Abstract
Spontaneous malignant mesothelioma was found in a 104-week-old male Crj:CD(SD) rat. The tumor was scattered on the surface of the lung, heart, mediastinal pleura and thoracic wall and metastasized to the alveolar septa. Histopathologically, small flattened or cuboidal tumor cells proliferated with stroma, formed almost normal papillary structures and reacted positively to colloidal iron stain and immunohistochemical staining for mesothelin. Round hyalinous stromata were pronounced, which is a characteristic feature, and the possible reason for this is as follows; at first, a small amount of collagen fibers was formed in the center of the clusters of several tumor cells, and then the cell clusters expanded like balloons with an increase in the collagen fibers.
Keywords: mesothelioma, round hyalinous stroma, thoracic cavity, spontaneous, Crj:CD(SD) rat
Mesotheliomas are aggressive tumors arising from the mesothelial cells of serosal surfaces. 1 In humans, these tumors are usually associated with exposure to asbestos and are most common in the pleura. 1 A wide range of fibers including asbestos and erionite, chemicals and metals have been shown to induce mesotheliomas in rodents. 2 However, although not so common, mesothelioma is one of the spontaneously occurring tumors in Fischer 344 rats, and the incidence is markedly higher in males as compared to that in females. 3 – 5 The most common site is the genital serosa, but it also grows and spreads into the peritoneal cavity in some cases. 6 – 8 To our knowledge, spontaneous mesotheliomas do not commonly occur in Sprague-Dawley rats, and only a few cases have been reported. 9 – 11 Recently, we encountered a spontaneous mesothelioma in the thoracic cavity of a mature male Sprague-Dawley rat. This paper describes the gross, histopathological and electron microscopical features of the mesothelioma.
The male rat was one of 200 specific-pathogen-free Crj:CD(SD) rats of both sexes purchased from Charles River Japan Inc. (Kanagawa, Japan) and was orally administered methylcellulose solution in a 2-year background data accumulation study for carcinogenicity studies. The rats used in this study were housed individually in wire-mesh cages in a barrier-sustained animal room maintained at a temperature of 22 ± 3°C with a humidity of 55 ± 20%, 6 to 20 air changes per hour and a 12-hour light and 12-hour dark cycle. They were given a commercial diet (CR-LPF, Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum. The animal was cared for according to the principles outlined in the guides for the care and use of laboratory animals prepared by the Japanese Association for Laboratory Animal Science and our laboratory.
The clinical signs of the affected rat were emaciation, hunchback position, decrease in locomotor activity, tachypnea and hypothermia before death at 104 weeks of age. After necropsy, all sampled organs and tissues were fixed in 10% phosphate-buffered formalin solution and were subjected to routine histological examination using hematoxylin and eosin (HE) stain. Sections of the lung were additionally stained with colloidal iron, Masson’s trichrome, periodic acid Schiff (PAS) reaction and von Kossa’s method. For immunohistochemical staining, a paraffin-embedded section of the lung was subjected to the labeled streptavidin biotin (LSAB) method using a commercial kit (Dako Japan, Kyoto, Japan) for anti-rat C-ERC/mesothelin (306) (×400, polyclonal, Immuno-Biological Laboratories, Takasaki, Japan). For electron microscopic examination, a surface and inner part of formalin-fixed lung tissue including mass or plaque was fixed in 1% osmium, routinely processed and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and examined under an electron microscope (H-7600, Hitachi, Tokyo, Japan).
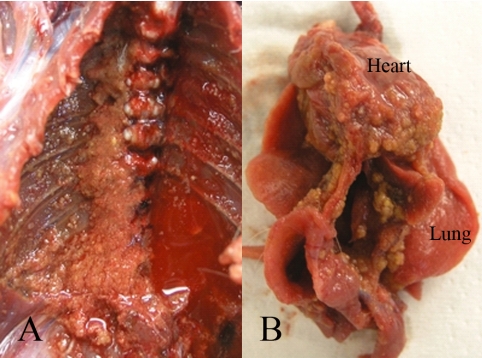
At necropsy, accumulation of approximately 30 mL red pleural effusion was observed. Numerous small whitish or brownish masses were scattered on the surfaces of the thoracic wall (Fig. 1A), lung, heart, mediastinal pleura and diaphragm (Fig. 1B); and the pericardium adhered to the heart. In the lung, small whitish plaque was observed in the inner parts as well as on the surface.
