogen/progesterone ratio is higher in bromocriptine-treated rats, which leads to squamous endometrial metaplasia. This physiological reaction facilitates endometritis and pyometra, which through irritation results in increased cell proliferation and, if sustained, may give rise to neoplasia.
No comparable uterine findings were detected in a 52 week dog study or in a carcinogenicity study in mice. Also, in clinical studies bromocriptine did not influence follicle stimulating hormone (FSH), LH, estradiol or progesterone levels in female patients. Endometrial biopsies of chronically treated patients did not show any drug-related changes 24 . The uterine APF in rats is without relevance for women and considered to be an exaggerated pharmacodynamic effect of bromocriptine specific for aging female rats 23 , 24 .
Leydig cell tumors: Mesulergine is an 8 α-aminoergoline with antagonistic and agonistic activity on the D-2 dopamine receptor. It was developed for the treatment of hyperprolactinemia and of Parkinson’s disease. For reasons not related to the APF discussed here, it did not reach the market, but a successor drug with similar properties has been registered as drug.
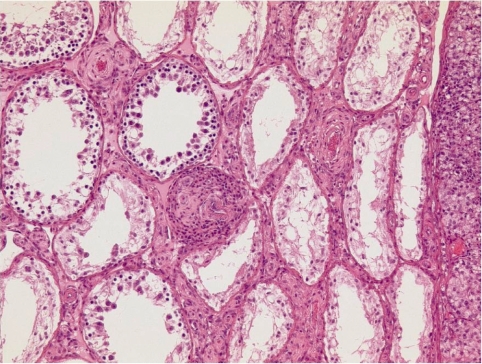
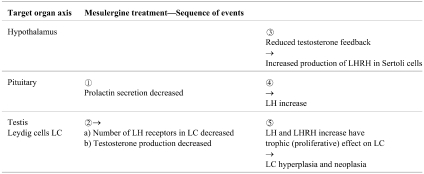
In the carcinogenicity study of 129 weeks with mesulergine at doses of 0.11, 0.42, and 1.7 mg/kg bw/day in feed admixture, male Wistar rats developed at all doses an excess of Leydig cell (LC) tumors morphologically not distinguishable from spontaneous tumors commonly seen in old male Wistar rats. The typical appearance of this tumor and the precursor lesion, namely focal and diffuse LC hyperplasia, is shown in Fig. 3. The seminiferous tubules are partly atrophic, which may be due both to pressure produced by the large LC tumors and the disturbed endocrine regulation.
Fig. 3.
Leydig cell hyperplasia (one focus in the center of the figure) and adenomas (one seen on the right side of the figure) of the testis of a Kfm:WIST rat treated in feed for 129 weeks with 1.7 mg/kg bw/day of mesulergine. H&E, lens 10×.
Other dopaminergic compounds induced also variants of LC tumors characterized by glandular and/or tubular structures (single columnar layer of cells, occasionally with papillary projections), including some with malignant features, such as cellular atypia and invasion of the capsule and/or blood vessels 25 . The glandular/tubular structures stained immunohistochemically as LC, thus confirming that this variant is a form of LC metaplasia.
The proposed MoA 26 , 27 is shown in Table 2 and is related to the PRL lowering effect of mesulergine and the resulting reduced sensitivity of LC to LH because of fewer LH receptors in LC. Rat LC also possess LH releasing hormone (LHRH) receptors 28 . Therefore, in addition to increased LH levels, increased levels of LHRH contributed to the hyperplastic and neoplastic response of the LC.
Table 2. Mesulergine Effect on Rat Leydig Cells (Modified after 23 ).
The occurrence of variants of LC tumors did not change the final assessment that the syndrome appears to be rat-specific, which was confirmed by members of a workshop on rodent LC adenomas and human relevance 29 . Similar changes are not found in other laboratory animal species or man. Increased LH levels in men are seen e.g. in the Klinefelter Syndrome, a numerical chromosome aberration during gametogenesis with one (occasionally several) additional X chromosome(s) (XXY or XXXY genotype), and do not result in LC tumors.
Other drugs—such as busereline, carbamazepine, cimetidine, finasteride, flutamide, gemfibrozil, histrelin, hydralazine, indomethacin, isradipine, lactitol, leuprolide, metronidazole, mesulergine, nafarelin, norprolac, and vidarabine—may also disturb the closed-loop feedback mechanism of the hypothalamo-hypophyseal-gonadal axis 28 . Although the precise cause of the perturbation may vary depending on the drug type, the end result appears to be similar 26 , 28 . However for new drug candidates the hypothesis of a hormonal disturbance in laboratory animals needs to be proven on a case-by-case basis 29 .
Pituitary tumors : Tumors of the pituitary are frequent in rodent bioassays, sometimes in a dose-dependent manner, suggesting a causal relationship with treatment. The following case triggered a number of additional investigations, but could not be completely resolved regarding the MoA. However, biomarkers identified during the various steps of addressing the APF allowed monitoring of man for the corresponding disturbance and allaying concern. In addition, clinical data—the drug was on the market for many years prior to the tumor finding—did not reveal any evidence that these rat pituitary tumors were relevant to man.
Salmon and porcine calcitonin are calcium-lowering hormones used e.g. for the treatment of hypercalcemia associated with bone metastases and postmenopausal osteoporosis. The drugs are also used for many other indications, often off-label, and therefore an increased incidence of tumors in the pars distalis of the pituitary in rats treated for 52 weeks with subcutaneous doses of 1.25, 5.0, or 80.0 IU/kg bw/day of salmon calcitonin was of concern: Pituitary tumors were seen in male rats at all doses and pituitary hyperplasic foci in female rats at the high dose 30 . In a follow-up study these tumors were found to be non-functioning, that is the serum levels of traditional pituitary hormones were not significantly altered with exception of a two-fold increase of TSH. However, immunohistochemical and in situ hybridization characterization of the tumors showed that these tumors produced significant amounts of the α-subunit of the pituitary glycoprotein hormones LH, FSH, and TSH 31 , 32 . This α-subunit is rarely found in control and spontaneously occurring hyperplastic or neoplastic lesions. A time course study revealed an increase of the serum α-subunit already after 24 weeks of treatment and was in parallel to the appearance of histopathological lesions. In treated human beings the glycoprotein α-subunit was always normal. After many years of repeated investigations it can be concluded that the drugs are safe.
Cardiovascular toxicity
It is important to carefully examine the early short-term studies for heart lesions 33 , though this does not replace functional testing, mostly measurement of the QT time (see first part of this review). Drug candidates can exert their toxicity via a direct action on myocytes, e.g. through cytotoxicity, which is the case e.g. for tyrosine kinase inhibitors 34 , or in connection with drug-induced metabolic disorders, e.g. diabetes mellitus, hyperthyroidism or phospholipidosis. Depending on the species, cardiotoxicity can manifest itself in different parts of the heart. E.g. minoxidil induces arteriopathic changes mainly in the right atrium in dogs, but in the left atrium in minipigs. This can possibly be explained by differences of the atrial vascularization: Minipigs are right coronary artery predominant, while the left coronary artery is dominant in most other species. As heart and skeletal muscles are partly significantly different 35 , skeletal statin myopathies are generally not associated with significant cardiac toxicity 36 – 38 . However, there is evidence that besides having a cardioprotective effect, statins may also be cardiotoxic 39 – 41 . In addition, it is suspected that agonists of peroxisome proliferator activated receptors (PPAR) may not only cause rhabdomyolysis, but also adversely affect the heart (see section on PPAR agonists below).
Direct cardiotoxicity observed in laboratory animals is often predictive for cardiac side-effects in man. However, commonly used laboratory animal species are in part quite prone to develop cardiovascular changes. Dogs are frequently affected by spontaneously occurring vasculitis, depending on the dog-colony. Such lesions may be difficult to distinguish from induced lesions 42 , 43 .
Hemodynamic effects of drug candidates associated with morphological cardiotoxicity are often due to exaggerated pharmacological effects on myocytes, including inotropic (force), chronotropic (rate), lusiotropic (relaxation), and dromotropic (conduction) effects. Inotropic and chronotropic effects lead to a work overload with underperfusion of areas supplied by end arteries and shortening of the diastolic period when blood perfusion takes place. Inotropic and chronotropic effects are seen e.g. with isoprenalin or drugs leading to hyperthyroidism. Dogs are highly sensitive to cardioactive and vasodilating drugs, particularly to drugs leading to tachycardia 44 , 45 . Lesions in dog hearts associated with tachycardia are without relevance to man. However, if similar findings also occur in monkeys, the issue becomes more serious and a sufficient safety ratio is necessary. Dromotropic effects of drug candidates on the conduction system of the heart, often the hERG channels, may lead to ventricular blocks and arrhythmias. QT measurements and hERG testing is mainly used for screening of such effects, but positive tests only mean that careful investigations in man are needed to check for human relevance 46 , 47 .
Hemodynamic effects also include effects on blood volume e.g. by PPAR agonists 48 . Vasoconstriction with increased blood pressure and decreased end artery perfusion can occur e.g. with noradrenalin or vasopressin. Vasodilation with decreased blood pressure, vascular wall distention, and potentially vascular wall necrosis occurs e.g. with phosphodiesterase (PDE) inhibitors and potassium channel openers. Most hemodynamic side-effects are not relevant to man 45 , but this needs to be demonstrated case-by-case by elucidating the MoA leading to the observed effect and demonstration of the absence of this effect in man. Over all, hemodynamic effects are more difficult to elicit in rodents, but age-related spontaneous cardiovascular diseases are frequent in these species. Also monkeys and minipigs are less sensitive than dogs to drug-induced hemodynamic-related cardiovascular toxicity.
The most frequent vascular toxicity is vasculitis, which is induced by a number of drugs such as potassium channel opener 49 , 50 , adenosine agonists 51 , endothelin receptor antagonists 52 , 53 , dopaminergic agonists 54 , and quite often by PDE inhibitors 55 – 60 . An example of a subacute arteritis/periarteritis in the kidney of a rat treated with an undisclosed drug is shown in Fig. 4.
Fig. 4.
Subacute arteritis/periarteritis in a SIV 50 rat renal artery with necrosis, inflammation, and proliferation of fibroblasts; undisclosed drug. H&E, lens 25× .
Vascular toxicity is difficult to detect during the in-life phase of safety studies and in clinical trials, because the available biomarkers (see below) are not that powerful and because the MoA is generally not well understood 61 . Vascular toxicity is often seen in mesenteric vessels of rats, which are also the site for spontaneous polyarteritis nodosa 62 , possibly because the surrounding adipose tissue may not sufficiently support dilated arteries and/or because these vessels have a higher number of vasoactive receptors. Also, increased shear stress on the arterial wall appears to play a role. If exaggerated vasodilation is suspected as MoA for vasculitis in a subacute study, it can be worthwhile to try blocking vasodilation e.g. with vasopressin.
Potentially useful biomarkers of cardiovascular injury in early and later clinical trials for hazard identification, characterization, and management in man are
-
●
Traditional physiological biomarkers such as arterial pressure and heart rate
-
●
Cardiac troponin I and T concentrations in serum to recognize myocardial membrane disruption and myocardial necrosis early
-
●
Von Willebrand factor (vWF) and vWF propetide (vWFpp) characteristic for endothelial cell function
-
●
Vascular endothelial growth factor, endothelin, caveolin-1, asymmetric dimethylarginine, nitric oxide, and circulating endothelial cells
-
●
Markers of inflammation such as high-sensitivity C-reactive protein (hs-CRP), interleukin-6, and other cytokines or markers identified by “-omics” and flow cytometry 63 .
Risk evaluation using a WoE approach for evaluation of vascular toxicity must be based on detailed pathology assessment including lesion distribution, if possible time course, EM investigations to establish the NOAEL, and number of species affected. The hemodynamic status needs to be investigated, e.g. blood pressure, heart rate, possibly local flow rates, vessel wall diameter and/or wall thickness, and other biomarkers as appropriate and mentioned above. Reversibility studies and mechanistic studies using an antagonist against the suspected MoA (see above) may be helpful. As usual, pharmacokinetic data including area under the curve (AUC) for unbound (free) drug concentrations and protein-binding data are needed. Theophylline, a well known anti-asthma drug, is an example of a drug on the market with significant vascular toxicity in laboratory animals, but after many years of use it is clear that theophylline does not adversely affect human vessels 62 . Also autopsies of minoxidil-treated patients did not reveal any evidence for cardiovascular side-effects. However, it can not be excluded that subtle drug-induced vascular adverse effects aggravate pre-existing conditions such as atherosclerosis or potentiate the effect of noxious stimuli such as tobacco consumption or hypertension, or promote atherogenesis through a local inflammatory cascade. It may therefore be necessary to conduct studies in atherosclerotic animal models to check for aggravation of the underlying disease by treatment with drug candidates showing vascular APF.
