Abstract
Aims: In addition to nitric oxide and carbon monoxide, hydrogen sulfide (H2S) is an endogenously synthesized gaseous molecule that acts as an important signaling molecule in the living body. Transcription factor hypoxia-inducible factor 1 (HIF-1) is known to respond to intracellular reduced oxygen (O2) availability, which is regulated by an elaborate balance between O2 supply and demand. However, the effect of H2S on HIF-1 activity under hypoxic conditions is largely unknown in mammalian cells. In this study, we tried to elucidate the effect of H2S on hypoxia-induced HIF-1 activation adopting cultured cells and mice. Results: The H2S donors sodium hydrosulfide and sodium sulfide in pharmacological concentrations reversibly reduced cellular O2 consumption and inhibited hypoxia- but not anoxia-induced HIF-1α protein accumulation and expression of genes downstream of HIF-1 in established cell lines. H2S did not affect HIF-1 activation induced by the HIF-α hydroxylases inhibitors desferrioxamine or CoCl2. Experimental evidence adopting von Hippel-Lindau (VHL)- or mitochondria-deficient cells indicated that H2S did not affect neosynthesis of HIF-1α protein but destabilized HIF-1α in a VHL- and mitochondria-dependent manner. We also demonstrate that exogenously administered H2S inhibited HIF-1–dependent gene expression in mice. Innovation: For the first time, we show that H2S modulates intracellular O2 homeostasis and regulates activation of HIF-1 and the subsequent gene expression induced by hypoxia by using an in vitro system with established cell lines and an in vivo system in mice. Conclusions: We demonstrate that H2S inhibits hypoxia-induced HIF-1 activation in a VHL- and mitochondria-dependent manner. Antioxid. Redox Signal. 16, 203–216.
Introduction
Gaseous molecules constitute a unique class of biomaterials that are indispensable for maintaining the homeostasis of biological systems (4, 21). Among the gases used in the body and pharmacologically administered, oxygen (O2) has been extensively studied with regard to the mechanisms of its transport, utilization, and metabolism, because O2 is essential for almost all higher living organisms and is not produced in the body. Nitric oxide (NO) has also been extensively studied and is considered to regulate a variety of biological events and physiological functions such as vascular tone, platelet aggregation, smooth muscle relaxation, and synaptic function. NO is also an important messenger molecule involved in many physiological and pathological processes within the mammalian body, both beneficial and detrimental. Carbon monoxide (CO) has recently attracted the interest of researchers as a novel signaling molecule in the regulation of neurovascular functions. Hydrogen sulfide (H2S) is generated through the degradation of cysteine and is also used to synthesize this amino acid in the cysteine-generating pathway in mammals (23). Another line of study indicates the possible physiological significance of H2S in the nervous, circulatory, respiratory, and gastrointestinal systems (24, 45). In addition, exogenous H2S exerts pharmacological and toxicological effects (19, 42).
Hypoxia causes a set of adaptive responses (16). At the cellular level, adaptation involves a switch of energy metabolism from oxidative phosphorylation to anaerobic glycolysis, increased glucose uptake, and the expression of stress proteins related to cell survival or death. One of the most important cellular factors involved in regulating the expression of the genes encoding these proteins, including vascular endothelial growth factor (VEGF), glucose transporter 1 (GLUT1), lactate dehydrogenase A (LDHA), pyruvate dehydrogenase kinase 1 (PDK-1), inducible NO synthase, and heme oxygenase-1, is hypoxia-inducible factor 1 (HIF-1) (46). HIF-1 is a heterodimer composed of a constitutively expressed β subunit (HIF-1β) and an inducibly expressed α subunit (HIF-1α) (46). The regulation of HIF-1 activity occurs at multiple levels in vivo. Among these, the mechanisms regulating HIF-1α protein expression and transcriptional activity have been most extensively analyzed. Von Hippel-Lindau (VHL) tumor suppressor is the HIF-1α–binding component of the ubiquitin protein ligase that targets HIF-1α for proteasomal degradation in nonhypoxic cells (30). Hypoxia induces changes in the hydroxylation status of well-conserved prolyl and asparaginyl residues of HIF-1α, thus resulting in protein stabilization and transcriptional activation of HIF-1α (14). The iron chelator desferroxamine (DFX) and the divalent cation Co2+ efficiently suppress both the hydroxylases for prolyl and asparaginyl residues, thus causing HIF-1α stabilization and transactivation even under normoxic conditions. Signaling through receptor tyrosine kinases can induce HIF-1 expression by an independent mechanism. HER2/neu activation increases the rate of HIF-1α protein synthesis via PI3 kinase and the downstream serine-threonine kinases Akt (protein kinase B) and FKBP/rapamycin-associated protein (FRAP), also known as mammalian target of rapamycin (mTOR) (26). In contrast, insulin-like growth factor-1–induced HIF-1α synthesis is dependent on the activity of both the PI3 kinase and MAP kinase pathways in cells (8). FRAP/mTOR phosphorylates and activates the translational regulatory proteins eukaryotic initiation factor 4E (eIF-4E)–binding protein 1 (4E-BP1) and p70 S6 kinase (p70 S6K). Phosphorylation of 4E-BP1 disrupts its inhibitory interaction with eIF-4E, whereas activated p70 S6K phosphorylates the 40S ribosomal protein S6. The effect of HER/neu signaling on the translation of HIF-1α protein depends on the presence of the 5′-untranslated region of HIF-1α mRNA. These pathways, thus, provide a molecular basis for the stimulation of HIF-1α protein synthesis in response to HER2/neu activation.
Innovation.
Gaseous molecules such as oxygen (O2), nitric oxide, and carbon monoxide constitute a unique class of biomaterials that are indispensable for maintaining the homeostasis of biological systems. In addition, a line of study indicates the possible physiological and pathophysiological significance of H2S in the nervous, circulatory, respiratory, and gastrointestinal systems. Hypoxia causes a set of adaptive responses. One of the most critical cellular factors involved in regulating the expression of the hypoxia-responsive genes is HIF-1. In this study, it is demonstrated that H2S inhibits cellular O2 consumption and hypoxia-induced HIF-1 activation by decreasing HIF-1α protein accumulation and that H2S does not inhibit anoxia- or HIF-α hydroxylase inhibitors-induced HIF-1 activation. Together with the evidence adopting VHL- or mitochondria-deficient cells, it is indicated that H2S does not affect neosynthesis of HIF-1α protein but destabilized HIF-1α in a VHL- and mitochondria-dependent manner. Thus, for the first time it is shown that H2S modulates intracellular O2 homeostasis and regulates activation of HIF-1 and the subsequent gene expression induced by hypoxia.
A series of reports indicate that the gaseous molecules NO and CO significantly enhance and inhibit both HIF-1 activity under normoxic conditions and HIF-1 activation under hypoxic conditions at physiological and pharmacological concentrations (3, 12, 17, 22, 29). H2S is increasingly recognized as an important signaling molecule in the cardiovascular and nervous systems (23). It exerts a host of biological effects on various targets, resulting in responses that include both cytotoxic and cytoprotective effects (31, 42). Although inhalation of high concentrations of H2S is toxic, breathing low concentrations reversibly reduces metabolism in rodents and improves survival after hemorrhagic shock in rats (9, 32). H2S can also partly inhibit cellular respiration by acting as an inhibitor of cytochrome c oxidase. Inhibition of cytochrome c oxidase is a probable mechanism for the regulation of cellular O2 consumption by H2S (4, 20, 24). Further, it was recently proposed that the metabolism of H2S may serve as an O2 sensor in vertebrate vascular smooth muscle (36, 38). Moreover, there is a report indicating that H2S activates HIF-1 independent of von Hippel–Lindau in Caenorhabditis elegans (C. elegans) under 20% O2 conditions (2) and also enhances HIF-1-dependent gene expression induced by CoCl2 in cells from vascular origin (27). However, the influence of H2S on HIF-1 activity and hypoxia-induced HIF-1 activation in mammalian cells is largely unknown at the moment. The available evidence prompted us to investigate how H2S donors affect HIF-1 activation under normoxic and hypoxic conditions. In this study using an in vitro system with established cell lines and an in vivo system in mice, we for the first time demonstrated that H2S inhibits hypoxia-induced HIF-1 activation in a VHL- and mitochondria-dependent manner.