Fig. 1.
Gross findings of this tumor. A: Numerous small whitish or brownish masses are scattered on the surface of the thoracic wall. B. Similar masses are also observed on the surfaces of the lung, heart, mediastinal pleura and diaphragm.
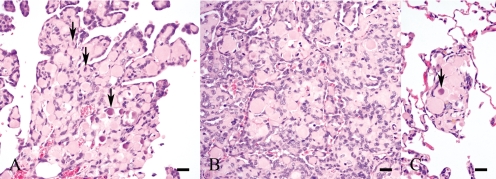
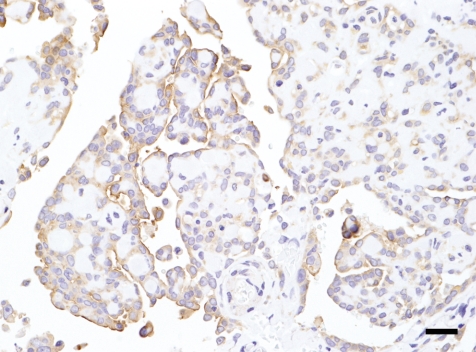
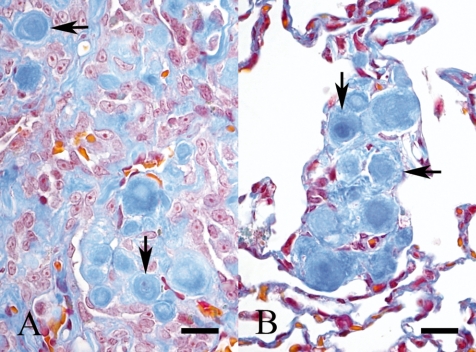
On histopathological observation of the masses, small flattened or cuboidal tumor cells proliferated with stroma and normally formed papillary structures that were more tightly-packed in the inner parts (Fig. 2B) than on the free surfaces (Fig. 2A). Furthermore, mitotic figures were infrequent. Mast cells and hemosiderin-laden macrophages were often present within the stroma. Tumor cells with the stroma were also observed in the alveolar septa (Fig. 2C). The stroma was mostly hyalinous and often small and round; this was a characteristic feature of this case (Figs. 2A –2C). Psammoma bodies were observed at the centers of some characteristic stromata (Figs. 2A , 2C). Colloidal iron-positive materials, which became negative or weaker by prior incubation with hyaluronidase, were found in the cytoplasm of tumor cells. Immunohistochemically, most tumor cells reacted positively for mesothelin (Fig. 3). The characteristic stromata were stained bluish with Masson’s trichrome (Figs. 4A , 4B) and reacted negatively to PAS reaction or colloidal iron. A concentric pattern of collagen fibers, which stained bluish with Masson’s trichrome, was frequently observed (Figs. 4A , 4B). Moreover, the psammoma bodies were positive for staining with the PAS reaction and von Kossa’s method.
Fig. 2.
Small flattened or cuboidal tumor cells proliferate with stroma and usually form papillary structures that are more tightly-packed in the inner parts of the tumor mass (B) than in the free surfaces (A). The stroma is mostly hyalinous and often small and round. Psammoma bodies are observed at the centers of some stromata (arrows). Although shown in B, psammoma bodies were also present in the inner parts. C. Tumor cells with a stroma are also observed in the alveolar septa. HE. Bars=20 μm.
Fig. 3.
Immunohistochemical staining for mesothelin. Tumor cells react positively for mesothelin. HE. Bar=20 μm.
Fig. 4.
Round hyalinous stromata are stained bluish with Masson’s trichrome. Concentric patterns of collagen fibers are observed (arrows). A: Inner part of the tumor mass. B: Characteristic stromata in the alveolar septa. Bars=20 μm.