If the MoA of the cardiovascular APF is known and qualitatively relevant to man, the next question to address is: Is the cardiovascular APF also quantitatively relevant under therapeutic conditions applicable to man? Generally, a safety ratio higher than 10 (ratio of the exposure of the most sensitive and relevant animal species at the NOAEL over highest therapeutic human exposure, see first part of this review) can be considered to reflect safety. Often and despite further investigations the MoA and therefore the relevance of a vascular APF for man are not known. Then, considerations are mainly limited to safety ratios in conjunction with indication, medical need, target population, and available alternatives on the market. Vascular toxicity continues to be a significant safety issue for drug sponsors and regulators, as in humans vasculitis can range from single organ involvement, mostly the skin, to life-threatening multi-organ vasculitis 64 .
Liver and kidney
Liver and kidney toxicity is relatively frequent and often an issue during drug development 65 . The liver is the main site of the metabolism of xenobiotics including drugs and expresses many cytochrome P450 isoforms. The metabolism generally results in detoxification and excretion of xenobiotics, but may occasionally also lead to a toxic intermediate including e.g. short lived reactive oxygen species. This toxification effect has been known for many years e.g. for paracetamol, also called acetaminophen 66 . Paracetamol increases nitric oxide (NO), which scavenges superoxide generated by reactive oxygen species to produce peroxynitrite. This then causes protein nitration and tissue injury 67 .
The kidney is crucial for excretion of drugs from the body and the urine concentration can pose a hazard to renal tubuli and the associated tissue. Rodents concentrate urine to a much higher degree than man or dog, and local exposure to toxic drugs or metabolites in the urine may therefore be higher. Oliguria in dehydrated patients, a frequent condition in the elderly, can also be associated with high urinary concentrations of drugs and metabolites and therefore must be regarded as a risk factor for adverse drug effects especially on the kidney.
Unless liver or kidney toxicity is chronic, it is usually reversible. Nevertheless, these toxicities can be serious in the clinic: Patients, particularly the elderly, often also suffer from a pre-existing liver or kidney disease, because of enzyme induction or inhibition associated with the diet, with alcohol or with concomitant drug therapy, or because of genetic variances affecting e.g. drug metabolism 68 . Preclinical studies do not always allow detecting liver and kidney toxicity 69 , though numerous and well established screening methods exist, and liver and kidneys are extensively examined in toxicity studies. Toxicogenomics may help to improve detection of liver and kidney toxicity in preclinical safety studies and provide biomarkers for human monitoring 70 – 73 . The chances to elucidate the MoA of a drug candidate with liver or kidney toxicity are relatively high, because these toxicities are well studied.
Renal papillary necrosis is seen mostly in animals with a range of drugs and chemicals 74 . In man it is infrequent and occurs mainly in conjunction with polypharmacy and preexisting renal diseases. Of greatest concern are analgesics and non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclo-oxygenases and therefore inhibit the formation of vasodilatory prostaglandins by papillary interstitial cells 75 . Renal papillary necrosis was reported also for tyrosine kinase inhibitors and serotonin (5-HT1A) receptor agonists 76 . Recently, renal papillary antigen (RPA-1) was proposed as biomarker to detect early renal papillary lesions in rats 77 . RPA-1 is an antibody to an unknown epitope of an antigen in the rat renal papilla, specifically of the collecting ducts. The human equivalent of RPA-1 is not yet known 77 . Overall renal papillary necrosis in laboratory animals treated with a drug candidate is of clinical concern and needs careful monitoring of the renal function in clinical studies.
Drugs interfering with the renal renin-angiotensin regulation, such as inhibitors of the angiotensin-converting enzyme or angiotensin II antagonists, can affect the kidney in various ways including hypertrophy and hyperplasia of the juxtaglomerular apparatus. This finding is regarded as exaggerated pharmacological effect 78 – 80 and has also been described in humans. A number of agents, including drug candidates, are known to induce α2μ globulin nephropathy in male rats. α2μ globulin is not found in significant amounts or is completely absent in female rats, mice, guinea pigs, dogs, monkeys, and humans. Therefore, α2μ globulin nephropathy is a species- and sex-specific finding without relevance to humans 81 .
Although liver and kidneys are common target organs, toxicity rarely leads to termination of drug development in the preclinical phase. Generally both toxicities can be monitored in man with sensitive enzyme assays and potentially toxic drug candidates can proceed with the necessary precautions to clinical trials. Recently the Predictive Safety Testing Consortium has examined the issue of renal biomarkers in urine 82 . FDA and EMA (former EMEA) agreed that the following biomarkers qualify and are useful for detecting glomerular and/or tubular kidney toxicity in animals and partly also in man: KIM 1 (kidney injury molecule-1), albumin, CLU (clusterin), TFF3 (trefoil factor 3), total protein, cystatin C, and β2-microglobulin 82 .
The liver is the most common target organ in rodent bioassays 83 . Liver tumors are often due to an epigenetic MoA, that is increased DNA replication. Liver tumors are often not relevant to man, but sustained liver toxicity and regenerative proliferation is relevant to the evaluation of human cancer risk 84 . Increased cell proliferation (e.g. labeling index for DNA replication) and potentially associated preneoplastic changes (e.g. liver weight increase, hepatocellular necrosis or hypertrophy) can generally be detected in subacute toxicity studies, e.g. in 13 week studies 85 .
Gastro-intestinal tract
The tegaserod case : Tegaserod, a 5HT4-receptor partial agonist for the indication of irritable bowl syndrome, was associated in the high dose group (600 mg/kg bw/day p.o.) of the mouse lifetime bioassay with mucosal hyperplasia and an increased incidence of adenocarcinomas in the small intestine of 8 animals (7 in jejunum, 1 in ileum, affected sex not stated) 86 . The high dose corresponded to approximately 100 times the expected human exposure. There was no evidence of carcinogenicity at lower doses of up to approximately 35 times human exposure. Mucosal hyperplasia was also found in a subacute mice study after 2 weeks of 400 mg/kg bw/day p.o. Despite the relatively high safety ratio, the lesion was investigated in detail, among other reasons because the irritable bowl syndrome is not life-threatening, though a highly unpleasant disease to suffer from.
The hypothesis that inhibition of the diamine oxidase explained the MoA was not supported by the available data. An involvement of 5-HT receptors could be excluded by further experimental studies. However, toxicogenomic investigations provided evidence that high doses of tegaserod caused cellular stress most likely by increased gut peristaltic movements. Such stress may have triggered a proliferative response in the intestinal mucosa, resulting in mucosal hyperplasia following subacute treatment and tumors after lifetime exposure. The additional data also suggested that the hyperplastic response was due to local tegaserod exposure. At the NOEL (150 mg/kg bw/day p.o. in mice) systemic exposure (AUC) was 18-times higher than clinical exposure in man and local gut exposure several 100-times higher. These exposure differences between animals and humans were considered sufficient by the European Medicines Agency (EMA) to conclude that the APF is of no concern for the intended clinical use 86 . The drug was on the market, but was then withdrawn in a number of countries: In clinical studies involving over 18,600 patients a very small, but statistically significant increase in the incidence of cardiovascular ischemic events (13 out of 11,614 patients with tagaserod; 1 out of 7,031 with placebo) was seen. The withdrawal was requested by some health authorities, although, according to press statements, apparently most patients with CV adverse events had at least one CV risk factor or a pre-existing cardiovascular disease.
An unusual finding with a VEGF-receptor inhibitor : The drug, a vascular endothelial growth factor (VEGF) receptor inhibitor and inhibitor of angiogenesis in development as anticancer drug, was without special findings in preclinical studies up to 4 weeks duration. During necropsies of the 26-week rat toxicity study the duodenal diameter was found increased in the high dose group (100 mg/kg bw/day p.o.). The histological evaluation of this study revealed epithelial hyperplasia and infiltration of mucosal glands into the lamina muscularis and partly into the peritoneum and gastric wall at the high dose. The lesion regressed, but did not disappear following a 13 week recovery period. Because of its invasive behavior, the lesion was first diagnosed as being malignant and clinical studies were put on hold 87 . Figure 5 shows the typical appearance with ectactic and partly hyperplastic glands infiltrating the deeper layers of the duodenal wall.
Fig. 5.
Adenosis in the duodenum of a Wistar rat after a 26 week oral treatment with 100 mg/kg bw/day of a VEGF-receptor inhibitor. H&E, lens 10×.
External preclinical experts had not seen a similar lesion before, but considered it not to be malignant, also because it regressed to some degree following termination of exposure. It was proposed to label the lesion as adenosis with reversible hyperplasia. Physiological differences of the gastro-intestinal tract between rats and other species may be responsible for the observed susceptibility of the rat: The gastro-intestinal tract of rats continues to grow including fissioning of crypts also after birth, a behavior not present in mice, dogs or humans under non-pathological conditions. In fact, it could be shown that the lesion occurs only in rats, but not in mice or dogs, and can therefore be considered to be species-specific. However, it was not possible to establish the exact MoA of the drug candidate. It also remained unclear if the observed proliferation was a primary effect or a secondary reactive event. The hypothesis is that the drug acts on mesenchymal components 88 , facilitating the downgrowth of epithelial cells into lower layers of the duodenal wall. In view of the indication in cancer treatment, clinical trials, accompanied by careful monitoring of patients, were considered to be ethically acceptable. Additional preclinical studies to further characterize the lesion were done, but the results are not available to the public. The drug candidate is still in clinical development and well tolerated in humans.
Phospholipidosis
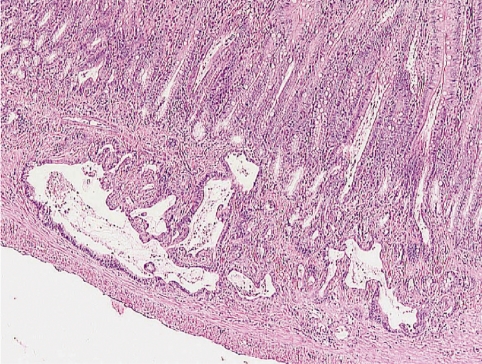
Drug-induced phospholipidosis (PLD) is a storage disease characterized by lysosomal accumulation of polar lipids, visible at microscopic level as intracellular inclusion bodies and in the EM as concentrically lamellar bodies 89 , 90 . Typically it is a consequence of treatment with cationic amphiphilic drugs and seen in cells with high membrane turnover, such as macrophages, or in tissues with high lipid or phospholipid biosynthesis such as adrenals, retina or lung. Figure 6 shows an example of phospholipidosis following 16 daily doses of chlorphentermine, an appetite suppressor drug now withdrawn from the market for other reasons.
Fig. 6.
Phospholipidosis with foam cells in lungs of a SIV 50 rat following 16 daily oral doses of 40 mg/kg bw/day of chlorphentermine. H&E, lens 25×.
Occurrence and severity vary between species, strain, and age of laboratory animals. Often a number of organs is affected. It can take just a few doses or many months of treatment to induce PLD. PLD, if not associated with secondary reactions, generally resolves, but this may take considerable time. PLD must be distinguished from lipidoses 91 . Despite extensive PLD, laboratory animals generally seem to be well. However, effects on cell function are possible 92 , 93 and include impairment of cell metabolism or of pino/endocytosis. Some drugs such as propranolol and verapamil were reported to lead to lactate dehydrogenase (LDH) release from hepatocytes, while other drugs, such as chloroquine and amiodarone, may be associated with myopathy.
Some authors 94 distinguish between different types of PLD. Macrophage-dominant PLD is characterized by foamy, enlarged macrophages in lungs, particularly in the subpleural area, and in lymphnodes, but also in liver, spleen, thymus and/or bone marrow. In the parenchymal cell-dominant PLD phospholipids accumulate in hepatocytes, renal and bile duct epithelia, endocrine cells, striated, and smooth muscle cells, endothelial cells and/or nerve cells. Localized PLD is a special type of parenchymal form and probably reflects exposure of the affected organ to a higher concentration of the drug. Possible mechanisms of phospholipid accumulation are inhibition of lysosomal phospholipase activity, altered phospholipid biosynthesis and/or impaired delivery of phospholipid degrading enzymes to lysosomes.