Results
H2S donors inhibited 1% O2-induced HIF-1 protein expression and HIF-1–dependent gene expression
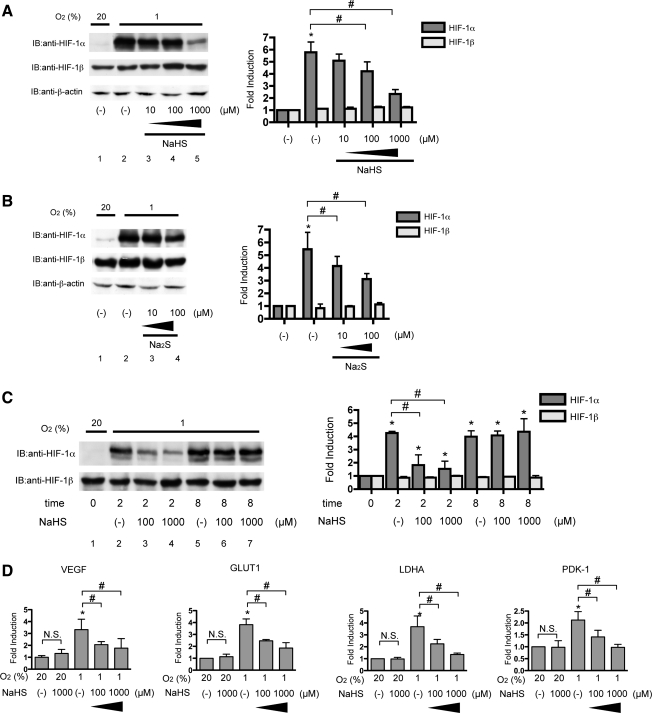
We used two H2S donors, sodium hydrosulfide (NaHS) and sodium sulfide (Na2S), to study the effect of H2S on HIF-1 activation. These donors promptly release H2S in solution. NaHS dissociates to Na+ and HS− in solution, then HS− associates with H+ to produce H2S. Hep3B cells were exposed to NaHS under 20% and 1% O2 conditions for 4 h, harvested, and subjected to immunoblot analysis by using anti-HIF-1α or anti-HIF-1β antibody (Fig. 1A). NaHS did not affect HIF-1α or HIF-1β protein expression under the 20% O2 condition. On the other hand, NaHS suppressed HIF-1α protein induction under 1% O2 in a concentration-dependent manner up to 1000 μM. HIF-1β expression was not affected by 1000 μM NaHS under 1% O2. Expression of β-actin protein was affected by neither NaHS treatment nor the 1% O2 condition. Na2S also inhibited 1% O2-induced HIF-1α protein accumulation (Fig. 1B).
FIG. 1.
Hydrogen sulfide donors inhibited 1% O2-induced HIF-1 protein expression and HIF-1–dependent gene expression. Hep3B cells were exposed to the indicated concentrations of NaHS (A) or Na2S (B) for 4 h under 20% or 1% O2. After treatment, cells were harvested, and whole-cell lysates were subjected to an immunoblot assay for HIF-1α, HIF-1β, or β-actin protein expression. (C) Hep3B cells were exposed to the indicated concentrations of NaHS for 2 or 8 h under 20% or 1% O2. Experiments were repeated thrice. Representative immunoblots are shown (A, B, and C; left panels). Band intensities were densitometrically analyzed. Fold induction relative to lane 1 was plotted as mean±SD (A and B; right panels). *p<0.05 compared with control; #p<0.05 for comparisons between the indicated groups. (D) Hep3B cells were exposed to the indicated concentrations of NaHS for 8 h under 20% or 1% O2 and harvested for semi-quantitative RT-PCR for vascular endothelial growth factor (VEGF), glucose transporter 1 (GLUT1), Lactate dehydrogenase A (LDHA), and pyruvate dehydrogenase kinase-1 (PDK-1). Experiments were repeated at least thrice in triplicate. *p<0.05 compared with the control (20% and no treatment), #p<0.05 for comparisons between the indicated groups. N.S., not statistically significant; HIF-1, hypoxia-inducible factor 1; NaHS, sodium hydrosulfide; Na2S, sodium sulfide.
Next, we performed a time-course study on the inhibitory effect of NaHS. Hep3B cells were exposed to 1000 μM NaHS for the indicated times under the 1% O2 condition, and then harvested for immunoblot analysis. NaHS inhibited HIF-1α protein accumulation until 2 h (Fig. 1C, lanes 2–4); however, after 8 h, a suppressive effect was no longer observed (lanes 5–7), thus indicating that the effect is reversible. In contrast, HIF-1β expression was constant.
Next, we investigated whether NaHS inhibited 1% O2-induced gene expression downstream of HIF-1 in Hep3B cells by semi-quantitative RT-PCR (Fig. 1D). Expression of VEGF, GLUT1, LDHA, and PDK-1 mRNA was induced under 1% O2, and all inductions were inhibited by NaHS in a concentration-dependent manner, as in the case of HIF-1α protein expression. Notably, NaHS did not affect basal expression of genes under 20% O2 conditions.
Inhibition of HIF-1 by NaHS was observed in cells from various kinds of tissues
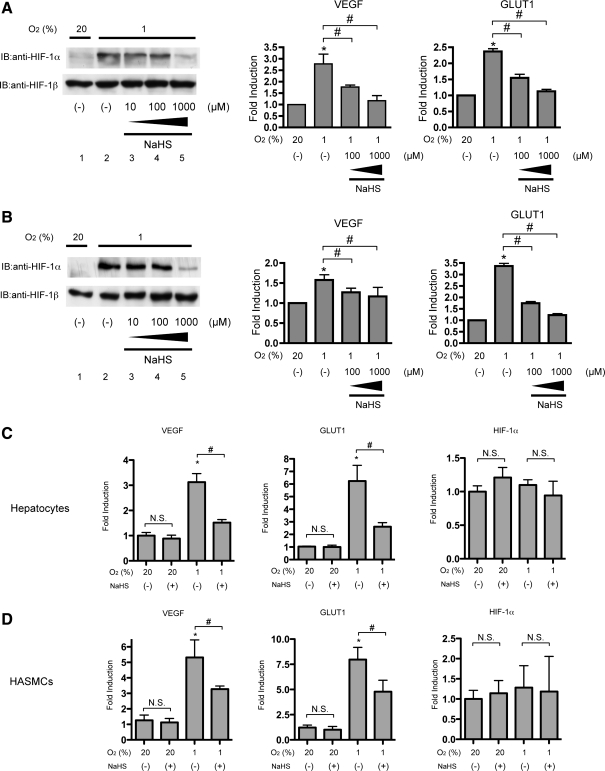
To investigate whether the effect of NaHS is observed in other types of cells, we tested neuronal SH-SY5Y cells (Fig. 2A) and HeLa cells (Fig. 2B). Cells of both cell lines were exposed to 1% O2 with or without 1000 μM NaHS for 4 h. Induction of HIF-1α protein expression was inhibited, but that of HIF-1β was not affected by NaHS treatment, as in the case of Hep3B cells (Fig. 2A, B; left panels). Induction of VEGF and GLUT1 mRNA expression was also inhibited by NaHS, as in the case of HIF-1α protein and Hep3B cells (Fig. 2A, B; right panels).
FIG. 2.
Inhibition of HIF-1 by NaHS was observed in cells from various kinds of tissues. SH-SY5Y cells (A) or HeLa cells (B) were exposed to the indicated concentrations of NaHS for 4 h under 20% or 1% O2. After treatment, cells were harvested for Western blot analysis, and whole-cell lysates were subjected to an immunoblot assay for HIF-1α and HIF-1β (A and B). Representative immunoblots are shown (A and B; left panels). SH-SY5Y cells (A; right panel), HeLa cells (B; right panel), primary cultured mouse hepatocytes (C), or human aortic smooth muscle cells (HASMCs) (D) were exposed to the indicated concentrations of NaHS for 8 h under 20% or 1% O2 and harvested for semi-quantitative RT-PCR by using primer pairs for indicated genes. Experiments were repeated at least thrice in triplicate. *p<0.05 compared with the control (20% and no treatment), #p<0.05 for comparisons between the indicated groups.