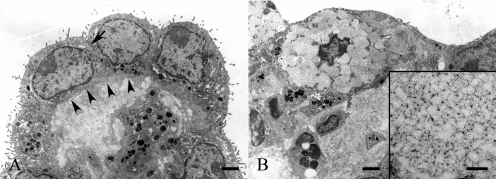
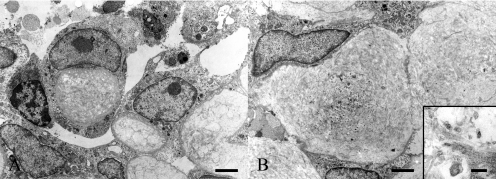
On electron microscopic observation, tumor cells had numerous microvilli on their surfaces and intercellular junctions (desmosome-like structures). The tumor cells were attached to collagenous connective tissues by means of a basement membrane (Fig. 5A). Some tumor cells had mucous secretory granules resembling proteoglycans characterized by network formation consisting of particles and filaments (Fig. 5B). 12 The stroma was composed mainly of compactly arranged collagen fibers. In addition, a small amount of proteoglycan-like materials was also contained in the stroma. As a characteristic feature, clusters of several tumor cells surrounding round collagen-rich stromata were often observed. Some of the clusters had a small amount of stroma (Fig. 6A). Others had a relatively-abundant stroma, and they adhered to one another (Fig. 6B). Membranous structures, which were suspected to be cytoplasmic organelles, accumulated at the centers of some of the round stromata (Fig. 6B).
Fig. 5.
Electron micrographs of tumor cells in this tumor mass. A: Tumor cells have numerous microvilli and intercellular junctions (arrow). The tumor cells are attached to collagenous connective tissues by means of a basement membrane (arrowheads). Bar=2 μm. B: The tumor cell has mucous secretory granules. Bar=2 μm. Inset: Secretory granules are characterized by network formation consisting of particles and filaments. Bar=0.2 μm.
Fig. 6.
Electron micrographs of tumor cells in this tumor mass. Clusters of several tumor cells surrounding round collagen-rich stromata are often observed. A: Clusters contain a small amount of stroma. Bar=2 μm. B: Clusters have a relatively-abundant stroma, and they adhere to one another. Bar=2 μm. Inset: Membranous structures suspected to be cytoplasmic organelles accumulate at the center of a round stroma. Bar=0.2 μm.
The following morphological findings in this case indicated a diagnosis of malignant mesothelioma: (1) gross masses or plaques scattered on the surfaces of the thoracic organs and tissues; (2) histopathologically, tumor cells proliferated with stroma and formed papillary structures; (3) electron microscopically, microvilli, intercellular junctions and basement membranes were observed; and (4) tumor cells metastasized to the alveolar septa. As the tumor cells reacted positively to colloidal iron stain and had mucous secretory granules resembling proteoglycans, it appeared that the tumor cells produced hyaluronic acid. Additionally, the tumor cells reacted positively for mesothelin. These findings also supported the diagnosis.
A round hyalinous stroma was the characteristic feature in this case. The stroma in mesotheliomas varies from myxoid to densely fibrous with or without hyalinization, and the myxoid area is positive for colloidal iron stain due to the existence of hyaluronic acid. 1 As the stroma in this case was mainly composed of collagen fibers and little hyaluronic acid, it would appear hyalinous on HE. As a reason why the small round stroma was pronounced, the following hypothesis was considered. At first, a small amount of collagenous stroma formed in the center of the clusters of tumor cells, and then the cell clusters expanded like balloons with an increase in the stroma. However, the expansion was limited because the clusters were composed of too few tumor cells.
Psammoma bodies are laminated spherical concretions that occur in a variety of tumors and in approximately 5% of human mesotheliomas. 1 , 13 To our knowledge, however, there has been no report concerning rat mesotheliomas with psammoma bodies. Psammoma bodies are thought to be produced by degenerating tumor cells, secretions from these cells or a mixture of both. 13 In this case, membranous structures suspected to be cytoplasmic organelles accumulated at the centers of round stromata where the psammoma bodies were observed. As the membranous structures may be derived from tumor cells, the psammoma bodies may occur from tumor cell degeneration.