The occurrence of PLD in preclinical studies is always troublesome, though it is not the end for a drug candidate. PLD inducers are often effective drugs, as their lipophilicity facilitates permeability into various tissues. Screening for PLD potential is possible e.g. based on the physicochemical properties of the drug candidate, quantitative structural activity relationship (QSAR) modeling, and using in vitro screens with flow cytometry or in vivo studies followed by EM 95 . More recently, also toxicogenomic investigations were used as screening tool 96 . Risk evaluation of PLD inducing drug candidates needs to take into account all relevant factors from preclinical studies including degree of PLD, progression, reversibility, functional effects, and site of accumulation. PLD in non-regenerative tissues such as the nervous system increases concern. Other aspects to be considered during the WoE analysis are the number of species affected, the availability of biomarkers, and of course safety ratios. However, as PLD in laboratory animals may not be predictive for man, further development is not excluded even in case of a potentially insufficient safety ratio, depending mainly upon the indication of the drug candidate. More caution is recommended in case of indications needing treatment over longer periods of time. Monitoring for PLD in clinical studies is possible e.g. by examining peripheral white blood cells, especially lymphocytes, for lysosomal lamellar bodies by EM or flow cytometry. However, the relationship between tissue burden by PLD and appearance of lysosomal lamellar bodies in lymphocytes is not well known. Also Nile Red coloration can be used to show lymphocyte lipid inclusions. Urinary bis-monoglycerol phosphate (BMD) was reported to correlate with PLD, at least in rats 97 . For a recent overview of possible strategies to develop drug candidates with a potential for inducing PLD see also 95 .
Partly subtle lesions
It is easy to miss subtle lesions in early preclinical safety studies. If picked up, the question always is: Is it just a stone in the desert (incidental finding) or is it the tip of a pyramid buried under sand (significant problem)? MEK inhibitors are developed as anticancer drugs. The name MEK is derived from a combination of MAPK/ERK kinase, where MAPK (MAPK1) stands for mitogen-activated protein kinase 1 and ERK (ERK2) for extracellular signal-regulated kinase 2. MEK inhibitors are now known to be associated with multifocal mineralization in different tissues 98 , in particular also in the gastric mucosa of rats, already 15 days after a single dose of 500 μmol/kg99. The mineralizations are partly preceded by increased plasma levels of 1,25-dihydroxyvitamin D and hyperphosphatemia. MEK is involved in the vitamin D-induced transcriptional activation of the cytochrome P450C24 (CYP-24) promoter. The induced CYP-24 is responsible for degradation of active vitamin D3 metabolites. If MEK is inhibited, vitamin D is no longer inactivated 100 . Vitamin D metabolism differs between humans and rats 101 . Furthermore only minor mineralization is seen in dogs and monkeys. Therefore rodents may not be predictive for this type of adverse effect in man: So far, clinical administration of MEK inhibitors was not found to be associated with metastatic mineralization. However, measurement of plasma calcium, phosphorus, and other electrolytes is recommended at least during early clinical trials and during trials of longer duration.
Metastatic mineralization can also be caused by a MoA associated with hypercalcemia, e.g. increased secretion of parathyroid hormone in case of parathyroid tumors leading to bone resorption. Similarly, destruction of bone by primary tumors such as multiple myeloma or by extended skeletal metastasis, vitamin D intoxication or renal failure with secondary hyperparathyroidism through retention of phosphate can lead to mineralized foci.
Hypertrophic osteopathy
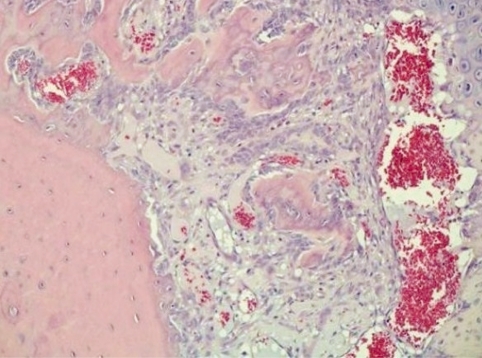
The following bone lesion was not interpretable at the time it was detected. As follow-up drug candidates were available, the involved drug candidate, an isoquinoline derivative with bronchodilating anti-inflammatory activity through inhibition of PDE IV, was not further developed. The lesion was first seen in a reproductive study, where the male rats were treated for up to 15 weeks and the females for up to 7 weeks with SDZ MNS 949 (6,7-dimethoxy-3-methyl-1-[3',5'-bis (methoxyethoxy) phenyl-isoquinoline). At the high dose of 130 mg/kg bw/day in feed, swollen legs were seen in around 40% of rats of both sexes. Histologically males showed hyperostosis in the distal part of the tibias and/or a bony shell formation arranged in a peripheral circle, partly interspersed with fat marrow. About half of the females had the same changes, while the other half showed a high grade of chondrogenesis with an inflammatory component as illustrated in Fig. 7. Some animals also had hyperostosis of the tail root vertebrae.
Fig. 7.
Hypertrophic osteopathy in a female Wistar rat treated orally for 47 days with 130 mg/kg bw/day of the PDE IV inhibitor SDZ MNS 949. H&E, lens 10×
These lesions were further characterized in an additional 26 week study 102 . Hypertrophic osteopathies are known to occur in various species, generally as a consequence of other diseases including intrathoracic neoplasias. Release of vasodilating endogenous substances is likely to be involved in the pathogenesis. Vasodilation could also have played a major role in the lesions described here. It was assumed that the process begins with an inflammation initiating chondrogenesis, which in turn leads to hyperostosis with shell formation.
PPAR agonists
APFs with PPAR agonists are a much debated issue these days 103 – 105 . PPAR stands for peroxisome proliferator- activated receptors, which are nuclear hormone receptors acting as ligand-activated transcription factors. Commonly three subtypes are distinguished: α (alpha), δ (delta, sometimes also called β beta) and γ (gamma). α receptors are mainly involved in fatty acid oxidation in livers and muscles during the fasting response. δ receptors are important for fatty acid oxidation and energy uncoupling in fat tissue and muscles. γ receptors play a role in lipogenesis and lipid storage in livers and fat tissues as well as in the regulation of insulin sensitivity of the muscles 106 , 107 . PPAR α agonists such as fibrates improve dyslipidemia, while PPAR γ agonists such as thiazolidinediones improve insulin resistance and therefore diabetes mellitus. The complementary action of PPAR α and γ agonists is beneficial for patients suffering from metabolic syndrome and renders the development of dual PPAR α/γ agonists attractive in spite of safety concerns 108 .
Besides drugs, other compounds such as phthalate ester plasticizers, pesticides, and industrial solvents are known to increase size and number of peroxisomes in laboratory animals 109 . Peroxisome proliferation under exposure to these compounds is generally most marked in rats and mice, less in hamsters, and only slightly, if at all, in guinea pigs, monkeys, and humans. Less than half of the over 100 known peroxisome proliferating compounds were tested in rodent bioassays and shown to be carcinogenic primarily in livers by activation of the α receptor. It is unlikely that older compounds such as clofibrate 110 are hepatocarcinogens at expected levels of exposure of humans.
However, PPAR α is also present in human cells, though at much lower concentrations, and the data do not permit to exclude that the animal MoA is plausible in humans 111 . Events in rodent livers are activation of PPAR α, peroxisome proliferation with increased acyl-CoA oxidase, and microsomal fatty acid oxidation resulting in excessive production of hydrogen peroxide, Kupffer-cell-mediated events, interference with cell proliferation, and apoptosis of hepatocytes as well as selective clonal hepatocyte expansion. PPAR α agonists may also affect other organs such as the testis 111 : The MoA in rat LC also involves PPAR α activation in the liver, resulting in changes of the metabolism of LC relevant hormones and their precursors. Testicular testosterone biosynthesis may also be directly inhibited. Similarly, proliferative changes in rat pancreatic acinar cells start with PPAR α activation in the liver and are associated with changes in the bile synthesis and composition. Today’s PPAR α agonists in development are 10–1000 fold more potent human PPAR α agonists than traditional fibrates, and humans are often significantly more sensitive to their pharmacological action than rodents 112 . These new facts change the toxicological assessment of modern PPARs fundamentally.
PPAR agonists submitted to date are not genotoxic in the standard ICH genotoxicity battery, but are carcinogenic in various rodent species and strains, in both sexes, and various organs. In particular the following tumors can be observed with PPAR γ and α/γ dual agonists: Hemangiosarcomas in mice, urothelial bladder tumors particularly in male rats, adipose tissue tumors in rats, liver tumors particularly in female mice, cervix and gall bladder tumors in mice and stomach tumors in male rats 106 . Therefore PPAR agonists are classified as “probable human carcinogens” according to the criteria of the Environmental Protection Agency (EPA) and the International Agency for Research on Cancer (IARC). Further adverse effects seen in humans and partly also in laboratory animals are signs of myopathy and rhabdomyolysis, weight gain, fluid retention, peripheral edema, and potentially increased risk of cardiac failure 108 . Nevertheless, PPAR agonists are promising drugs. Risk evaluation must be done for each adverse effect separately and case-by-case.
For starting clinical trials in man FDA’s recommendations are consistent with those of other regulatory authorities and include 113 : For clinical trials exceeding 6 months duration preliminary results from rodent bioassays are needed. Transgenic mouse models are not accepted. If animal tumors are observed, mechanistic data are welcome. If rodent tumors occur only at exposures above the 10-fold therapeutic exposures at the maximal recommended human dose (MRHD), phase 3 studies over 6 months are approved, provided that the receptor transactivation potency in animals and humans is comparable. If rodent tumors occur at lower exposure or if for toxicity reasons doses leading to exposure levels in excess of the 10 times human exposure levels at the MRHD are not possible, the review will occur on a case-by-case basis 113 .
The following example serves as illustration for the risk assessment process of a PPAR agonist, namely the dual PPAR α/γ agonist muraglitazar 114 . Two-year studies were conducted in mice with doses of 1, 5, 20, and 40 mg/kg bw/day and in rats with doses of 1, 5, 30, and 50 mg/kg bw/day. Gallbladder mucosal hyperplasia and some benign gallbladder adenomas were observed in male mice at the two higher doses corresponding to over 60 times the area-under-the-curve (AUC) at human exposure with daily doses of 5 mg. The incidence of transitional cell papilloma and carcinoma of the urinary bladder in male rats was increased in a dose-related fashion starting at 5 mg/kg bw/day corresponding to approximately 8 times the human therapeutic AUC and was mediated by urolithiasis. Incidences of subcutaneous liposarcoma in male rats and subcutaneous lipoma in female rats were increased at the high dose only corresponding to approximately 50 times the human therapeutic exposure at 5 mg/day. These mesenchymal tumors were attributed to persistent pharmacologic stimulation of preadipocytes, leading also to hyperplastic and metaplastic adipocytes in mice and rats. Relevant non-neoplastic changes consisted of thinning of cortical bone in mice. As muraglitazar is not genotoxic, the authors concluded that the observed tumorigenic effects in mice and rats have no clinical relevance, since they occurred at either clinically not relevant exposures (gallbladder and adipose tumors) or by a species-specific MoA (urinary bladder tumors).
In 2005, the Health and Environmental Sciences Institute (HESI) PPAR Agonist Project Committee was established by pharmaceutical companies in an effort to better understand the MoA and human relevance of rodent tumors induced by PPAR agonists 115 . The working group concluded that the most likely MoA for vascular tumors including the mesenchymal component in mice and hamsters, with mice being also predisposed to spontaneous development of hemangiosarcoma, is stimulation of adipogenesis with subsequent release of cytokines and growth factors leading to mesenchymal and/or endothelial cell proliferation and neoplasia 116 . This hypothesis is based on the following observations:
-
●
Adipocytes are an important source of cytokines and growth factors
-
●
Adipogenesis and angiogenesis are linked
-
●
PPAR γ agonists induce hemangiosarcomas primarily in adipose tissue
While some of the above factors and events may also play a role for the induction of sarcomas in rats, a specific MoA has so far not been proven for this species. Stimulation of DNA synthesis in subcutaneous adipose tissues of rats treated with the dual PPAR α/γ agonist tesaglitazar is not mediated via activation of PPAR receptors in these cells, but may involve proliferation of undifferentiated mesenchymal cells in subcutaneous tissues 117 .
Another prominent feature of PPAR γ and dual α/γ agonists in rodent carcinogenicity studies is the occurrence of urinary bladder carcinomas 118 . Urolithiasis was identified as the inciting event in the MoA due to urinary changes 119 . Demonstration of urinary solids following PPAR agonists treatment has not always been possible, but this is likely due to methodological issues 120 . Abnormal crystalluria or calculus formation does not occur in humans in response to PPAR agonists. Lesions were also reported in the monkey urothelium, but the pathology working group of the HESI PPAR Agonist Project Committee ascertained that the suspect findings were normal in the monkey urothelium and epithelial hyperplasia was absent 121 .
PPAR α agonists are also known to induce muscle lesions, both in animals and in humans 122 . In animals edema and striated skeletal muscle fiber lesions are found. In humans fibrate treatment can be associated with myalgias and more rarely rhabdomyolysis, notably in combination treatment with statins 123 . Such adverse findings are more likely with gemfibrozil than e.g. with fenofibrate and can be monitored using CK and AST levels. The MoA is unclear, but may also involve effects on kidneys with increased blood volume and aldosterone 48 . Of particular concern are cardiac effects which can be monitored in man using troponin levels. To cover the latter issue, the FDA has published a draft guidance in 2008 for development of diabetes drugs 124 . Muscle lesions must be considered to be relevant to man and sufficient safety ratios are needed to proceed with development of drug candidate in man.