Next, we investigated the effect of NaHS on HIF-1-dependent gene expressions in primary cultured mouse hepatocytes (Fig. 2C) and human aortic smooth muscle cells (HASMCs) (Fig. 2D). Exposure to 1% O2 conditions induced expression of GLUT1 and VEGF in both primary cultured hepatocytes and HASMCs. In contrast, the basal expression of those genes was not affected by NaHS treatment under 20% O2 conditions. The induction was suppressed by 1 mM NaHS treatment. Expression of HIF-1α mRNA was not affected under 1% O2 conditions or NaHS treatment.
H2S donors inhibited hypoxia-induced HIF-1α stabilization
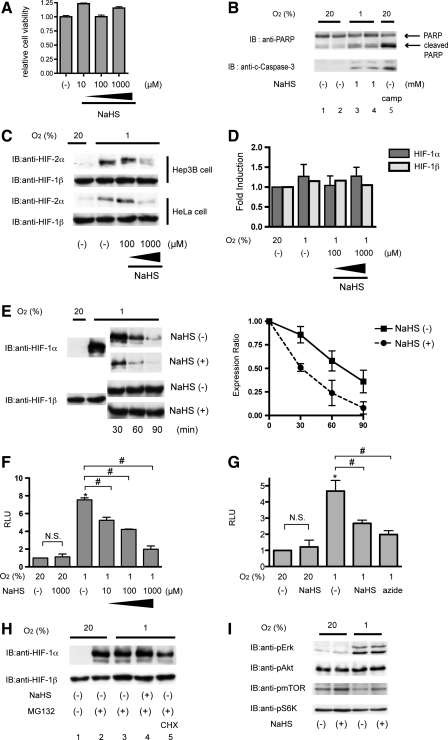
To explore the molecular mechanism by which H2S affects the accumulation of HIF-1α during hypoxia, we first examined the cytotoxic effect of H2S on Hep3B cells. A cytotoxicity assay using MTS/phenazine ethosulfate (PES) indicated that 1000 μM NaHS did not exert cytotoxicity within 4 h (Fig. 3A). Using the trypan blue exclusion dye assay, no significant cell death was observed at all the time points and concentrations tested (data not shown). In addition, cleaved-PARP and cleaved caspase-3 expression, which had been increased by hypoxic treatment, did not increase by exposure to 1000 μM NaHS (Fig. 3B). Thus, the inhibitory effect of H2S is not due to the cytotoxic effect to cells.
FIG. 3.
NaHS inhibited hypoxia-induced HIF-1α stabilization. (A and B) Hep3B cells were exposed to the indicated concentrations of NaHS for 4 h under 20% O2 conditions and subjected to a cytotoxicity assay by using MTS/PES as described in Materials and Methods section (A), and cells were subjected to an immunoblot assay for PARP and cleaved Caspase 3 expression (B). c-caspase-3; cleaved Caspase 3 (C) Hep3B cells or HeLa cells were exposed to the indicated concentrations of NaHS for 4 h under 20% or 1% O2. After treatment, cells were harvested for Western blot analysis, and whole-cell lysates were subjected to an immunoblot assay for HIF-1α or HIF-2α. (D) Hep3B cells were exposed to the indicated concentrations of NaHS for 8 h under 20% or 1% O2 and harvested for semi-quantitative RT-PCR by using primer sets for HIF-1α and HIF-1β. (E) Hep3B cells were exposed to 1% O2 for 4 h, and then cycloheximide (CHX) was added to a final concentration of 100 μM with or without 100 μM NaHS in a hypoxic workstation. Cells were incubated for 0–90 min, and whole-cell lysates were subjected to an immunoblot assay by using anti-HIF-1α or anti-HIF-1β antibodies (left panel). Relative intensities of HIF-1α signals were quantified by densitometric analysis of the immunoblots. Normalized values to time 0 as 1 were plotted against time (right panel). A representative blot is shown in the left panel. Results are mean±SD of three independent experiments. (F) Hep3B cells were transfected with the HIF-1–dependent reporter gene p2.1, encoding firefly luciferase, downstream of an HRE and SV40 promoter, and pRL-SV40, encoding Renilla luciferase. After 6-h incubation, cells were treated with the indicated concentrations of NaHS for 4 h. The ratio of firefly to Renilla luciferase activity was normalized to the value obtained for untreated cells to obtain a relative light unit (RLU). Results are mean±SD of three independent transfections. *p<0.05 compared with respective control; #p<0.05 for comparisons between the indicated groups (G) HeLa/ODD-Luc cells were exposed to 20% or 1% O2 conditions under treatment with 1000 μM NaHS or 2000 μM sodium azide for 4 h. Cells were harvested, and luciferase activity in each well was measured by using the same amount of cell lysate in the dual-luciferase reporter assay system (Promega). The luciferase activity was normalized to the value obtained for untreated cells to obtain a relative light unit (RLU). Results are mean±SD of three independent wells. *p<0.05 compared with respective control under 20% O2 conditions; #p<0.05 for comparisons between the indicated groups (H) Hep3B cells were treated with 1000 μM NaHS, 10 μM MG132, and/or 100 μM CHX for 4 h under 1% O2 and harvested for immunoblot assays for HIF-1α and HIF-1β. (I) Hep3B cells were exposed to 1000 μM NaHS under 20% and 1% O2 for 1 h. Cells were harvested, and lysates were subjected to an immunoblot assay. The phosphorylation status of Erk1/2, Akt, mTOR, and p70 S6K was examined in an immunoblot assay with anti-phosphospecific antibodies raised against the respective kinases. HRE, hypoxia-response element.
Next, we examined the effect of H2S on HIF-2α protein accumulation. In both Hep3B cells and HeLa cells, hypoxia-induced HIF-2α protein expression was also suppressed by NaHS treatment, thereby suggesting that the pathway common with both HIF-1α and HIF-2α is the target of H2S (Fig. 3C). The steady state of either HIF-1α mRNA or HIF-1β mRNA did not differ by exposure to 1% O2 or NaHS in Hep3B cells (Fig. 3D).
Since the primary mechanism leading to HIF-1α accumulation during hypoxia involves stabilization of HIF-1α proteins, we examined whether NaHS affected the intracellular stability of HIF-1 protein. For this purpose, Hep3B cells were incubated for 4 h under 1% O2 before the addition of cycloheximide (CHX) to block ongoing protein synthesis with or without NaHS treatment under the continued 1% O2 condition in a hypoxic workstation (Fig. 3E). After 30, 60, and 90 min, cells were harvested, and lysates were subjected to immunoblot analysis by using anti-HIF-1α and –HIF-1β antibodies (Fig. 3E; left panel). The HIF-1α protein level in the cells decreased significantly more rapidly with NaHS treatment than without NaHS treatment (Fig. 3E; right panel). Hep3B cells were transfected with the reporter p2.1 containing an HIF-1–dependent hypoxia response element (HRE). NaHS also inhibited hypoxia-induced HRE-dependent gene expression but not the basal expression under normoxia (Fig. 3F). Oxygen-dependent degradation domain (ODD) of HIF-1α fused to luciferase was expressed in the cells, which were then treated with NaHS (Fig. 3G). Exposure to 1% O2 induced luciferase activity. Treatment with 1 mM NaHS inhibited induction of luciferase activity by hypoxia as well as 2 mM sodium azide, thus indicating that NaHS decreased the stability of HIF-1α protein in an ODD-dependent manner under 1% O2. Together, this evidence demonstrates that H2S reduces the half life of HIF-1α protein in cells under hypoxic conditions.