Mesothelioma only occurred in this rat in this background data accumulation study. To our knowledge, there have been a few reports concerning spontaneous mesotheliomas in Sprague-Dawley rats. Based on the descriptions, the primary sites were the atriocaval location and ovary. 9 – 11 The exact primary site in this case was unclear, but it was evident that the tumor originated in the mesothelium of the thoracic organs or tissues. Thus, it was suggested that the site of predilection for mesothelioma may be different between the Sprague-Dawley rat and Fischer 344 rat.
Acknowledgments
We thank Ms. Mika Nagaike (Ishihara Sangyo Kaisha, Ltd.) for her cooperation in regard to the mesothelin immunohistochemical staining. We also thank Mr. Takayoshi Ito for his excellent technical assistance and Mr. Steve Yamakami for language editing.
References
- 1. Weiss SW, Goldblum JR.Mesothelioma. In: Soft Tissue Tumors, 5th ed. Weiss SW and Goldblum JR (eds), Elsevier, Amsterdam, 789–823,2008.
- 2.Kane AB. Animal models of malignant mesothelioma. Inhal Toxicol. 2006;18:1001–1004. doi: 10.1080/08958370600835393. [DOI] [PubMed] [Google Scholar]
- 3.Maekawa A, Kurosawa Y, Takahashi M, Kokubo T, Ogiu T, Onodera H, Tanigawa H, Ohno Y, Furukawa F, Hayashi Y. Spontaneous tumors in F-344/DuCrj rats. Gann. 1983;74:365–372. [PubMed] [Google Scholar]
- 4.Solleveld HA, Haseman JK, McConnell EE. Natural history of body weight gain, survival, and neoplasia in the F344 rat. J Natl Cancer Inst. 1984;72:929–940. [PubMed] [Google Scholar]
- 5.Iwata H, Hirouchi Y, Koike Y, Yamakawa S, Kobayashi K, Yamamoto T, Kobayashi K, Inoue H, Enomoto M. Historical control data of non-neoplastic and neoplastic lesions in F344/DuCrj rats. J Toxicol Pathol. 1991;4:1–24. [Google Scholar]
- 6.Gould DH. Mesotheliomas of the tunica vaginalis propria and peritoneum in Fischer rats. Vet Pathol. 1977;14:372–379. doi: 10.1177/030098587701400409. [DOI] [PubMed] [Google Scholar]
- 7.Tanigawa H, Onodera H, Maekawa A. Spontaneous mesotheliomas in Fischer rats—a histological and electron microscopic study. Toxicol Pathol. 1987;15:157–163. doi: 10.1177/019262338701500205. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya K, Tajima M, Yamate J. Histological classification of 62 spontaneous mesotheliomas in F344 rats. Jpn J Vet Sci. 1990;52:1313–1317. doi: 10.1292/jvms1939.52.1313. [DOI] [PubMed] [Google Scholar]
- 9.Chandra M, Davis H, Carlton WW. Naturally occurring atriocaval mesotheliomas in rats. J Comp Pathol. 1993;109:433–437. doi: 10.1016/s0021-9975(08)80306-4. [DOI] [PubMed] [Google Scholar]
- 10.Peano S, Conz A, Carbonatto M, Goldstein J, Nyska A. Atriocaval mesothelioma in male Sprague-Dawley rat. Toxicol Pathol. 1998;26:695–698. doi: 10.1177/019262339802600516. [DOI] [PubMed] [Google Scholar]
- 11.Lewis DJ. Ovarian neoplasia in the Sprague-Dawley rat. Environ Health Perspect. 1987;73:77–90. doi: 10.1289/ehp.877377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghadially FN.Ultrastructural Pathology of the Cell and Matrix. 3rd ed. Butterworths, London.1988. [Google Scholar]
- 13.Miyoshi I, Komatsu N, Daibata M, Kobayashi M, Hiroi M, Ohtsuki Y, Taguchi H. Psammoma bodies in malignant pleural mesothelioma. Intern Med. 2007;46:529–530. doi: 10.2169/internalmedicine.46.6103. [DOI] [PubMed] [Google Scholar]