In conclusion, PPAR α and dual α/γ ligands are in widespread clinical use for the treatment of dyslipidemia and insulin resistance and in future may also be used as anti-inflammatory drugs. When involved in the development of PPAR agonists, one has to be prepared to solve a number of issues. The carcinogenic alert increases with an increasing number of positive parameters as listed below:
-
●
The increase in tumor incidence is dose-related and well above the control range
-
●
Tumors occur at much younger age than in the controls
-
●
There is a shift to less differentiated tumors, possibly with impact on survival
-
●
Tumors are associated with an early increase in preneoplastic lesions
-
●
Insufficient safety ratio, e.g. below 10, but this limit needs to be decided on a case-by-case basis
Genotoxicity
For reasons of simplicity genotoxicity and mutagenicity are subsequently summarized under the heading of genotoxicity, as this term also covers mutagenicity. The area is discussed only to the extent the informed general toxicologist and toxicologic pathologist should be familiar with. If significant issues arise in this area, it is advisable to consult with a specialized colleague. In case of a genotoxicity or carcinogenicity issue it is good to remember that one third of the drugs listed in Physicians’ Desk Reference PDR 18 have positive or equivocal genotoxicity or carcinogenicity results (for details see below). Two-thirds of compounds positive for genotoxicity, particularly those positive in in vitro mammalian cell assays only, act in rodent bioassays by epigenetic mechanisms.
In their update on genotoxicity and carcinogenicity testing of marketed pharmaceuticals Brambilla and Martelli recently reported the follwing findings 125 : Of 838 drugs used for continuous therapy of at least 6 months or intermittently over extended periods, 366 (43.7%) did not have retrievable genotoxicity or carcinogenicity data. For the remaining 472 (56.3%) at least one genotoxicity or carcinogenicity test result was available, but only 208 drugs (24.8%) had all data required by current guidelines. Of 449 drugs with at least one genotoxicity test, 183 (40.8%) had at least one positive finding. Of the 338 drugs with at least one carcinogenicity test, 160 (47.3%) showed at least one positive result. Of 315 drugs with both genotoxicity and carcinogenicity data, 116 (36.8%) are neither genotoxic nor carcinogenic, 50 (15.9%) are not carcinogenic but positive in at least one genotoxicity assay, 75 (23.8%) are carcinogenic in at least one sex of mice or rats but negative in genotoxicity assays, while 74 (23.5%) are both genotoxic and carcinogenic.
Similar findings were published in 2001 for 467 marketed drugs, which were partly for short-term use only 126 . Anticancer drugs, nucleosides, steroids, biologicals, and peptide-based drugs were excluded from the analysis, because they are genotoxic by their MoA (anticancer and steroid drugs), and/or interfere with bacterial tests (nucleosides, biologics including peptide-like drugs), and are therefore generally not tested for genotoxicity. Where test results were available, bacterial mutagenesis assays were positive in 8.3%, in vitro cytogenetic tests in 24.8%, the mouse lymphoma assay (MLA) in 25%, and in vivo cytogenetic tests in 11.5%. Among the various tests the sister chromatid exchange assay (SCE) had the largest percentage of positives (43.5%) and mammalian mutagenesis assays (excluding MLA) the lowest (2.2%). Obviously, the predictive value of genotoxicity findings for two year bioassay outcomes is limited, since carcinogenicity can occur also via non-genotoxic mechanisms. The authors concluded that no combination of genotoxicity assays was superior in predicting rodent carcinogenesis bioassay results than the bacterial mutagenicity test alone. Similar results were also published later 127 . Further analyses of published genotoxicity and carcinogenicity data are also available for analgesics, anti-inflammatory drugs, and antipyretics 128 as well as for other classes of compounds.
Entacapone, a COMT inhibitor used as adjunct to levodopa and dopa decarboxylase (DDC) inhibitors for treatment of Parkinson’s disease, is an example of a drug on the market with both in vitro genotoxicity and in vivo rat carcinogenicity 129 . The MLA was found to be positive at concentrations of 25–50 μg/ml. The most likely reason is chromosome damage as also suggested by the chromosomal aberration test in human lymphocytes: It was positive with S-9 mix at concentrations of 100 μg/ml and showed also increased numbers of aberrant cells at 400 μg/ml. Ames tests, the in vitro DNA binding study, the in vivo micronucleus test and the rat liver UDS test were negative. Entacapone caused also renal adenomas and carcinomas in males at the high dose (400 mg/kg) in the two year rat study, while the mouse study did not allow adequate conclusions due to a high incidence of premature mortality at the high dose. Chromosome damage in vitro only was not regarded as impediment to market the drug. In addition, the renal neoplastic findings appeared to be due to nephrotoxicity seen in a one year toxicity study in male rats only, at plasma levels 20 times higher than those seen in man. The carcinogenic potential of entacapone administered in combination with carbidopa-levodopa has not been evaluated.
Positive genotoxicity results must be scrutinized. Reasons for false positive Ames tests can be an over-induction of phase 1 metabolism with increased activation of drug candidates to genotoxic metabolites, or a lack of or insufficient induction of phase 2 metabolism responsible for deactivation of genotoxic drug metabolites. Dose-dependent and reproducible in vitro but negative in vivo tests can indicate that in vivo no genotoxic products are formed, or that such genotoxic products are inactivated or do not reach the target cell. A positive Ames test and a positive chromosome aberration test in human lymphocytes indicate a critical, but not hopeless situation, particularly if the chromosome aberration test is only positive at toxic concentrations, which are known to induce apoptosis and release of endonucleases leading to chromosome aberrations and micronuclei in vitro.
No further testing for genotoxicity is generally needed if a statistically significant positive response is still within the historical negative control range or the positive response is observed only at the high dose and only under one experimental condition, but is not reproducible under equivalent or similar experimental conditions for the same endpoint. In vitro mammalian cell assays are frequently positive, particularly under non-physiological culture conditions such as high osmolarity or low pH. A positive micronucleus test is relevant if the positivity is due to a clastogenic effect. However, if the positive result is attributable to aneuploidy, the genotoxic risk is considered to be negligible. This is also true for clastogenic effects due to an interaction of the xenobiotic with proteins, such as topoisomerase, because such interactions have a threshold. If there is insufficient WoE to classify a positive result of an in vitro mammalian cell assay as not relevant to man, additional genotoxicity studies are indicated. Such studies can be done either in vitro to provide mechanistic information and/or in vivo generally on different tissues.
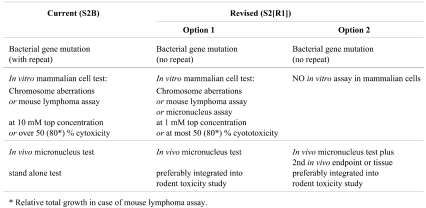
The ICH step 2 2008 draft consensus guideline “Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use S2(R1)” 130 is to replace the older S2A and S2B guidelines mainly for the following reasons:
-
●
High rate of irrelevant positive findings in vitro mammalian cell tests
-
●
Integration of newer test methods, in particular the in vitro micronucleus test, in vivo models in a variety of tissues, e.g. by using the single cell gel electrophoresis (Comet) assay or transgenic animals, and the use of rat blood for micronucleus evaluation
-
●
Efforts for further improvement of animal welfare
Table 3 summarizes the new genotoxicity test strategy proposed in the 2008 draft consensus guideline.
Table 3. ICH Guidance on Genotoxicity Testing 130 .
Further genotoxicity testing in case of positive or equivocal findings in standard genotoxicity tests may also include
-
●
Genotoxicity markers from longer-term studies
-
°
Micronucleated normochromatic erythrocytes from mouse blood
-
°
Metaphase analysis of cultured blood lymphocytes from rat or monkey
-
°
-
●
Analysis of DNA adducts
-
●
Comet assay
-
●
Transgenic mouse mutation assay
-
●
Short-term carcinogenicity study e.g. using p53 +/– transgenic (knockout) mice, particularly if there is residual concern that genotoxic activity may contribute to tumorigenesis. This test is sometimes requested by certain regulatory authorities prior to repeat-dose clinical trials 131 , but it is not universally accepted.
A potential alternative is the rasH2 model, considered to be appropriate for testing of genotoxic and non-genotoxic compounds and accepted as alternative to two-year mouse carcinogenicity studies by the U.S. Food and Drug Administration, the Japanese Ministry of Health, Labor, and Welfare, and the EMA for regulatory submission 132 – 137 . “Short-term” 26 week bioassays with transgenic mice may permit a more timely assessment of the carcinogenic potential of drug candidates before starting long-term clinical trials 138 .
A further test option—though at present not well accepted, at least not in Japan—is examining peripheral blood lymphocytes for micronuclei from humans in clinical trials 139 . Peripheral blood lymphocytes do not divide and have a low capacity for DNA repair. As DNA damage is not lethal in resting lymphocytes, the damage accumulates and is expressed e.g. as micronuclei following stimulation to divide in vitro.
Moreover, cell transformation assays, e.g. on Syrian hamster embryo cells, are sometimes mentioned as test options and may be requested by certain regulatory authorities 131 . The test may be used to generate additional data, but the molecular basis of cell transformation is not well understood. In particular, the relationship between genotoxicity and transformation remains unclear. Therefore, the results of cell transformation assays may be difficult to interpret.
Hazard characterization based on genotoxicity results must include among others the following aspects: Type and number of tests with positive results, reproducibility, dose-response relationship, consideration of cytotoxicity for a positive outcome, magnitude of the positive result. Particularly, if a repeat test with a new batch turns out positive, the possibility of a contamination with by-products must be considered. For the final risk evaluation of genotoxicity and carcinogenicity, the following factors are of particular importance 131 :
-
●
Experimental data generated during genotoxicity, general toxicity, and rodent bioassay studies, ADME parameters as well as pharmacology data of the drug in question
-
●
Drug indication: Hard vs. soft indications, as higher treatment-related risks are more acceptable for severe life-saving indications than for relatively harmless sicknesses
-
●
Target population, in particular the age of the targeted patient population
-
●
Duration of treatment: Single, multiple or long-term. In general, single-dose clinical studies are permitted regardless of the genotoxicity results (see also next paragraph)
-
●
Importance of the drug: Are there alternatives and what are the additional benefits offered by the drug in question
It may not be possible to eliminate all impurities in a drug substance 140 . A staged threshold of toxicological concern (TTC) analysis is then a pragmatic approach to balance duration of clinical trials, availability of analytical methods, maturity of synthetic schemes, and the potential risk to humans. Toxicological concerns can be addressed e.g. by controlling daily intake. The acceptable daily intake of genotoxic substances varies between approximately 1.5 μm/day for long-term (“lifetime”) intake and approximately 60 μm/day for treatments of up to 1 month. Based on scientific reasoning, these virtually safe intake values do not pose an unacceptable risk to either human volunteers or patients at any stage of clinical development and marketing of a pharmaceutical product 141 . The limit of 1.5 μm/day for lifetime intake is also mentioned in the EMA “Guideline on the limits of genotoxic impurities” 142 and in the FDA draft guideline “Guidance for industry. Genotoxic and carcinogenic impurities in drug substances and products: Recommended approaches” 143 .
Experimental safety data from animals are superseded by clinical data obtained in man. However, clinical data on genotoxicity and carcinogenicity are difficult or impossible to obtain and weak effects are likely to go unnoticed. Genotoxicity and carcinogenicity effects are considered to be essentially irreversible. Therefore, preclinical data on genotoxicity and carcinogenicity retain their value even for drugs on the market. Only long-term well-controlled epidemiological studies on large cohorts of patients would have more weight than preclinical data, but such studies are potentially unethical, difficult, and resource-intensive to conduct and are therefore generally not available. This needs to be taken into account during the risk evaluation process.