Next, the effect of NaHS on HIF-1α protein synthesis was examined (Fig. 3H). Exposure of cells to 10 μM of the proteasome inhibitor Z-Leu-Leu-Leu-al (MG132) resulted in accumulation of HIF-1α protein (Fig. 3H, lanes 1 and 2), because MG132 inhibits HIF-1α protein degradation. The translation inhibitor CHX, which blocks ongoing protein synthesis, inhibited the accumulation of HIF-1α induced by MG132 (lane 5). In contrast, NaHS did not affect the HIF-1α protein expression induced by MG132 (lanes 3 and 4), thus indicating that H2S does not affect the neosynthesis of HIF-1α protein. Moreover, NaHS did not affect the phosphorylation status of Erk1/2, Akt, mTOR, or p70 S6K, which play a significant role in HIF-1α protein neosynthesis under normoxia or hypoxia (Fig. 3I).
NaHS inhibited hypoxia-induced but not anoxia-induced HIF-1 activation
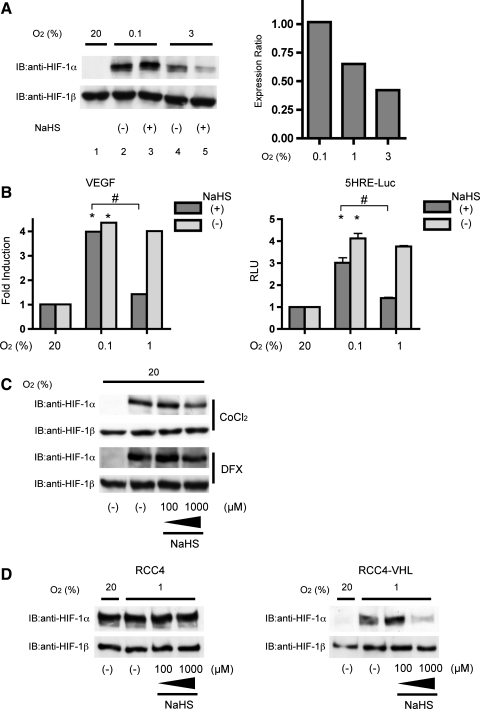
We investigated the effect of H2S on HIF-1α protein expression under 0.1% and 3% O2 conditions in Hep3B cells. As shown in Figure 4A, an inhibitory effect of NaHS was not observed under the 0.1% O2 conditions. In contrast, an inhibitory effect was detected under the 3% O2 conditions (lanes 4 and 5). The inhibitory effect of NaHS on HIF-1α protein expression was O2-tension dependent (Fig. 4A, right panel). Hep3B cells were more sensitive to exposure to NaHS under 3% O2 than under 1% O2. Next, we examined the O2 tension dependence of the effect of NaHS on VEGF mRNA expression and HRE-dependent gene expression. Exposure to hypoxia significantly induced VEGF mRNA expression in 8 h in Hep3B cells. No difference was detected between the 0.1% and 1% O2 conditions. However, an inhibitory effect of NaHS was detected only under the 1% O2 condition in Hep3B cells (Fig. 4B; left panel). As in the case of VEGF mRNA, similar phenomena were observed in HRE-dependent gene expression in Hep3B cells. The suppressive effect of NaHS was more significant under 1% O2 than under 0.1% O2 (Fig. 4B; right panel). We also examined the effect of H2S on DFX- and CoCl2-induced HIF-1α expression under the 20% O2 condition. In contrast to its effect under the 1% O2 condition, NaHS did not significantly inhibit DFX- or CoCl2-induced HIF-1α protein expression (Fig. 4C).
FIG. 4.
NaHS inhibited hypoxia-induced but not anoxia-induced HIF-1α protein expression. (A) Hep3B cells were exposed to 1000 μM NaHS for 4 h under 20%, 3%, 1%, or 0.1% O2. Cells were harvested for Western blot analysis after treatment. Whole-cell lysates were subjected to an immunoblot assay for HIF-1α and HIF-1β. A representative immunoblot is shown (A; left panel). Band intensities were densitometrically analyzed from two independent immunoblots, and mean relative expression ratios of band density of NaHS-treated cells to those of cells without NaHS treatment are shown (A; right panel). (B) Hep3B cells were exposed to 1000 μM NaHS for 8 h under 20%, 1%, or 0.1% O2 and harvested for semi-quantitative RT-PCR with a primer set for VEGF. Results are mean±SD of three independent experiments (B; left panel). HeLa/5HRE-Luc cells were treated with the 1000 μM NaHS for 8 h under 20%, 1%, and 0.1% O2. The firefly luciferase activity was determined and normalized to the value required for untreated cells to obtain relative light units (RLU) (B; right panel). Results are mean±SD of three independent transfections. *p<0.05 compared with the control, #p<0.05 for comparisons between the indicated groups. (C) Hep3B cells were exposed to 100 μM CoCl2 or 100 μM DFX with or without the indicated concentrations of NaHS for 4 h. (D) RCC4 and RCC4-von Hippel-Lindau cells were exposed to 20% and 1% O2 with or without 1000 μM NaHS for 4 h. After treatment, cells were harvested, and whole-cell lysates were subjected to immunoblot assay for HIF-1α and HIF-1β.
Next, we investigated whether the inhibitory effect of H2S was VHL dependent. RCC4 cells, which genetically lack VHL, and their derivatives, RCC4-VHL cells, were tested. RCC4 cells expressed HIF-1α protein even under the 20% O2 conditions. Expression was unchanged under the 1% O2 conditions and was insensitive to NaHS up to 1000 μM (Fig. 4D; left panel). In contrast, RCC4-VHL cells, which exogenously express VHL, expressed HIF-1α minimally under the 20% O2 conditions. HIF-1α protein expression was induced under 1% O2, and induction was inhibited by 1000 μM NaHS (Fig. 4D; right panel), thus indicating that VHL is necessary for the suppressive effect of NaHS on HIF-1α protein accumulation under hypoxia.
Mitochondria are necessary for the inhibition of hypoxia-induced HIF-1 activation by H2S
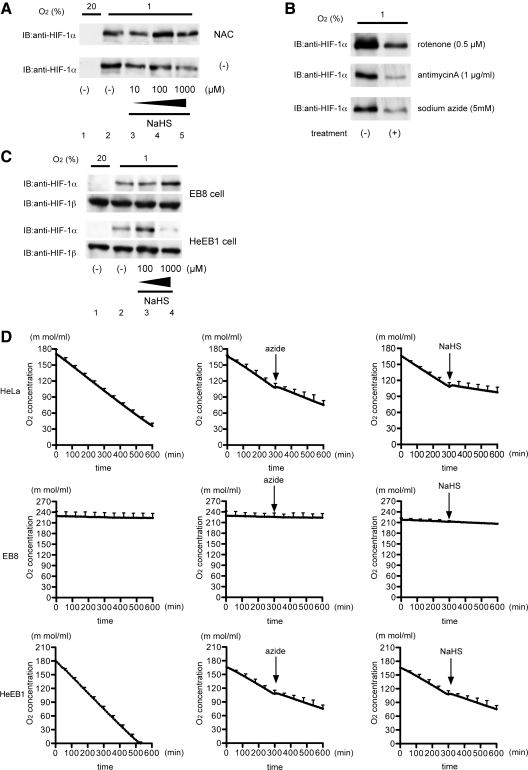
Since mitochondria play an essential role in hypoxia-induced HIF-1 activation and are target organelles of H2S in cells, we investigated the possible involvement of mitochondria in the inhibitory effect of H2S on HIF-1 activation. Reactive oxygen species (ROS), produced to different degrees by the various complexes of the mitochondrial electron transport chain (ETC) under hypoxic conditions, have been implicated in the regulation of HIF-1α protein stability. 10 mM N-acetylcysteine (NAC) treatment did not affect the hypoxia-induced HIF-1α accumulation (Fig. 5A, lane 2), but did reverse the inhibition of HIF-1α accumulation by NaHS (Fig. 5A, lanes 4 and 5). We tested the effects of various inhibitors of ETC on Hep3B cells under the 1% O2 condition for 4 h. HIF-1α protein accumulation was prevented by treatment with the different inhibitors including rotenone, antimycin A, and sodium azide as well as NaHS in Hep3B cells under the 1% O2 condition, thus indicating that inhibition of respiration at any site within ETC prevented HIF-1α protein accumulation (Fig. 5B). To investigate the involvement of mitochondria in inhibitory effect of H2S, we used EB8 cells and HeEB1 cells. Hypoxic treatment induced HIF-1α protein expression in both EB8 and HeEB1 cells (Fig. 5C; lanes 1 and 2). However, NaHS inhibited HIF-1α protein induction in only HeEB1 cells (lane 4), thus suggesting that mitochondria have an essential role in the inhibitory effect of H2S on HIF-1 activation. Since mitochondria are major O2 consumers in cells, we examined the impact of NaHS on O2 consumption in HeLa cells. As indicated in Figure 5C, 1 mM NaHS significantly suppressed O2 consumption to the same extent as did 2 mM sodium azide. O2 consumption was also examined in EB8 cells (HeLa cells lacking mtDNA) and HeEB1 cells (a hybrid clone of EB8 cells with mtDNA from wild-type HeLa cells).
