Abstract
This study aimed to determine whether vital exhaustion (VE) was associated with BMI cross-sectionally and after 3 and 6 years of follow-up. Extant data from the Atherosclerosis Risk in Communities (ARIC) study were used to examine the relationship between VE and BMI among 13,727 white and African-American adults cross-sectionally (baseline) and longitudinally (3 and 6 years later). We used adjusted and nonadjusted general linear regression models. Associations with excess weight gain (≥5.0%) were also examined using logistic regression. Results showed that BMI was significantly higher among both white and African-American men and women in the highest VE quartile compared to those with no VE. Similarly, high VE at baseline was associated with higher BMI 3 and 6 years later, although VE was not able to predict future BMI after adjusting for baseline BMI. Baseline VE predicted future excess weight gain in white men and women, but not in African Americans. These results suggest that reducing VE levels may play an important role in reducing the prevalence of obesity. High VE was associated with higher current BMI (all races) and excess weight gain (whites only). Although high VE predicted future weight gain without baseline BMI adjustment, the magnitude of change in BMI over time was similar among those with low and high VE; suggesting that any relationship between VE and BMI was already established at baseline. Assessment of VE and BMI over time would help to elucidate uncertainties between the temporal nature of the relationship between them.
INTRODUCTION
Some studies suggest that obesity and negative psychological states may be related, although currently the evidence is not conclusive (1). Understanding the relationship between obesity and psychological distress has important clinical implications in improving the quality of life for overweight individuals. In addition, poor psychological states often interfere with the success of weight loss intervention programs (2–5) and may be negatively associated with maintenance of weight loss (6). One weakness of this literature is that most studies have been cross-sectional. Furthermore, different effects have been observed by gender, with the relationship between overweight and poor psychological state usually greater in women than men (7,8). Associations are also often dependent on the level of obesity, with some studies only finding a relationship in individuals with morbid levels of obesity (1).
Most of the research to date on the association between obesity and negative emotions has focused on depression, with less known about the association between obesity and other psychological pathologies. Limited evidence indicates that obesity may be linked to other psychological states. For example, overweight individuals have been found to have a higher than average prevalence of anxiety, low self-esteem, eating disorders, hostility, and poor quality of life (5,9–11).
Vital exhaustion (VE) is a negative psychological state characterized by excessive fatigue, irritability, and feelings of demoralization (12). Although VE is correlated with depression (13), it is often considered conceptually distinct (14). Understanding the relationship between VE and obesity is of value because the disorders share similar comorbidities. For example, it is estimated that between 30 and 60% of cardiac events are preceded by VE independent of clinical indicators of disease severity (12,15), and obesity is independently associated with psychological stress and cardiovascular disease (CVD) (16–18). Huijbrechts et al. (19) studied the relationship between a variety of mental states (including VE) and BMI (kg/m2) in 166 participants who had been hospitalized for myocardial infarction (MI). Of the eight personality characteristics studied, VE was the only factor which significantly predicted increased BMI 5 months after the MI event (β = 0.14, P = 0.000). Thus, it is possible that obesity mediates the relationship between VE and CVD. For example, VE may be higher in the obese because of increased psychological stress, leading to further increased CVD risk. This theory has not been tested and there is limited evidence that there is an association between VE and obesity.
We examined the association between obesity and VE using data from the Atherosclerosis Risk in Communities (ARIC) study. We aimed to determine whether the level of VE was associated with: (i) current BMI; (ii) BMI 3 and 6 years later; and (iii) excess weight gain over 3 and 6 years. We hypothesized that VE would be positively related to BMI and excess weight gain in white and African-American men and women.
METHODS AND PROCEDURES
The ARIC study is a prospective investigation of the natural history and etiology of atherosclerosis and CVD. A community based sample of 15,792 white and African-American men and women 45– 64 years of age were recruited from four communities in the United States: Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the northwestern suburbs of Minneapolis, Minnesota. Three follow-up visits occurred at ~3-year intervals. The follow-up rates among participants who were alive at each visit were 92.7, 85.6, and 80.8% at visits 2, 3, and 4, respectively. Full details of the ARIC data collection methodology are described elsewhere (20,21). This study was approved by the University of North Carolina at Chapel Hill Public Health Institutional Review Board on research involving human subjects and by at Institutional Review Boards at all participating centers.
Measures and covariates
VE was assessed using the Maastricht questionnaire at the second ARIC examination (1990–1992). This examination is referred to as “baseline” hereon. The Maastricht questionnaire is a 21-item questionnaire used to characterize excessive fatigue, irritability, and feelings of demoralization (12,15). Items were selected during the development of the questionnaire as those found to discriminate between future cases of coronary heart disease and a healthy group of controls in men (12,15) and women (22). Other studies have since demonstrated a relationship between scores and future incidence of MI (15,22–24). VE scores were derived based on the response categories: 0 = no; 1 = do not know; and 2 = yes (except for two items, which were negatively scored: 0 = yes; 1 = do not know; and 2 = no). Scores were obtained for each item and summed to produce an overall score. On the basis of the overall population distribution of VE scores for the whole sample, we created quartiles of VE; none (≤3); low (4–8); moderate (9–15); and high (≥16).