Reactions to positive genotoxicity data may differ between different regulatory divisions and agencies. For clinical trials, particularly in hard indications, that is in severe, potentially life-saving indications, genotoxicity may be acceptable, but the risk for healthy volunteers must be minimal, as explained above. If questionable genotoxicity is observed, development of the drug candidate generally proceeds, though, if in the later lifetime bioassays questionable tumor findings are present, the situation may become difficult depending on the indication of the drug, the patient population, and available alternatives (but see also the entacapone case summarized above). In case of a strong genotoxicity signal in the absence of good evidence that the result is not relevant to man, it is wise to stop development, unless the drug is for a hard indication such as cancer treatment. With few exceptions a “No go” situation arises, if genotoxicity is observed in vitro and in one in vivo test, and (potentially) treatment-related tumors occur in rodent bioassays which may be relevant to man. The situation is even more serious if precursor lesions are already present in shorter-term studies. However, one should not forget that the widely used over-the-counter drug paracetamol tests positive in the chromosome aberration test in lymphocytes and mouse bone marrow cells and leads to DNA adducts, while Ames and V79 micronucleus tests are negative 144 . In addition, at high doses paracetamol induces liver and bladder tumors in rodents. However, these doses cause hepatotoxi-city, which is a plausible explanation for the liver tumors, and the urinary bladder tumors are likely to be due to urolithiasis 145 . Available data suggest three possible MoA for paracetamol-induced genotoxicity: (1) Inhibition of ribonucleotide reductase; (2) increase in cytosolic and intranuclear Ca2+ levels; (3) DNA damage caused by N-acetyl-p-benzo-quinone imine (NAPQI) after glutathione depletion 145 . All MoA have thresholds, and genotoxic effects of paracetamol appear only at dosages toxic to the bone marrow and not achieved at therapeutic dosages.
Reproductive toxicity
As said above regarding genotoxicity, also reproductive toxicity is discussed here only to the extent to which the informed general toxicologist and toxicologic pathologist should be familiar with. If significant reproductive toxicity issues arise, a specialized colleague should be involved. Reproductive toxicity studies serve to test for adverse effects on the following parameters 146 , 147 .
-
●
Fertility of both sexes, estrus cycle and tubal transport in females, implantation and development of pre-implantation stages of the embryo, as well as functional effects on mating behavior in both sexes and epididymal sperm maturation in males
-
●
Pregnancy and development of the embryo and fetus to detect embryotoxicity and/or teratogenicity, which may be expressed as increased occurrence of resorptions, abortions, stillbirth, malformations, gender distribution of the pups, and effects on fetal weight
-
●
Pregnancy, parturition, lactation, pre-natal embryo-fetal, and peri- and post-natal development of the offspring until sexual maturity
Alternatives to the conventional reproductive toxicity tests are still under evaluation 148 .
Reproductive toxicity is a highly sensitive issue. The assessment of adverse effects on fertility depends to some degree on the affected sex: Recovery of spermatogenic toxicity is possible as long as spermatogonia type A survive. However, there is no oocyte regeneration: Oocyte destruction permanently reduces the number of oocytes available. Absence of oocytes means permanent female infertility, similar to menopause initiated in humans by exhaustion of available oocytes. Adverse fertility effects of a drug candidate have consequences for the further development of that drug depending on targeted patient population, indication, and available alternatives. Collection of human data for the assessment of preclinical findings is possible to a limited extent in men by sperm analysis and in women by observation of the menstrual cycle.
Embryotoxicity and particularly teratogenicity are serious findings. If unequivocal embryotoxic or teratogenic effects are seen in animal species as e.g. with endothelin antagonists 149 , safety ratios are generally not helpful: It is rarely possible to establish the MoA and our understanding of these types of APF is generally too limited to allow extrapolation of such animal data to humans. Such findings always lead to a contraindication to the effect that women in child-bearing age can only be treated if not pregnant and if under reliable contraceptive treatment. In case of pregnancy, the potential benefits of treatment of the expecting mother must outweigh the potential risks to the fetus (see below). Whether the drug is viable depends on scientific judgment, marketing prospects, legal aspects because of possible damage claims and the company strategy.
Teratogenicity in animals is regarded as being predictive to man with the exception of cortisone-induced cleft palates in mice, a species known to be particularly sensitive to cortisone during pregnancy 150 , 151 . Questionable findings potentially indicating embryotoxicity and/or teratogenicity need to be assessed in more detail, e.g. by investigating if the observed effect is reproducible and/or in case of embryotoxicity reversible. A potential embroytoxic or teratogenic effect also needs to be distinguished from retardation of development as e.g. associated with maternal toxicity, as well as from artifacts or natural variation. For drugs that may have developmental toxicity in humans, a careful risk management and mitigation strategy needs to be implemented for clinical trials and later for marketing.
Particularly delicate, but not that rare, are potentially teratogenic findings in the high dose group, such as isolated malformations in some fetuses from single litters. A good historical database and absence of a broader teratogenic pattern of the observed malformations help in risk evaluation, which also needs to take other findings such as maternal observations into account. Data from the literature or other sources about the finding and possibly about the drug class have to be reviewed and experts to be involved as needed. The findings should be discussed with regulators. Additional studies may be warranted case-by-case to conclude the integrative assessment.
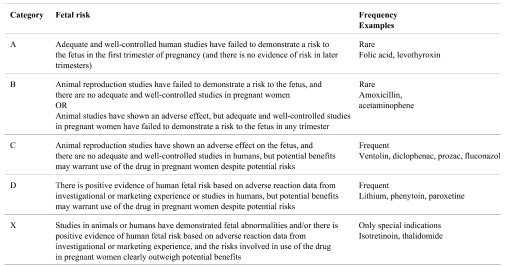
Regulators classify the fetal risk of drugs, but there are some differences in the criteria used by different countries. The Food and Drug Administration FDA categories and their criteria 152 are shown in Table 4. They can be found in detail in the Code of Federal Regulations Title 21, Part 201 - Labeling - Subpart B 153 .
Table 4. FDA Categories for Fetal Risk (Adapted from 153 ).
Another example for pregnancy categorization is e.g. the Australian categorization, which is partly more detailed 154 . There is no official categorization of drug risks to the fetus in Japan, but the information about fetal risk is described in the package insert.
Reliable human data are generally missing and therefore only few drugs can be assigned to the category with least concern for teratogenicity (category A of the FDA classification). When faced with a therapeutic decision for a woman at child-bearing age, the treating physician may often not find it easy to select the optimal drug based on the available classification. Therefore, FDA is currently proposing to amend this regulation and to require that labeling includes a summary of the risks of using a drug during pregnancy and lactation: A discussion of the data together with the relevant clinical information supporting that summary will be requested. The proposal would eliminate the current categories A, B, C, D, and X 155 . Various reference guides for and epidemiological studies about the use of drugs in pregnancy and lactation are published 156 – 160 .
Effects on pre-, peri-, and post-natal fetal development including parturition and lactation need to be investigated as to the possible underlying pathogenesis and reversibility. The MoA is often not known and, unless it is not relevant to humans for obvious reasons, knowledge of the MoA is also not very helpful in contrast to a sufficiently high safety ratio.
In summary: Embryotoxicity and teratogenicity do not result in termination of the development of a drug, but lead to modifications of its use, that is to restrictions such as exclusion of women in child-bearing age without contraceptive therapy and the exclusion of pregnant women, particularly during the first trimester, and limitation to hard indications for treatment of the aforementioned patient population. Adverse fertility effects and delayed, but reversible fetal development may be handled similarly to general toxicity findings.
Conclusions
Toxicologists and toxicologic pathologists need to be prepared to deal with APFs. Toxicity and carcinogenicity studies are designed more for sensitivity (avoiding false negative results, that is missing toxicity) than for specificity (avoiding false positive results). Unexpected APFs are frequent, if not the rule, when testing potent drugs. To resolve significant issues, experts should be asked to join a working group specifically constituted for addressing the APF issue in question. This working group should be an integral element of the drug development process to effectively support decision-making. Good management of the working group preferably by an experienced and competent company associate is crucial for assuring an objective-oriented process taking both scientific and business relevant aspects into account.
Additional tailor-made studies to further characterize an APF and to obtain insights into the MoA of the drug candidate must be carefully designed to obtain interpretable results. As shown in the first part of this review, the most important parameters in the overall WoE approach regarding relevance of APFs for man are
-
●
Safety ratios comparing animal exposure at the NOAEL and human therapeutic exposure, permitting a quantitative prediction of the relevance of the finding for man.
-
●
MoA helping to understand the qualitative relevance of the finding for man, where possible. If the MoA proves that an APF is not relevant to man, then no safety ratios are necessary.
The MoA of drug candidates with potential genotoxicity and teratogenicity can often not be established. Generally no clinical data will become available to supersede preclinical data. While a sufficient safety ratio may help in case of equivocal genotoxicity, this is not the case for potential teratogens, thus limiting the use of such drugs in women of childbearing potential or pregnant women.
Acknowledgments
We thank Dr. Jean-Claude Schaffner, Novartis Pharma AG, NIBR PCS, Pathology, Postfach, 4057 Basel, Switzerland, for Fig. 5. Many thanks to Regula Ettlin-Meier, 14 Mittelweg, CH-4142 Muenchenstein, Switzerland, for editorial assistance.
References
- 1.Dorato MA, Engelhardt JA. The no-observed-adverse-effect-level in drug safety evaluations: Use, issues, and definition(s) Regul Toxicol Pharmacol. 2005;42:265–274. doi: 10.1016/j.yrtph.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SM, Klaunig J, Meek ME, Hill RN, Pastoor T, Lehman-McKeeman L, Bucher J, Longfellow DG, Seed J, Dellarco V, Fenner-Crisp P, Patton D. Evaluating the human relevance of chemically induced animal tumors. Toxicol Sci. 2004;78:181–186. doi: 10.1093/toxsci/kfh073. [DOI] [PubMed] [Google Scholar]
- 3.Proceedings of the Eighteenth International Symposium of the Society of Toxicologic Pathologists, Washington DC, June 13–17, 1999. Toxicologic Pathology of the Nervous System. Toxicol Pathol. 2000;28:3–214. [Google Scholar]
- 4. Kaufmann W.Cerebellar neurotoxins I. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 5. Haworth R.Cerebellar neurotoxins II. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 6.Shirai N, Nii A, Horii I. Neuronal necrosis in a dog following exposure to an NMDA receptor antagonist. J Toxicol Pathol. 2008;21:185–188. [Google Scholar]
- 7.Olney JW, Labruyere J, de Gubareff T. Brain damage in mice from voluntary ingestion of glutamate and aspartate. Neurobehav Toxicol. 1980;2:125–129. [PubMed] [Google Scholar]
- 8.McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- 9.Park CH, Choi SH, Piao Y, Kim S, Lee YJ, Kim HS, Jeong SJ, Rah JC, Seo JH, Lee JH, Chang K, Jung YJ, Suh YH. Glutamate and aspartate impair memory retention and damage hypothalamic neurons in adult mice. Toxicol Lett. 2000;115:117–125. doi: 10.1016/s0378-4274(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 10.Auer RN. Hypoglycemic brain damage. Metab Brain Dis. 2004;19:169–175. doi: 10.1023/b:mebr.0000043967.78763.5b. [DOI] [PubMed] [Google Scholar]
- 11.Gazit V, Ben-Abraham R, Coleman R, Weizman A, Katz Y. Cysteine-induced hypoglycemic brain damage: An alternative mechanism to excitotoxicity. Amino Acids. 2004;26:163–168. doi: 10.1007/s00726-003-0045-5. [DOI] [PubMed] [Google Scholar]
- 12.Perez J, Perentes E. Light-induced retinopathy in the albino rat in long-term studies. An immunohistochemical and quantitative approach. Exp Toxicol Pathol. 1994;46:229–235. doi: 10.1016/S0940-2993(11)80088-6. [DOI] [PubMed] [Google Scholar]
- 13. Schraermeyer U, Heiduschka P.Retinal toxins. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 14.NTP toxicology and carcinogenesis studies of nalidixic acid (CAS No. 389-08-2) in F344/N rats and B6C3F1 mice (feed studies) Natl Toxicol Program Tech Rep Ser. 1989;368:1–195. [PubMed] [Google Scholar]
- 15.Theil PL. Ophthalmological examination of patients in long-term treatment with tranexamic acid. Acta Ophthalmol (Copenh) 1981;59:237–241. doi: 10.1111/j.1755-3768.1981.tb02985.x. [DOI] [PubMed] [Google Scholar]
- 16.Yam JC, Kwok AK. Ocular toxicity of hydroxychloroquine. Hong Kong Med J. 2006;12:294–304. [PubMed] [Google Scholar]
- 17.Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol. 2008;23:201–209. doi: 10.1080/08820530802049962. [DOI] [PubMed] [Google Scholar]
- 18.Physicians’ desk reference. Thomson PDR, 64th ed. Montvale. 2010. [Google Scholar]
- 19.Rosolen SG, Kolomiets B, Varela O, Picaud S. Retinal electrophysiology for toxicology studies: Applications and limits of ERG in animals and ex vivo recordings. Exp Toxicol Pathol. 2008;60:17–32. doi: 10.1016/j.etp.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Perlman I. Testing retinal toxicity of drugs in animal models using electrophysiological and morphological techniques. Doc Ophthalmol. 2009;118:3–28. doi: 10.1007/s10633-008-9153-6. [DOI] [PubMed] [Google Scholar]
- 21.Leblanc B, Jezequel S, Davies T, Hanton G, Taradach C. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regul Toxicol Pharmacol. 1998;28:124–132. doi: 10.1006/rtph.1998.1243. [DOI] [PubMed] [Google Scholar]
- 22.Capen CC. Pathogenic mechanisms of endocrine disease in domestic and laboratory animals. J Toxicol Pathol. 2002;15:1–6. [Google Scholar]
- 23. Ettlin RA, Junker U, Prentice DE.Dopamine agonists. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 24. Richardson BP, Turkalj I, and Flückiger E. Bromocriptine. In: Safety Testing of New Drugs : Laboratory Predictions and Clinical Performance. DR Laurence, AEM McLean, and M Weatherall (eds). Academic Press, London, Orlando. 19–63. 1984.