FIG. 5.
Involvement of mitochondria in inhibition of HIF-1 activation by NaHS. (A) Hep3B cells were exposed to the indicated concentrations of NaHS with or without 10 mM NAC treatment for 4 h and harvested for immunoblot assays with anti-HIF-1α. (B) Hep3B cells were exposed to 1% O2 with (+) or without (−) treatment with the indicated reagents (0.5 μM rotenone, 1 μg/ml antimycin A, and 5 mM sodium azide). After treatment, cells were harvested, and whole-cell lysates were subjected to an immunoblot assay for HIF-1α. (C) EB8 and HeEB1 cells were exposed to 20% and 1% O2 and treated with the indicated concentrations of NaHS for 4 h. After treatment, the cells were harvested, and whole-cell lysates were subjected to an immunoblot assay for HIF-1α and HIF-1β. (D) Oxygen concentration curves were generated by using a Clark electrode for HeLa, EB8, and HeEB1 cell suspensions. Arrows indicate addition of 2 mM sodium azide or 1 mM NaHS. The slope of the curve is a measure of the rate of O2 consumption.
NaHS inhibited HIF-1–dependent gene expression in vivo
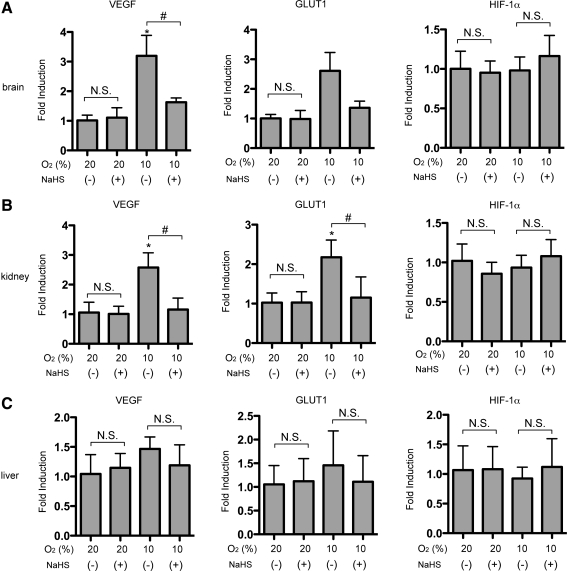
To explore whether H2S can influence the activation of HIF-1 or downstream gene expression in vivo, NaHS was intraperitoneally (i.p.) administered to mice under 20% or 10% O2 atmosphere for 4 h, and VEGF, GLUT-1, and HIF-1α mRNA levels in brain (Fig. 6A), kidney (Fig. 6B), and liver (Fig. 6C) were examined. Hypoxia-induced VEGF mRNA was inhibited in brain and kidney, and was also inhibited by NaHS (Fig. 6A, B; left panels). Hypoxic treatment of mice increased GLUT-1 mRNA levels in brain and kidney, and induction was significantly inhibited by concomitant use of NaHS (2.0 mg/kg) (Fig. 6A, B, middle panels). In liver, significant induction of either GLUT1 or VEGF mRNA was not observed at 10% O2 atmosphere, and mRNA expression was not affected by NaHS treatment. NaHS did not affect the gene expression under 20% O2 conditions (Fig. 6C). HIF-1α mRNA expression was not affected by either hypoxic treatment or NaHS exposure. There were no significant differences between control and NaHS groups in the vital parameters of blood pressure, heart rate, and SpO2 during the experiments (data not shown), thus suggesting that modulation of HIF-1 activation by H2S was not due to alteration of the respiratory or circulatory system.
FIG. 6.
NaHS inhibited HIF-1–dependent gene expression in vivo. NaHS (2.0 mg/kg) was intraperitoneally administered to mice under 20% and 10% O2 atmosphere for 4 h. VEGF, GLUT-1, and HIF-1α mRNA levels in brain (A), kidney (B), and liver (C) were analyzed by quantitative real-time RT-PCR. Results are mean±SD of three independent mice. *p<0.05 compared with the control (20% and no treatment), #p<0.05 for comparisons between the indicated groups.
Discussion
In this study, we demonstrated that the H2S donors NaHS and Na2S inhibited hypoxia-induced HIF-1 activation. Hypoxia-induced HIF-1α protein accumulation and HIF-1-downstream gene expression were suppressed by treatment with the H2S donors both in vitro and in vivo in a VHL- and mitochondria-dependent manner.
We used these H2S donors rather than gaseous H2S itself exclusively in this study. According to previous reports, the concentrations of the H2S donors NaHS and Na2S tested in this study were greater than physiological concentrations but within pharmacological concentrations. As for concentration of NaHS or Na2S adopted in this study, the concentration was from 10 to 1000 μM in the case of NaHS or from 10 to 100 μM in the case of Na2S. 100 μM of NaHS or 10 μM of Na2S showed a significant inhibitory effect. Multiple studies have demonstrated the cytoprotective effect of H2S that is observed from 10 μM to 5 mM in an in vitro setting and from 1 to 20 mg/kg i.p in an in vivo setting when administrated as NaHS (42). Thus, doses adopted in this study are within pharmacological dose of H2S donors. On the other hand, it is reported that values of labile H2S in plasma and blood vary mostly between 20 and 300 μM (20, 47, 48). Less than one-fifth of H2S is reported to exist in the undissociated form (H2S), which can evaporate from the medium; the remaining four-fifths existing as HS− at equilibrium with H2S in aqueous solutions with pH 7.4 at 37°C (24, 39). Another report claims that one-third of H2S+HS− remains after 15 min of administration, and >90% evaporates from the medium by 30 min (25). Although it has not been possible to determine the active form of H2S, the term “hydrogen sulfide” has been used (24). As demonstrated in Figure 1C, NaHS inhibited hypoxia-induced HIF-1α accumulation as early as 2 h (Fig. 1C, lanes 2–4), but the inhibitory effect was not observed at 8 h (Fig. 1C, lanes 6 and 7); re-administration of NaHS after 8 h inhibited HIF-1α accumulation (data not shown). Evidence indicates that the inhibitory effect of H2S is reversible, and the kinetics of inhibition is consistent with the half life of H2S in the medium. A cytotoxicity assay and trypan blue staining indicated that even 1 mM NaHS did not elicit cell death within 8 h of treatment (Fig. 3A), although H2S gas is known to be toxic (39, 42). An assay to examine the expression of cleaved PARP and caspase 3 also indicated that treatment with 1 mM NaHS does not induce apoptosis in cells (Fig. 3B).