- 25.Qureshi SR, Perentes E, Ettlin RA, Kolopp M, Prentice DE, Frankfurter A. Morphologic and immunohistochemical characterization of Leydig cell tumor variants in Wistar rats. Toxicol Pathol. 1991;19:280–286. doi: 10.1177/019262339101900311. [DOI] [PubMed] [Google Scholar]
- 26.Prentice DE, Siegel RA, Donatsch P, Qureshi S, Ettlin RA. Mesulergine induced Leydig cell tumours, a syndrome involving the pituitary-testicular axis of the rat. Arch Toxicol Suppl. 1992;15:197–204. doi: 10.1007/978-3-642-77260-3_27. [DOI] [PubMed] [Google Scholar]
- 27.Dirami G, Teerds KJ, Cooke BA. Effect of a dopamine agonist on the development of Leydig cell hyperplasia in Sprague-Dawley rats. Toxicol Appl Pharmacol. 1996;141:169–177. doi: 10.1006/taap.1996.0273. [DOI] [PubMed] [Google Scholar]
- 28.Prentice DE, Meikle AW. A review of drug-induced Leydig cell hyperplasia and neoplasia in the rat and some comparisons with man. Hum Exp Toxicol. 1995;14:562–572. doi: 10.1177/096032719501400703. [DOI] [PubMed] [Google Scholar]
- 29.Clegg ED, Cook JC, Chapin RE, Foster PM, Daston GP. Leydig cell hyperplasia and adenoma formation: Mechanisms and relevance to humans. Reprod Toxicol. 1997;11:107–121. doi: 10.1016/s0890-6238(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 30.Brown WR, Fetter AD, Van Ryzin RJ, Langloss JM. Proliferative pituitary lesions in rats treated with salmon or porcine calcitonin. Toxicol Pathol. 1993;21:81–86. doi: 10.1177/019262339302100110. [DOI] [PubMed] [Google Scholar]
- 31.Jameson JL, Weiss J, Polak JM, Childs GV, Bloom SR, Steel JH, Capen CC, Prentice DE, Fetter AW, Langloss JM. Glycoprotein hormone alpha-subunit-producing pituitary adenomas in rats treated for one year with calcitonin. Am J Pathol. 1992;140:75–84. [PMC free article] [PubMed] [Google Scholar]
- 32.Osamura RY, Murakoshi M, Inada R, Horiuchi T, Watanabe K. Biological aspects of pituitary tumors induced by synthetic salmon calcitonin (TZ-CT) in Sprague-Dawley rats. Immunohistochemical and ultrastructural studies. Acta Pathol Jpn. 1992;42:401–407. doi: 10.1111/j.1440-1827.1992.tb03244.x. [DOI] [PubMed] [Google Scholar]
- 33.Hanton G. Preclinical cardiac safety assessment of drugs. Drugs R D. 2007;8:213–228. doi: 10.2165/00126839-200708040-00002. [DOI] [PubMed] [Google Scholar]
- 34.Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009;48:964–970. doi: 10.1080/02841860903229124. [DOI] [PubMed] [Google Scholar]
- 35.Forner F, Foster LJ, Campanaro S, Valle G, Mann M. Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Mol Cell Proteomics. 2006;5:608–619. doi: 10.1074/mcp.M500298-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Mor A, Mitnick HJ, Pillinger MH, Wortmann RL. Drug-induced myopathies. Bull NYU Hosp Jt Dis. 2009;67:358–369. [PubMed] [Google Scholar]
- 37.Dalakas MC. Toxic and drug-induced myopathies. J Neurol Neurosurg Psychiatry. 2009;80:832–838. doi: 10.1136/jnnp.2008.168294. [DOI] [PubMed] [Google Scholar]
- 38.Klopstock T. Drug-induced myopathies. Curr Opin Neurol. 2008;21:590–595. doi: 10.1097/WCO.0b013e32830e2774. [DOI] [PubMed] [Google Scholar]
- 39.Rabkin SW, Kong JY. Lovastatin-induced cardiac toxicity involves both oncotic and apoptotic cell death with the apoptotic component blunted by both caspase-2 and caspase-3 inhibitors. Toxicol Appl Pharmacol. 2003;193:346–355. doi: 10.1016/j.taap.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Jasinska M, Owczarek J, Orszulak-Michalak D. The influence of simvastatin at high dose and diltiazem on myocardium in rabbits, the biochemical study. Acta Pol Pharm. 2006;63:386–390. [PubMed] [Google Scholar]
- 41.Golomb E, Nyska A, Schwalb H. Occult cardiotoxicity— toxic effects on cardiac ischemic tolerance. Toxicol Pathol. 2009;37:572–593. doi: 10.1177/0192623309339503. [DOI] [PubMed] [Google Scholar]
- 42.Clemo FA, Evering WE, Snyder PW, Albassam MA. Differentiating spontaneous from drug-induced vascular injury in the dog. Toxicol Pathol. 2003;31(Suppl):25–31. doi: 10.1080/01926230390174904. [DOI] [PubMed] [Google Scholar]
- 43.Jokinen MP, Lieuallen WG, Johnson CL, Dunnick J, Nyska A. Characterization of spontaneous and chemically induced cardiac lesions in rodent model systems: The national toxicology program experience. Cardiovasc Toxicol. 2005;5:227–244. doi: 10.1385/ct:5:2:227. [DOI] [PubMed] [Google Scholar]
- 44.Sandusky GE, Means JR, Todd GC. Comparative cardiovascular toxicity in dogs given inotropic agents by continuous intravenous infusion. Toxicol Pathol. 1990;18:268–278. doi: 10.1177/019262339001800205. [DOI] [PubMed] [Google Scholar]
- 45.Dogterom P, Zbinden G, Reznik GK. Cardiotoxicity of vasodilators and positive inotropic/vasodilating drugs in dogs: An overview. Crit Rev Toxicol. 1992;22:203–241. doi: 10.3109/10408449209145324. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad K, Dorian P. Drug-induced QT prolongation and proarrhythmia: An inevitable link? Europace. 2007;9(Suppl 4):iv16–iv22. doi: 10.1093/europace/eum167. [DOI] [PubMed] [Google Scholar]
- 47.Sager PT. Key clinical considerations for demonstrating the utility of preclinical models to predict clinical drug-induced torsades de pointes. Br J Pharmacol. 2008;154:1544–1549. doi: 10.1038/bjp.2008.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blasi ER, Heyen J, Hemkens M, McHarg A, Ecelbarger CM, Tiwari S.Effects of chronic PPAR-agonist treatment on cardiac structure and function, blood pressure, and kidney in healthy sprague-dawley rats. PPAR Res.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mesfin GM, Piper RC, DuCharme DW, Carlson RG, Humphrey SJ, Zins GR. Pathogenesis of cardiovascular alterations in dogs treated with minoxidil. Toxicol Pathol. 1989;17:164–181. doi: 10.1177/019262338901700113. [DOI] [PubMed] [Google Scholar]
- 50.Mesfin GM, Robinson FG, Higgins MJ, Zhong WZ, DuCharme DW. The pharmacologic basis of the cardiovascular toxicity of minoxidil in the dog. Toxicol Pathol. 1995;23:498–506. doi: 10.1177/019262339502300406. [DOI] [PubMed] [Google Scholar]
- 51.Metz AL, Dominick MA, Suchanek G, Gough AW. Acute cardiovascular toxicity induced by an adenosine agonist-antihypertensive in beagles. Toxicol Pathol. 1991;19:98–107. doi: 10.1177/019262339101900203. [DOI] [PubMed] [Google Scholar]
- 52.Jones HB, Macpherson A, Betton GR, Davis AS, Siddall R, Greaves P. Endothelin antagonist-induced coronary and systemic arteritis in the beagle dog. Toxicol Pathol. 2003;31:263–272. doi: 10.1080/01926230390204298. [DOI] [PubMed] [Google Scholar]
- 53.Stephan-Gueldner M, Inomata A. Coronary arterial lesions induced by endothelin antagonists. Toxicol Lett. 2000;112–113:531–535. doi: 10.1016/s0378-4274(99)00218-0. [DOI] [PubMed] [Google Scholar]
- 54.Ikegami H, Kajikawa S, Miura H, Ito K, Okamiya H, Nakayama H, Doi K. Endothelial hypertrophy in acute phase of arteritis induced by fenoldopam, a vasodilator, in rats. J Toxicol Pathol. 2002;15:119–122. [Google Scholar]
- 55.Joseph EC. Arterial lesions induced by phosphodiesterase III (PDE III) inhibitors and DA(1) agonists. Toxicol Lett. 2000;112–113:537–546. doi: 10.1016/s0378-4274(99)00221-0. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Herman EH, Knapton A, Chadwick DP, Whitehurst VE, Koerner JE, Papoian T, Ferrans VJ, Sistare FD. SK&F 95654-induced acute cardiovascular toxicity in Sprague-Dawley rats—histopathologic, electron microscopic, and immunohistochemical studies. Toxicol Pathol. 2002;30:28–40. doi: 10.1080/01926230252824680. [DOI] [PubMed] [Google Scholar]
- 57.Losco PE, Evans EW, Barat SA, Blackshear PE, Reyderman L, Fine JS, Bober LA, Anthes JC, Mirro EJ, Cuss FM. The toxicity of SCH 351591, a novel phosphodiesterase-4 inhibitor, in Cynomolgus monkeys. Toxicol Pathol. 2004;32:295–308. doi: 10.1080/01926230490431493. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Herman EH, Robertson DG, Reily MD, Knapton A, Ratajczak HV, Rifai N, Honchel R, Blanchard KT, Stoll RE, Sistare FD. Mechanisms and biomarkers of cardiovascular injury induced by phosphodiesterase inhibitor III SK&F 95654 in the spontaneously hypertensive rat. Toxicol Pathol. 2006;34:152–163. doi: 10.1080/01926230600588562. [DOI] [PubMed] [Google Scholar]
- 59.Weaver JL, Snyder R, Knapton A, Herman EH, Honchel R, Miller T, Espandiari P, Smith R, Gu YZ, Goodsaid FM, Rosenblum IY, Sistare FD, Zhang J, Hanig J. Biomarkers in peripheral blood associated with vascular injury in Sprague-Dawley rats treated with the phosphodiesterase IV inhibitors SCH 351591 or SCH 534385. Toxicol Pathol. 2008;36:840–849. doi: 10.1177/0192623308322310. [DOI] [PubMed] [Google Scholar]
- 60. Sobry C, George C.PDE4 inhibitors I: Vasculopathies. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 61.Louden C, Brott D, Katein A, Kelly T, Gould S, Jones H, Betton G, Valetin JP, Richardson RJ. Biomarkers and mechanisms of drug–induced vascular injury in non-rodents. Toxicol Pathol. 2006;34:19–26. doi: 10.1080/01926230500512076. [DOI] [PubMed] [Google Scholar]
- 62.Nyska A, Herbert RA, Chan PC, Haseman JK, Hailey JR. Theophylline-induced mesenteric periarteritis in F344/N rats. Arch Toxicol. 1998;72:731–737. doi: 10.1007/s002040050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dagues N, Pawlowski V, Sobry C, Hanton G, Borde F, Soler S, Freslon JL, Chevalier S. Investigation of the molecular mechanisms preceding PDE4 inhibitor-induced vasculopathy in rats: Tissue inhibitor of metalloproteinase 1, a potential predictive biomarker. Toxicol Sci. 2007;100:238–247. doi: 10.1093/toxsci/kfm161. [DOI] [PubMed] [Google Scholar]
- 64.Merkel PA. Drug-induced vasculitis. Rheum Dis Clin North Am. 2001;27:849–862. doi: 10.1016/s0889-857x(05)70239-8. [DOI] [PubMed] [Google Scholar]
- 65. Scientific Advisory Panel Sub-Groups on Hepatotoxicity Recommendations from the Scientific Advisory Panel Sub-Groups on Hepatotoxicity: Hepatotoxicity of health products. 2004. hc-sc.gc.ca/dhp-mps/prodpharma/activit/sci-consult/hepatotox/sap_gcs_hepatotox_2004-07-26-eng.php#foreword.