The intracellular expression of HIF-1α protein is determined by the balance between its degradation and synthesis or stability and translation rate (15, 40). The results of the experiment using the protein translation inhibitor CHX indicated that H2S facilitated the degradation of HIF-1α protein under the 1% O2 conditions (Fig. 3E). Evidence that NaHS did not affect HIF-1α protein expression in cells treated with MG132 strongly suggest that H2S did not affect HIF-1α protein neosynthesis under the 1% O2 condition (Fig. 3H). Moreover, the phosphorylation status of the protein kinases Erk, Akt, mTOR, and p70 S6 kinase, which play a critical role in maintaining the machinery whereby HIF-1α mRNA is translated into protein, was not affected by NaHS treatment under both normoxic and hypoxic conditions (Fig. 3I). Taken together with the data showing that NaHS did not influence HIF-1α mRNA expression (Fig. 3D) and that degradation of ODD-luciferase was facilitated by NaHS (Fig. 3G), our results indicate that H2S prevents cells from stabilizing HIF-1α protein even under hypoxia, without affecting the translation of HIF-1α mRNA into protein. Notably, NaHS also inhibited the accumulation of HIF-2α protein, the stability of which is regulated by almost the same mechanism as that regulating the stability of HIF-1α protein in Hep3B cells under 1% O2 condition (28) (Fig. 3C). The O2 tension-dependent stability of HIF-α, including HIF-1α and HIF-2α, is mainly regulated by hydroxylation of HIF-α–specific peptides by the specific oxygenases PHD1–3 and the subsequent recognition of the hydroxylated HIF-α peptide by the tumor suppressor VHL (14). As indicated in Figure 4D, NaHS did not affect HIF-1α protein expression in VHL-deficient RCC4 cells. However, insensitivity was reversed by the introduction of VHL into RCC4 cells. Evidence strongly suggests that H2S affects cellular processes upstream of HIF-1α recognition by VHL in the signaling pathway from hypoxia to HIF-1α protein accumulation (11).
HIF-α stabilization during anoxia, such as that provided by the 0.1% O2 condition, or in response to DFX or CoCl2 does not seem to require mitochondria, because these conditions and reagents directly inhibit PHDs (11), thereby preventing recognition by protein complexes, including VHL. Interestingly, H2S did not inhibit the HIF-1α protein stabilization induced by these interventions (Fig. 4A–C). In contrast, H2S did inhibit HIF-1α protein stabilization under hypoxic conditions such as 1% and 3% O2, in which mitochondria are closely involved in HIF-1α stabilization. Although the bona fide nature of intracellular hypoxia sensors is still controversial, mitochondria play a critical role in hypoxia-induced HIF activation (6). Figure 5C demonstrates that mitochondria were essential organelles for the H2S-induced suppression of hypoxia-induced HIF-1 activation. In ρ0 EB8 cells, HIF-1α protein accumulation was observed in response to exposure to 1% O2, but H2S did not affect the protein accumulation (Fig. 5D). On the other hand, HeBE1 cells harboring mtDNA from wild-type HeLa cells were sensitive to H2S treatment, as were wild-type HeLa cells (Fig. 5D). Moreover, we demonstrated that both 1 mM NaHS and 2 mM sodium azide inhibited O2 consumption in a mitochondria-dependent manner (Fig. 5C). Taken together, our results suggest that H2S acts as a regulator of O2 consumption of mitochondria and intracellular O2 metabolism in mammalian cells and that H2S destabilizes HIF-1α protein under hypoxic conditions on inhibition of mitochondrial respiration, as indicated by the inhibition of HIF-1α protein stabilization in hypoxia by NO (12). NaHS did not affect the phosphorylation status of Erk1/2, Akt, mTOR, or p70 S6K, which play a significant role in HIF-1α protein neosynthesis (Fig. 3I), although it is reported that the H2S donor diallyl sulfide (DAS) induced hemeoxygenase-1 through MAPK pathway (10). Gong et al. reported that DAS activates ERK in an ROS-dependent manner. In contrast, we indicated that induction of HIF-1α protein accumulation by NaHS is not inhibited by NAC treatment (Fig. 5A). It is still elusive that DAS-dependent MAPK activation is totally dependent on H2S.
By analogy with protein S-nitrosylation, protein S-sulfhydration has been proposed as a mechanism for the modification of cellular function mediated by H2S (33, 37). H2S physiologically modifies cysteines in a large number of proteins by S-sulfhydration, thus converting sulfhydryl (P-SH) groups to persulfide groups (P-SSH), which appears to be another type of posttranslational modification of proteins. It is known that the covalent modification in S-sulfhydration is reversed by reducing agents, such as NAC and DTT, similar to nitrosylation by NO (33). In the current study, treatment with the antioxidant NAC attenuated the H2S-elicited inhibition of HIF-1α protein accumulation (Fig. 5A). Thus, disturbance of the intracellular redox balance by H2S may affect the hypoxia signaling pathway to HIF-1 activation.
In an in vivo study demonstrated as Figure 6, we did not observe the induction of HIF-1-dependent gene expression inhibitory effect of H2S in liver under 10% O2 atmosphere. On the other hand, we also demonstrated that H2S inhibited HIF-1-dependent gene expression in primary cultured mouse hepatocytes (Fig. 2C). One of the reasons of the discrepancy may be that 10% O2 atmosphere, which induces HIF-1 activation in brain and kidney, is not low enough to induce the HIF-1 activation in liver.
A recent report describes that the 300 μM of NaHS increases expression of HIF-1α protein and VEGF induced by CoCl2 not by hypoxia in rat brain capillary endothelial cells and rat aortic vascular smooth muscle cells within 1 h (27). In the study, the effect of NaHS on hypoxia-induced HIF-1 activation is not investigated. In contrast, we find that NaHS does inhibit hypoxia-induced HIF-1 activation in HASMCs (Fig. 2D) and human umbilical vein endothelial cells (data not shown) in the dose of 300 μM (data not shown) and 1000 μM of NaHS. The mechanisms behind the discrepancy are largely elusive at this moment and should be investigated as a further study. A previous study reported that H2S elicited HIF-1 activation in a C. elegans model (2). Worms were exposed to 50 ppm gaseous H2S for 45 min, and the protein expression of HIF-1α was examined. Exposure to 50 ppm H2S increased HIF-1α protein expression as effectively as exposure to a 0.5% O2 atmosphere in a VHL-independent but EGLN-1–dependent manner. However, the effects of H2S on hypoxia-induced HIF-1α protein accumulation and gene expression were not investigated in the study. We did not detect an increase in HIF-1α protein or HIF-1–dependent gene expression on treatment with NaHS under the normoxic condition in Hep3B or SH-SY5Y cells. The difference in species may explain the discrepancy between our results and the previous results in C. elegans, although there is no experimental evidence for this at this moment.
Metabolic “hypoxia,” a pathophysiological state in which the use of O2 in mitochondrial respiration is prevented, is known to affect HIF-1 activation under hypoxic conditions, despite the presence of O2 (12). In this study, we demonstrated that inhibition of mitochondrial O2 consumption by H2S, resulted in a destabilization of HIF-1α protein in a VHL-dependent manner. The physiological and pathophysiological implications of these observations remain to be investigated as future studies.
Materials and Methods
Cell culture and reagents
Human hepatoma Hep3B cells and human cervical carcinoma HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Human neuroblastoma SH-SY5Y cells were maintained in RPMI 1640 medium that contained 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. The characteristics of EB8 cells (HeLa cells lacking mtDNA) and HeEB1 cells (a hybrid clone of EB8 cells with mtDNA from wild-type HeLa cells) were described elsewhere (7). They were cultured in RPMI 1640 supplemented with heat-inactivated FBS, 50 g/ml uridine, and 0.1 mg/ml pyruvate. RCC4 cells stably transfected with pcDNA3-VHL (RCC4/VHL) or empty vector (RCC4) were maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. HASMCs were obtained from Kurabo (Osaka, Japan). Primary cultured mouse hepatocytes were prepared by following a protocol previously described (1).
The H2S donors NaHS and Na2S, the iron chelator DFX, sodium azide, antimycin A, and rotenone were obtained from Sigma (St. Louis, MO). The protein synthesis inhibitor CHX and the cell-permeable proteasome inhibitor MG132 were obtained from Calbiochem (San Diego, CA). Anti-HIF-1α antibodies were obtained from BD Biosciences (San Jose, CA), and anti-HIF-1β antibody was obtained from Novus Biologicals (Littleton, CO). Anti-β-actin antibody was purchased from Sigma. Anti-phosphorylated p44/42MAPK (Thr-202/Tyr-204), Akt (Ser-473), the mTOR (Ser 2448), and p70 S6 kinase (S6K) (Thr 389) antibodies were obtained from Cell Signaling Technology (Beverly, MA). Anti-poly-(ADP-ribose) polymerase (PARP) and-cleaved Caspase-3 antibodies were from Cell Signaling Technology.
Hypoxic treatment
Cells were maintained in a multigas incubator (APMW-36; Astec, Fukuoka, Japan) and exposed to hypoxic conditions (1% O2–5% CO2–94% N2) (34, 35, 44).
Immunoblot assays
Whole-cell lysates were prepared as previously described (43). Briefly, whole-cell lysates were prepared by using ice-cold lysis buffer [0.1% SDS, 1% Nonidet P-40 (NP-40), 5 mM EDTA, 150 mM NaCl, 50 mM Tris-Cl (pH 8.0), 2 mM DTT, 1 mM sodium orthovanadate, and Complete Protease Inhibitor™ (Roche Diagnostics, Tokyo, Japan) by using a protocol previously described (49). Samples were centrifuged at 10,000 ×g to form a pellet from the cell debris. For HIF-1α and HIF-1β, 100 μg of protein was fractionated by SDS-PAGE (7.5% gel) and subjected to an immunoblot assay by using primary antibodies at 1:1000 dilution. Anti-β-actin mouse monoclonal antibody (Sigma) was used as a control at 1:5000 dilution. Horseradish peroxidase-conjugated to sheep anti-mouse IgG (GE Healthcare, Piscataway, NJ) was used as a secondary antibody at 1:1000 dilution. The signal was developed by using enhanced chemiluminescence reagent (GE Healthcare). Experiments were repeated at least thrice. The intensity of each band was quantified with Image J software [Tanaka, 2010 #232;Wakamatsu, 2009 #173] for statistical analysis.
Quantitative reverse transcriptase-PCR analysis
RNA was purified by using RNeasy™ (Qiagen, Valencia, CA) and treated with DNase. First-strand synthesis and real-time PCR were performed by using the QuantiTect SYBR green PCR kit (Qiagen) according to the manufacturer's protocol. PCR and detection were performed by using a 7300 real-time PCR system (Applied Biosystems, Foster City, CA). PCR primers were purchased from Qiagen. The relative change in expression of each target mRNA relative to 18S rRNA was calculated (43, 44).
Reporter gene assay
Reporter plasmid p2.1, harboring a 68-bp HRE from the human enolase 1 gene inserted upstream of an SV40 promoter and Photinus pyralis (firefly) luciferase coding sequences, was previously described (18, 22). Cells were plated (5×104 per well) on the day before transfection. In each transfection, 200 ng of reporter gene plasmid p2.1 and 50 ng of the control plasmid pRL-SV40 (Promega, Madison, WI) containing the SV40 promoter upstream of Renilla reniformis (sea pansy) luciferase coding sequences were premixed with Fugene 6 transfection reagent (Roche Diagnostics). Cells were exposed to each condition as indicated and harvested. Luciferase activity was determined by using the dual-luciferase reporter assay system™ (Promega). The ratio of firefly to sea pansy luciferase activity was determined (22). When indicated, HeLa cells stably harboring reporter gene plasmid pGL3/5HRE-Luc (HeLa/5HRE-Luc) (44) were subjected to each condition and treated with each reagent for 4 h. Cell lysates were subjected to luciferase assay (Promega) as previously described.
At least two independent transfections were performed in triplicate for each experiment.
HIF-1α stability assay using reporter construct containing HIF-1α-ODD domain
pGL3/ODD-Luc plasmid (13) was digested with both XhoI and XbaI, and the resultant DNA fragment composed of SV40 promoter and ODD-Luc coding sequence was inserted between XhoI-XbaI site of p5HRE-Luc plasmid (pEF/ODD-Luc) to produce pGL3/5HRE-Luc plasmid. HeLa/ODD-Luc cells were subjected to each condition and treated with each reagent for 4 h. Cells were harvested, and luciferase activity in each well was measured by using the same amount of cell lysate in the dual-luciferase reporter assay system (Promega). At least two independent experiments were performed in triplicate for each experiment, and representative data are shown.
Cytotoxicity assay
Changes in the cellular viability of NaHS-treated cells were determined by the CellTiter 96® AQueous One Solution cell proliferation assay (Promega). The assay uses a colorimetric method to determine the number of viable cells in cytotoxicity assays. Hep3B cells were seeded in 96-well plates at a density of 2.0×104 per well (in 100 μl medium). After 24 h, NaHS was added at different concentrations. After an additional 4 h of incubation, MTS ([3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt]/PES solution was added, and incubation was continued for 30 min. The absorbance of individual wells was then measured at a wavelength of 490 nm corrected to 650 nm by using a Thermo Max™ microplate reader (Molecular Devices, Sunnyvale, CA).
Measurement of total cellular O2 consumption
Cells were trypsinized and suspended at 1×107 cells per ml in DMEM with 10% FBS and 25 mM HEPES buffer. For each experiment, equal numbers of cells suspended in 0.4 ml were pipetted into the chamber of an Oxytherm electrode unit (Hansatech Instruments, Norfork, United Kingdom), which uses a Clark-type electrode to monitor the dissolved O2 concentration in the sealed chamber over time (49). The data were exported to a computerized chart recorder (Oxygraph; Hansatech Instruments) that calculated the rate of O2 consumption. The temperature was maintained at 37°C during measurement. The O2 concentration in 0.4 ml of DMEM medium without cells was also measured over time to provide background values. O2 consumption experiments were repeated at least thrice. Data were expressed as mean±SD.
Animal study
Six-week-old male BALB/CA mice were purchased from CLEA Japan (Shiga, Japan). Food and water were available ad libitum, and mice were maintained under controlled environmental conditions (24°C, 12 h light/dark cycle) (43). Mice were divided into 4 groups: normoxia (exposed to 20% O2 for 4 h), hypoxia (exposed to 10% O2 for 4 h), normoxia+NaHS (exposed to 20% O2 with NaHS i.p. 2.0 mg/kg for 4 h), and hypoxia+NaHS (exposed to 10% O2 with NaHS i.p. 2.0 mg/kg for 4 h). Mice were placed in a polypropylene chamber, and O2 and N2 mixed gas was delivered to the chamber at a flow rate of 3 L/min by using an anesthetic machine (Custom50; Aika, Tokyo, Japan). Animal protocols were approved by the Animal Research Committee of Kyoto University, and all experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Blood pressure, heart rate, and peripheral O2 saturation (SpO2) during experiments were measured with a tail-cuff sphygmomanometer (model MK-1030; Muromachi Kikai, Tokyo, Japan) (41) and a MouseOx pulse oximeter (Starr Life Sciences, Oakmont, PA) (5). At the end of the experiments, mice were killed by cervical dislocation. The brains, kidneys, and livers were rapidly removed, frozen in liquid nitrogen, and stored at −80°C for subsequent determinations.
Statistical analysis
Data were expressed as mean±SD and analyzed by one-way analysis of variance followed by the Newman–Keuls test using Prism version 4c (Graphpad Inc., La Jolla, CA). A p-value<0.05 was considered significant (43).
Abbreviations Used
- CHX
cycloheximide
- DAS
diallyl sulfide
- DFX
desferroxamine
- DMEM
Dulbecco's modified Eagle's medium
- ETC
electron transport chain
- FBS
fetal bovine serum
- FRAP
FKBP/rapamycin-associated protein
- GLUT1
glucose transporter 1
- HASMC
human aortic smooth muscle cells
- H2S
hydrogen sulfide
- HIF-1
hypoxia-inducible factor 1
- HRE
hypoxia response element
- LDHA
lactate dehydrogenase A
- MG132
Z-Leu-Leu-Leu-al
- mTOR
mammalian target of rapamycin
- Na2S
sodium sulfide
- NAC
N-acetylcysteine
- NaHS
sodium hydrosulfide
- PDK-1
pyruvate dehydrogenase kinase 1
- PES
phenazine ethosulfate
- ROS
reactive oxygen species
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant #223902980 and 22659283) to K.H.