- 66.Boyd EM, Bereczky GM. Liver necrosis from paracetamol. Br J Pharmacol Chemother. 1966;26:606–614. doi: 10.1111/j.1476-5381.1966.tb01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 68.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 69.Grattagliano I, Bonfrate L, Diogo CV, Wang HH, Wang DQ, Portincasa P. Biochemical mechanisms in drug-induced liver injury: Certainties and doubts. World J Gastroenterol. 2009;15:4865–4876. doi: 10.3748/wjg.15.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamadeh HK, Amin RP, Paules RS, Afshari CA. An overview of toxicogenomics. Curr Issues Mol Biol. 2002;4:45–56. [PubMed] [Google Scholar]
- 71.Pennie W, Pettit SD, Lord PG. Toxicogenomics in risk assessment: An overview of an HESI collaborative research program. Environ Health Perspect. 2004;112:417–419. doi: 10.1289/ehp.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiyosawa N, Ando Y, Manabe S, Yamoto T. Toxicogenomic biomarkers for liver toxicity. J Toxicol Pathol. 2009;22:35–52. doi: 10.1293/tox.22.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Russmann S, Kullak-Ublick GA, Grattagliano I. Current concepts of mechanisms in drug-induced hepatotoxicity. Curr Med Chem. 2009;16:3041–3053. doi: 10.2174/092986709788803097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bach PH, Nguyen TK. Renal papillary necrosis—40 years on. Toxicol Pathol. 1998;26:73–91. doi: 10.1177/019262339802600110. [DOI] [PubMed] [Google Scholar]
- 75. Betton GR, Rinke M, Davies DT, Somers RL, Floettman EJ.Compounds causing papillary necrosis. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 76.Rinke M, Bomhard EM, Hildebrand H, Leser KH, Loof I, Ruehl-Fehlert C. Serotonin (5-HT1A-receptor) agonist-induced collecting duct vacuolation and renal papillary necrosis in the rat. Toxicol Pathol. 1998;26:152–159. doi: 10.1177/019262339802600118. [DOI] [PubMed] [Google Scholar]
- 77.Price SA, Davies D, Rowlinson R, Copley CG, Roche A, Falkenberg FW, Riccardi D, Betton GR. Characterization of renal papillary antigen 1 (RPA-1), a biomarker of renal papillary necrosis. Toxicol Pathol. 2010;38:346–358. doi: 10.1177/0192623310362246. [DOI] [PubMed] [Google Scholar]
- 78.Zaki FG, Keim GR, Takii Y, Inagami T. Hyperplasia of juxtaglomerular cells and renin localization in kidney of normotensive animals given captopril. Electron microscopic and immunohistochemical studies. Ann Clin Lab Sci. 1982;12:200–215. [PubMed] [Google Scholar]
- 79.Dominick MA, Bobrowski WF, Metz AL, Gough AW, MacDonald JR. Ultrastructural juxtaglomerular cell changes in normotensive rats treated with quinapril, an inhibitor of angiotensin-converting enzyme. Toxicol Pathol. 1990;18:396–406. doi: 10.1177/019262339001800306. [DOI] [PubMed] [Google Scholar]
- 80.Jackson DG, Jones HB. Histopathological and ultrastructural changes in the juxtaglomerular apparatus of the rat following administration of ZENECA ZD6888 (2-ethyl-5,6,7,8-tetrahydro-4-[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)- methoxy]quinoline), an angiotensin II antagonist. Toxicol Pathol. 1995;23:7–15. doi: 10.1177/019262339502300102. [DOI] [PubMed] [Google Scholar]
- 81.Hakoi K, Hayashi T, Irimura K, Hayashi S, Suzuki S, Yamaguchi S, Konishi N, Fukushima S. Alpha2u-globulin nephropathy in rats treated with a glycosaminoglycan extracted from sea cucumbe. J Toxicol Pathol. 2001;14:13–18. [Google Scholar]
- 82.Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, Sultana S, Gerhold DL, Phillips JA, Maurer G, Carl K, Laurie D, Harpur E, Sonee M, Ennulat D, Holder D, Andrews-Cleavenger D, Gu YZ, Thompson KL, Goering PL, Vidal JM, Abadie E, Maciulaitis R, Jacobson-Kram D, Defelice AF, Hausner EA, Blank M, Thompson A, Harlow P, Throckmorton D, Xiao S, Xu N, Taylor W, Vamvakas S, Flamion B, Lima BS, Kasper P, Pasanen M, Prasad K, Troth S, Bounous D, Robinson-Gravatt D, Betton G, Davis MA, Akunda J, McDuffie JE, Suter L, Obert L, Guffroy M, Pinches M, Jayadev S, Blomme EA, Beushausen SA, Barlow VG, Collins N, Waring J, Honor D, Snook S, Lee J, Rossi P, Walker E, Mattes W. Renal biomarker qualification submission: A dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 83.Gold LS, Manley NB, Slone TH, Rohrbach L, Garfinkel GB. Supplement to the Carcinogenic Potency Database (CPDB): Results of animal bioassays published in the general literature through 1997 and by the National Toxicology Program in 1997–1998. Toxicol Sci. 2005;85:747–808. doi: 10.1093/toxsci/kfi161. [DOI] [PubMed] [Google Scholar]
- 84.Holsapple MP, Pitot HC, Cohen SM, Boobis AR, Klaunig JE, Pastoor T, Dellarco VL, Dragan YP. Mode of action in relevance of rodent liver tumors to human cancer risk. Toxicol Sci. 2006;89:51–56. doi: 10.1093/toxsci/kfj001. [DOI] [PubMed] [Google Scholar]
- 85.Cohen SM. Evaluation of possible carcinogenic risk to humans based on liver tumors in rodent assays: The two-year bioassay is no longer necessary. Toxicol Pathol. 2010;38:487–501. doi: 10.1177/0192623310363813. [DOI] [PubMed] [Google Scholar]
- 86. European Medicines Agency (EMA) Refusal assessment report for Zelnorm. 2006. ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000621/WC500058849.pdf.
- 87. Schaffner JC, Ernst R, Junker U, Thomas H, Germann PG.Vascular endothelial growth factor inhibitors (VEGF inhibitors). In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 88.Roorda BD, Ter Elst A, Diks SH, Meeuwsen-de Boer TG, Kamps WA, de Bont ES. PTK787/ZK 222584 inhibits tumor growth promoting mesenchymal stem cells: Kinase activity profiling as powerful tool in functional studies. Cancer Biol Ther. 2009;8:1239–1248. doi: 10.4161/cbt.8.13.8688. [DOI] [PubMed] [Google Scholar]
- 89.Lullmann-Rauch R. Drug-induced lysosomal storage disorders. Front Biol. 1979;48:49–130. [PubMed] [Google Scholar]
- 90. Berg AL, Ciaccio P.Compounds causing phospholipidosis. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 91.Obert LA, Sobocinski GP, Bobrowski WF, Metz AL, Rolsma MD, Altrogge DM, Dunstan RW. An immunohistochemical approach to differentiate hepatic lipidosis from hepatic phospholipidosis in rats. Toxicol Pathol. 2007;35:728–734. doi: 10.1080/01926230701481956. [DOI] [PubMed] [Google Scholar]
- 92.Reasor MJ, Kacew S. Drug-induced phospholipidosis: Are there functional consequences? Exp Biol Med (Maywood) 2001;226:825–830. doi: 10.1177/153537020122600903. [DOI] [PubMed] [Google Scholar]
- 93.Bredehorn T, Clausen M, Duncker G, Lullmann-Rauch R. Morphological and functional changes due to drug-induced lysosomal storage of sulphated glycosaminoglycans in the rat retina. Graefes Arch Clin Exp Ophthalmol. 2001;239:788–793. doi: 10.1007/s004170100361. [DOI] [PubMed] [Google Scholar]
- 94.Nonoyama T, Fukuda R. Drug-induced phospholipidosis —pathologicalaspects and its prediction. J Toxicol Pathol. 2008;21:9–24. [Google Scholar]
- 95.Chatman LA, Morton D, Johnson TO, Anway SD. A strategy for risk management of drug-induced phospholipidosis. Toxicol Pathol. 2009;37:997–1005. doi: 10.1177/0192623309352496. [DOI] [PubMed] [Google Scholar]
- 96.Sawada H, Takami K, Asahi S. A toxicogenomic approach to drug-induced phospholipidosis: Analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 2005;83:282–292. doi: 10.1093/toxsci/kfh264. [DOI] [PubMed] [Google Scholar]
- 97.Baronas ET, Lee JW, Alden C, Hsieh FY. Biomarkers to monitor drug-induced phospholipidosis. Toxicol Appl Pharmacol. 2007;218:72–78. doi: 10.1016/j.taap.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 98.Brown AP, Reindel JF, Grantham L, Loi CM, Van Becelaere K, Leopold J, Graziano MJ. Administration of a MEK inhibitor results in tissue mineralization in the rat due to dysregulation of phosphorus and calcium homeostasis. Toxicologist. 2005;84:108. [Google Scholar]
- 99.Q6. McKay J.MEK Inhibitors. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 100.Dwivedi PP, Hii CS, Ferrante A, Tan J, Der CJ, Omdahl JL, Morris HA, May BK. Role of MAP kinases in the 1,25-dihydroxyvitamin D3-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter. Specific functions for ERK1/ERK2 and ERK5. J Biol Chem. 2002;277:29643–29653. doi: 10.1074/jbc.M204561200. [DOI] [PubMed] [Google Scholar]
- 101.Hamamoto H, Kusudo T, Urushino N, Masuno H, Yamamoto K, Yamada S, Kamakura M, Ohta M, Inouye K, Sakaki T. Structure-function analysis of vitamin D 24-hydroxylase (CYP24A1) by site-directed mutagenesis: Amino acid residues responsible for species-based difference of CYP24A1 between humans and rats. Mol Pharmacol. 2006;70:120–128. doi: 10.1124/mol.106.023275. [DOI] [PubMed] [Google Scholar]
- 102.Laengle UW, Brueggemann S, Prentice DE, Ettlin RA, Richardson B, Naef R, Cordier A. Hypertrophic osteopathy in rats following chronic administration of SDZ MNS 949, an isoquinoline. Exp Toxicol Pathol. 1994;45:473–479. doi: 10.1016/s0940-2993(11)80506-3. [DOI] [PubMed] [Google Scholar]
- 103.Peters JM, Cheung C, Gonzalez FJ. Peroxisome proliferator-activated receptor-alpha and liver cancer: Where do we stand? J Mol Med. 2005;83:774–785. doi: 10.1007/s00109-005-0678-9. [DOI] [PubMed] [Google Scholar]
- 104.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol Sci. 2006;90:269–295. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- 105.Peters JM. Mechanistic evaluation of PPAR{alpha}-mediated hepatocarcinogenesis: Are we there yet? Toxicol Sci. 2008;101:1–3. [Google Scholar]
- 106.Aoki T. Toxicity profiles of peroxisome proliferator-activated receptor (PPAR) agonists and preclinical safety profile for muraglitazar. J Toxicol Pathol. 2007;20:197–202. [Google Scholar]
- 107.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 108.Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007;1771:1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 109.Lai DY. Rodent carcinogenicity of peroxisome proliferators and issues on human relevance. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2004;22:37–55. doi: 10.1081/GNC-120038005. [DOI] [PubMed] [Google Scholar]
- 110.Reddy JK, Qureshi SA. Tumorigenicity of the hypolipidaemic peroxisome proliferator ethyl-alpha-p-chlorophenoxyisobutyrate (clofibrate) in rats. Br J Cancer. 1979;40:476–482. doi: 10.1038/bjc.1979.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JM, Roberts RA, Fenner-Crisp PA. PPARalpha agonist-induced rodent tumors: Modes of action and human relevance. Crit Rev Toxicol. 2003;33:655–780. doi: 10.1080/713608372. [DOI] [PubMed] [Google Scholar]
- 112. El Hage J.Peroxisome Proliferation-Activated Receptor Agonists: Carcinogenicity Findings and Regulatory Recommendations. International Atherosclerosis Society Symposium on PPAR, Monte Carlo.2005.