The authors thank Dr. Fumito Ichiniose at Harvard University for the critical reading of this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Azuma H. Hirose T. Fujii H. Oe S. Yasuchika K. Fujikawa T. Yamaoka Y. Enrichment of hepatic progenitor cells from adult mouse liver. Hepatology. 2003;37:1385–1394. doi: 10.1053/jhep.2003.50210. [DOI] [PubMed] [Google Scholar]
- 2.Budde M. Roth M. Hydrogen sulfide increases HIF-1 activity independent of VHL-1 in C. elegans. Mol Biol Cell. 2010;21:212–217. doi: 10.1091/mbc.E09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin BY. Jiang G. Wegiel B. Wang HJ. Macdonald T. Zhang XC. Gallo D. Cszimadia E. Bach FH. Lee PJ. Otterbein LE. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci USA. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper CE. Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 5.Early MA. Lishnevsky M. Gilchrist JM. Higgins DM. Orme IM. Muller WA. Gonzalez-Juarerro M. Schenkel AR. Non-invasive diagnosis of early pulmonary disease in PECAM-deficient mice using infrared pulse oximetry. Exp Mol Pathol. 2009;87:152–158. doi: 10.1016/j.yexmp.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emerling BM. Chandel NS. Oxygen sensing: getting pumped by sterols. Sci STKE. 2005;2005:pe30. doi: 10.1126/stke.2892005pe30. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto N. Koshikawa N. Gassmann M. Hayashi J. Takenaga K. Hypoxic induction of hypoxia-inducible factor-1alpha and oxygen-regulated gene expression in mitochondrial DNA-depleted HeLa cells. Biochem Biophys Res Commun. 2002;297:346–352. doi: 10.1016/s0006-291x(02)02186-1. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda R. Hirota K. Fan F. Jung YD. Ellis LM. Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 9.Ganster F. Burban M. de la Bourdonnaye M. Fizanne L. Douay O. Loufrani L. Mercat A. Cales P. Radermacher P. Henrion D. Asfar P. Meziani F. Effects of hydrogen sulfide on hemodynamics, inflammatory response and oxidative stress during resuscitated hemorrhagic shock in rats. Crit Care. 2010;14:R165. doi: 10.1186/cc9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong P. Hu B. Cederbaum AI. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch Biochem Biophys. 2004;432:252–260. doi: 10.1016/j.abb.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Guzy RD. Hoyos B. Robin E. Chen H. Liu L. Mansfield KD. Simon MC. Hammerling U. Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Hagen T. Taylor CT. Lam F. Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 13.Harada H. Kizaka-Kondoh S. Hiraoka M. Optical imaging of tumor hypoxia and evaluation of efficacy of a hypoxia-targeting drug in living animals. Mol Imaging. 2005;4:182–193. doi: 10.1162/15353500200505112. [DOI] [PubMed] [Google Scholar]
- 14.Hirota K. Semenza GL. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun. 2005;338:610–616. doi: 10.1016/j.bbrc.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 15.Hirota K. Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Hochachka PW. Buck LT. Doll CJ. Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang LE. Willmore WG. Gu J. Goldberg MA. Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–9044. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 18.Jiang BH. Rue E. Wang GL. Roe R. Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 19.Kabil O. Banerjee R. The redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajimura M. Fukuda R. Bateman RM. Yamamoto T. Suematsu M. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal. 2010;13:157–192. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiba M. Kajimura M. Goda N. Suematsu M. From O2 to H2S: a landscape view of gas biology. Keio J Med. 2002;51:1–10. doi: 10.2302/kjm.51.1. [DOI] [PubMed] [Google Scholar]
- 22.Kasuno K. Takabuchi S. Fukuda K. Kizaka-Kondoh S. Yodoi J. Adachi T. Semenza GL. Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–2558. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 23.Kimura H. Hydrogen sulfide as a biological mediator. Antioxid Redox Signal. 2005;7:778–780. doi: 10.1089/ars.2005.7.778. [DOI] [PubMed] [Google Scholar]
- 24.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 25.Kimura Y. Dargusch R. Schubert D. Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 26.Laughner E. Taghavi P. Chiles K. Mahon PC. Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X. Pan L. Zhuo Y. Gong Q. Rose P. Zhu Y. Hypoxia-inducible factor-1alpha is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol Pharm Bull. 2010;33:1550–1554. doi: 10.1248/bpb.33.1550. [DOI] [PubMed] [Google Scholar]
- 28.Loboda A. Jozkowicz A. Dulak J. HIF-1 and HIF-2 transcription factors—similar but not identical. Mol Cells. 2010;29:435–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 29.Mateo J. Garcia-Lecea M. Cadenas S. Hernandez C. Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maxwell P. Wiesener M. Chang G. Clifford S. Vaux E. Cockman M. Wykoff C. Pugh C. Maher E. Ratcliffe P. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 31.Minamishima S. Bougaki M. Sips PY. Yu JD. Minamishima YA. Elrod JW. Lefer DJ. Bloch KD. Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mok YY. Atan MS. Yoke Ping C. Zhong Jing W. Bhatia M. Moochhala S. Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br J Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustafa AK. Gadalla MM. Sen N. Kim S. Mu W. Gazi SK. Barrow RK. Yang G. Wang R. Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oda S. Oda T. Nishi K. Takabuchi S. Wakamatsu T. Tanaka T. Adachi T. Fukuda K. Semenza GL. Hirota K. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS One. 2008;3:e2215. doi: 10.1371/journal.pone.0002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oda T. Hirota K. Nishi K. Takabuchi S. Oda S. Yamada H. Arai T. Fukuda K. Kita T. Adachi T. Semenza GL. Nohara R. Activation of hypoxia-inducible factor 1 during macrophage differentiation. Am J Physiol Cell Physiol. 2006;291:C104–C113. doi: 10.1152/ajpcell.00614.2005. [DOI] [PubMed] [Google Scholar]
- 36.Olson K. Whitfield N. Bearden S. St Leger J. Nilson E. Gao Y. Madden J. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol. 2010;298:R51–R60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagliaro P. Moro F. Tullio F. Perrelli M-G. Penna C. Cardioprotective pathways during reperfusion: focus on redox signaling and other modalities of cell signaling. Antioxid Redox Signal. 2011;14:833–850. doi: 10.1089/ars.2010.3245. [DOI] [PubMed] [Google Scholar]
- 38.Peng Y-J. Nanduri J. Raghuraman G. Souvannakitti D. Gadalla MM. Kumar GK. Snyder SH. Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiffenstein RJ. Hulbert WC. Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 40.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology. 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 41.Shiuchi T. Cui TX. Wu L. Nakagami H. Takeda-Matsubara Y. Iwai M. Horiuchi M. ACE inhibitor improves insulin resistance in diabetic mouse via bradykinin and NO. Hypertension. 2002;40:329–334. doi: 10.1161/01.hyp.0000028979.98877.0c. [DOI] [PubMed] [Google Scholar]
- 42.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T. Wakamatsu T. Daijo H. Oda S. Kai S. Adachi T. Kizaka-Kondoh S. Fukuda K. Hirota K. Persisting mild hypothermia suppresses hypoxia-inducible factor-1alpha protein synthesis and hypoxia-inducible factor-1-mediated gene expression. Am J Physiol Regul Integr Comp Physiol. 2010;298:R661–R671. doi: 10.1152/ajpregu.00732.2009. [DOI] [PubMed] [Google Scholar]
- 44.Wakamatsu T. Tanaka T. Oda S. Nishi K. Harada H. Daijo H. Takabuchi S. Kai S. Fukuda K. Hirota K. The intravenous anesthetics barbiturates inhibit hypoxia-inducible factor 1 activation. Eur J Pharmacol. 2009;617:17–22. doi: 10.1016/j.ejphar.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 45.Wallace JL. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid Redox Signal. 2010;12:1125–1133. doi: 10.1089/ars.2009.2900. [DOI] [PubMed] [Google Scholar]
- 46.Wang GL. Jiang BH. Rue EA. Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whiteman M. Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J Cell Mol Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitfield NL. Kreimier EL. Verdial FC. Skovgaard N. Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H. Gao P. Fukuda R. Kumar G. Krishnamachary B. Zeller KI. Dang CV. Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]