- 113. El Hage J.Preclinical and Clinical Safety Assessments for PPAR Agonists. DIA meeting, Washington DC.2004. [Google Scholar]
- 114.Tannehill-Gregg SH, Sanderson TP, Minnema D, Voelker R, Ulland B, Cohen SM, Arnold LL, Schilling BE, Waites CR, Dominick MA. Rodent carcinogenicity profile of the antidiabetic dual PPAR alpha and gamma agonist muraglitazar. Toxicol Sci. 2007;98:258–270. doi: 10.1093/toxsci/kfm083. [DOI] [PubMed] [Google Scholar]
- 115.Hardisty JF, Elwell MR, Ernst H, Greaves P, Kolenda-Roberts H, Malarkey DE, Mann PC, Tellier PA. Histopathology of hemangiosarcomas in mice and hamsters and liposarcomas/fibrosarcomas in rats associated with PPAR agonists. Toxicol Pathol. 2007;35:928–941. doi: 10.1080/01926230701748156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cohen SM, Storer RD, Criswell KA, Doerrer NG, Dellarco VL, Pegg DG, Wojcinski ZW, Malarkey DE, Jacobs AC, Klaunig JE, Swenberg JA, Cook JC. Hemangiosarcoma in rodents: Mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111:4–18. doi: 10.1093/toxsci/kfp131. [DOI] [PubMed] [Google Scholar]
- 117.Hellmold H, Zhang H, Andersson U, Blomgren B, Holland T, Berg AL, Elebring M, Sjogren N, Bamberg K, Dahl B, Westerberg R, Dillner B, Tugwood J, Roberts R, Lundholm E, Camejo G, Skanberg I, Evans J. Tesaglitazar, a PPARalpha/gamma agonist, induces interstitial mesenchymal cell DNA synthesis and fibrosarcomas in subcutaneous tissues in rats. Toxicol Sci. 2007;98:63–74. doi: 10.1093/toxsci/kfm094. [DOI] [PubMed] [Google Scholar]
- 118.Dominick MA, White MR, Sanderson TP, Van Vleet T, Cohen SM, Arnold LE, Cano M, Tannehill-Gregg S, Moehlenkamp JD, Waites CR, Schilling BE. Urothelial carcinogenesis in the urinary bladder of male rats treated with muraglitazar, a PPAR alpha/gamma agonist: Evidence for urolithiasis as the inciting event in the mode of action. Toxicol Pathol. 2006;34:903–920. doi: 10.1080/01926230601072327. [DOI] [PubMed] [Google Scholar]
- 119.Suzuki S, Arnold LL, Pennington KL, Kakiuchi-Kiyota S, Wei M, Wanibuchi H, Cohen SM. Effects of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on the urine and urothelium of the rat. Toxicol Sci. 2010;113:349–357. doi: 10.1093/toxsci/kfp256. [DOI] [PubMed] [Google Scholar]
- 120.Cohen SM, Ohnishi T, Clark NM, He J, Arnold LL. Investigations of rodent urinary bladder carcinogens: Collection, processing, and evaluation of urine and bladders. Toxicol Pathol. 2007;35:337–347. doi: 10.1080/01926230701197115. [DOI] [PubMed] [Google Scholar]
- 121.Hardisty JF, Anderson DC, Brodie S, Cline JM, Hahn FF, Kolenda-Roberts H, Lele SM, Lowenstine LJ. Histopathology of the urinary bladders of cynomolgus monkeys treated with PPAR agonists. Toxicol Pathol. 2008;36:769–776. doi: 10.1177/0192623308323624. [DOI] [PubMed] [Google Scholar]
- 122.Q8. Lemarchand TX, Newton RK, Searfoss GH.Peroxisome proliferators II: Muscle lesions. In: Classic Examples in Toxicologic Pathology, 3rd ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology, Hannover. CD-ROM.2009.
- 123.Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120–122. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 124. USA FDA. Guidance for industry. Diabetes mellitus: Developing drugs and therapeutic biologics for treatment and prevention. Draft guidance. 2008.
- 125.Brambilla G, Martelli A. Update on genotoxicity and carcinogenicity testing of 472 marketed pharmaceuticals. Mutat Res. 2009;681:209–229. doi: 10.1016/j.mrrev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 126.Snyder RD, Green JW. A review of the genotoxicity of marketed pharmaceuticals. Mutat Res. 2001;488:151–169. doi: 10.1016/s1383-5742(01)00055-2. [DOI] [PubMed] [Google Scholar]
- 127.Snyder RD. An update on the genotoxicity and carcinogenicity of marketed pharmaceuticals with reference to in silico predictivity. Environ Mol Mutagen. 2009;50:435–450. doi: 10.1002/em.20485. [DOI] [PubMed] [Google Scholar]
- 128.Brambilla G, Martelli A. Genotoxicity and carcinogenicity studies of analgesics, anti-inflammatory drugs and antipyretics. Pharmacol Res. 2009;60:1–17. doi: 10.1016/j.phrs.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 129. Novartis Pharmaceuticals Canada Inc Prescribing information "Comtan" (entacapone) tablets, 200 mg. 2008. website: http://ask.novartispharma.ca/download.htm?res=comtan_scrip_e.pdf&resTitleId=102.
- 130. International Conference on Harmonization (ICH). ICH harmonized tripartite step 2 draft guideline. Guidance on genotoxicity testing and data interpretation for parmaceuticals intended for human use. S2(R1). 2008.
- 131.Jacobson-Kram D, Jacobs A. Use of genotoxicity data to support clinical trials or positive genetox findings on a candidate pharmaceutical or impurity.... now what? Int J Toxicol. 2005;24:129–134. doi: 10.1080/10915810590952933. [DOI] [PubMed] [Google Scholar]
- 132.Usui T, Mutai M, Hisada S, Takoaka M, Soper KA, McCullough B, Alden C. CB6F1-rasH2 mouse: Overview of available data. Toxicol Pathol. 2001;29(Suppl):90–108. doi: 10.1080/019262301753178500. [DOI] [PubMed] [Google Scholar]
- 133.Mitsumori K. Evaluation on carcinogenicity of chemicals using transgenic mice. Toxicology. 2002;181–182:241–244. doi: 10.1016/s0300-483x(02)00290-1. [DOI] [PubMed] [Google Scholar]
- 134.Mitsumori K. Possible mechanism on enhanced carcinogenesis of genotoxic carcinogens and unsolved mechanisms on lesser carcinogenic susceptibility to some carcinogens in rasH2 mice. J Toxicol Sci. 2003;28:371–383. doi: 10.2131/jts.28.371. [DOI] [PubMed] [Google Scholar]
- 135.Takaoka M, Sehata S, Maejima T, Imai T, Torii M, Satoh H, Toyosawa K, Tanakamaru ZY, Adachi T, Hisada S, Ueda M, Ogasawara H, Matsumoto M, Kobayashi K, Mutai M, Usui T. Interlaboratory comparison of short-term carcinogenicity studies using CB6F1-rasH2 transgenic mice. Toxicol Pathol. 2003;31:191–199. doi: 10.1080/01926230390183670. [DOI] [PubMed] [Google Scholar]
- 136.Jacobson-Kram D, Sistare FD, Jacobs AC. Use of transgenic mice in carcinogenicity hazard assessment. Toxicol Pathol. 2004;32(Suppl 1):49–52. doi: 10.1080/01926230490424761. [DOI] [PubMed] [Google Scholar]
- 137.Long GG, Morton D, Peters T, Short B, Skydsgaard M. Alternative mouse models for carcinogenicity assessment: Industry use and issues with pathology interpretation. Toxicol Pathol. 2010;38:43–50. doi: 10.1177/0192623309354107. [DOI] [PubMed] [Google Scholar]
- 138.Storer RD, Sistare FD, Reddy MV, DeGeorge JJ. An industry perspective on the utility of short-term carcinogenicity testing in transgenic mice in pharmaceutical development. Toxicol Pathol. 2010;38:51–61. doi: 10.1177/0192623309351718. [DOI] [PubMed] [Google Scholar]
- 139.Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, Kirsch-Volders M, Zeiger E, Ban S, Barale R, Bigatti MP, Bolognesi C, Cebulska-Wasilewska A, Fabianova E, Fucic A, Hagmar L, Joksic G, Martelli A, Migliore L, Mirkova E, Scarfi MR, Zijno A, Norppa H, Fenech M. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- 140. Mauthe R.Establishment of allowable concentrations of genotoxic impurities in drug substance and product. Winter Toxicology Forum2005.
- 141.Muller L, Mauthe RJ, Riley CM, Andino MM, Antonis DD, Beels C, DeGeorge J, De Knaep AG, Ellison D, Fagerland JA, Frank R, Fritschel B, Galloway S, Harpur E, Humfrey CD, Jacks AS, Jagota N, Mackinnon J, Mohan G, Ness DK, O'Donovan MR, Smith MD, Vudathala G, Yotti L. A rationale for determining, testing, and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity. Regul Toxicol Pharmacol. 2006;44:198–211. doi: 10.1016/j.yrtph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 142. EMA Guideline on the limits of genotoxic impurities. Washington DC. 2006. [Google Scholar]
- 143. USA FDA. Guidance for industry. Genotoxic and carcinogenic impurities in drug substances and products: Recommended approaches. 2008.
- 144.Tice RR, Stack HF, Waters MD. Human exposures to mutagens—an analysis using the genetic activity profile database. Environ Health Perspect. 1996;104(Suppl 3):585–589. doi: 10.1289/ehp.96104s3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bergman K, Muller L, Teigen SW. Series: Current issues in mutagenesis and carcinogenesis, No. 65. The genotoxicity and carcinogenicity of paracetamol: A regulatory (re)view. Mutat Res. 1996;349:263–288. doi: 10.1016/0027-5107(95)00185-9. [DOI] [PubMed] [Google Scholar]
- 146.Brent RL. Utilization of animal studies to determine the effects and human risks of environmental toxicants (drugs, chemicals, and physical agents) Pediatrics. 2004;113:984–995. [PubMed] [Google Scholar]
- 147.Chernoff N, Rogers EH, Gage MI, Francis BM. The relationship of maternal and fetal toxicity in developmental toxicology bioassays with notes on the biological significance of the "no observed adverse effect level". Reprod Toxicol. 2008;25:192–202. doi: 10.1016/j.reprotox.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 148.West PR, Weir AM, Smith AM, Donley EL, Cezar GG. Predicting human developmental toxicity of pharmaceuticals using human embryonic stem cells and metabolomics. Toxicol Appl Pharmacol. 2010;247:18–27. doi: 10.1016/j.taap.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 149.Attina T, Camidge R, Newby DE, Webb DJ. Endothelin antagonism in pulmonary hypertension, heart failure, and beyond. Heart. 2005;91:825–831. doi: 10.1136/hrt.2004.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Walker BE, Fraser FC. The embryology of cortisone-induced cleft palate. J Embryol Exp Morphol. 1957;5:201–209. [Google Scholar]
- 151.Barlow SM, McElhatton PR, Sullivan FM. The relation between maternal restraint and food deprivation, plasma corticosterone, and induction of cleft palate in the offspring of mice. Teratology. 1975;12:97–103. doi: 10.1002/tera.1420120202. [DOI] [PubMed] [Google Scholar]
- 152.Meadows M. Pregnancy and the drug dilemma. FDA Consum. 2001;35:16–20. [PubMed] [Google Scholar]
- 153. USA FDA CFR - Code of Federal Regulations Title 21, Part 201 - Labeling - Subpart B - Labeling requirements for prescription drugs and/or Insulin. Sec. 201.57 Specific requirements on content and format of labeling for human prescription drug and biological products described in 201.56(b)(1). 2009. From USA FDA website: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=201.57.
- 154. Australian Therapeutic Goods Administration (TGA). Prescribing medicines in pregnancy. An Australian categorisation of risk of drug use in pregnancy. 1999. Last amendment: 2007.
- 155.Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Regist. 2008;73:30831–30868. [PubMed] [Google Scholar]
- 156. Briggs GG, Freeman RK, Summer JY.Drugs in Pregnancy and Lactation. A Reference Guide to Fetal and Neonatal Risk. 8th ed. Lippincott, Williams & Wilkins, Philadelphia.2008. [Google Scholar]
- 157.Malm H, Martikainen J, Klaukka T, Neuvonen PJ. Prescription drugs during pregnancy and lactation—a Finnish register-based study. Eur J Clin Pharmacol. 2003;59:127–133. doi: 10.1007/s00228-003-0584-4. [DOI] [PubMed] [Google Scholar]
- 158.Buhimschi CS, Weiner CP. Medications in pregnancy and lactation: Part 2. Drugs with minimal or unknown human teratogenic effect. Obstet Gynecol. 2009;113:417–432. doi: 10.1097/AOG.0b013e31818d686c. [DOI] [PubMed] [Google Scholar]
- 159. Briggs GG, Nageotte MP.Diseases, Complications, and Drug Therapy in Obstetrics: A Guide for Clinicians. American Society of Health-System Pharmacists, Bethesda, Md.2009. [Google Scholar]
- 160.Anderson GD. Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert Opin Drug Metab Toxicol. 2006;2:947–960. doi: 10.1517/17425255.2.6.947. [DOI] [PubMed] [Google Scholar]