Participants were weighed without shoes in a scrub suit to the nearest pound (1 pound = 0.454 kg) using a beam balance scale at all visits. Height (without shoes) was measured to the nearest centimeter using a metal ruler attached to a wall and a standard triangular headboard. BMI (weight (kg)/height (m2)) was categorized as normal weight (18.5 to <25.0), overweight (25.0 to <30.0), or obese (≥30.0) based on World Health Organization criteria (25). We also created a >5% weight gain variable, because weight loss targets are commonly described as percentages in clinical settings (16) and the proposed definition of weight maintenance is ±3.0% (26). The 5.0% weight gain variable was created between baseline (2nd examination) and 3 years later, and between baseline and 6 years later.
Age (date of birth), race/ethnicity, and gender were self-reported during the recruitment phase and confirmed during the first clinical visit. Alcoholic beverage consumption and cigarette smoking status were self-reported at each clinic visit and defined as current, former, or never. Educational attainment level was ascertained at visit 1 and defined as: having less than high school education; high school graduate; or at least some college. Participants reported their marital status from the categories: married, widowed, divorced, separated, and never married, which we combined to form a dichotomous variable for married (married) or single (all other responses) because there were insufficient numbers of participants in some categories.
Exclusions
African Americans from Washington County, MD or Minneapolis, MN (n = 55) and participants that classified their ethnicity as other than white or African American (n = 48) were excluded because they were too small in number to allow ethnic and center-specific analyses. In addition, participants were excluded if they died before baseline (2nd examination; n = 310); did not attend the baseline examination (n = 1,122); were not administered the Maastricht questionnaire (n = 86), or did not fully complete all items (n = 248); missing BMI at baseline (n = 36); were underweight (BMI <18.5 kg/m2) at baseline (n = 119); or were missing any pertinent covariates at baseline (n = 41).
Statistical analysis
We found statistical significant interactions (P < 0.05) between race and gender and VE quartiles; therefore, all analyses were run separately for the four race–gender groups. General linear regression models (PROC GLM) were used to compare mean BMI levels across VE groups. The LSMEANS option was used to estimate the adjusted mean BMI for each VE group and to determine whether the mean BMI level among the “no VE” group was significantly different (P < 0.05) from the other VE groups (low, moderate, and high VE). Logistic regression models (PROC LOGISTIC) were used to determine whether excess weight gain over 3 and 6 years were associated with VE levels. Regression model 1 was adjusted for age, field center, level of education, smoking status, drinking status, and marital status. Model 2 was adjusted for the same variables as model 1, plus baseline BMI. Data were analyzed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Participant demographics are shown in Table 1. Overall, 55% were females and the mean age was 57.0 years. White and African-American men and women with moderate and high levels of VE were older, more likely to have less than a high school education, and be current smokers compared to adults with no VE in the same race–gender group. A smaller percentage of white women and men with moderate and high VE levels consumed alcoholic beverages compared to white men and women with no VE. There were no associations between VE and alcoholic beverage consumption among African-American women and men. White and African-American men and women with high VE levels were less likely to be married than those with low VE levels.
Table 1.
Mean (s.d.) or percentage distribution of selected demographic characteristics by race–gender groups; the Atherosclerosis Risk in the Communities (ARIC) study, 1987–1998
| Vital exhaustiona |
||||
|---|---|---|---|---|
| None | Low | Moderate | High | |
| White women (n) | 975 | 1,539 | 1,402 | 1,510 |
| Age (years, mean (s.d.)) | 56.2 (5.5) | 56.5 (5.6) | 57.1 (5.7)* | 57.2 (5.7)* |
| Education level (% <high school) | 8.3 | 11.2* | 14.9* | 23.6* |
| Smoking status (% current) | 16.4 | 17.5 | 22.3* | 25.9* |
| Drinking status (% current) | 67.9 | 63.9 | 60.0* | 51.9* |
| Marital status (% married) | 84.2 | 82.2 | 81.5 | 78.2* |
| African-American women (n) | 226 | 462 | 592 | 837 |
| Age (years, mean (s.d.)) | 55.1 (5.8) | 55.5 (5.6) | 56.3 (5.8)* | 56.6 (5.7)* |
| Education level (% <high school) | 19.0 | 29.2* | 37.2* | 49.0* |
| Smoking status (% current) | 17.7 | 16.7 | 21.1 | 22.6* |
| Drinking status (% current) | 25.2 | 27.9 | 24.7 | 22.5 |
| Marital status (% married) | 55.8 | 55.8 | 52.2 | 47.3* |
| White men (n) | 1,629 | 1,572 | 976 | 776 |
| Age (years) | 57.0 (5.6) | 57.8 (5.7)* | 57.9 (5.7)* | 58.2 (5.8)* |
| Education level (% <high school) | 10.9 | 15.4* | 18.3* | 28.4* |
| Smoking status (% current) | 17.5 | 19.8 | 23.6* | 28.1* |
| Drinking status (% current) | 72.4 | 68.2* | 66.0* | 61.1* |
| Married status (% married) | 91.2 | 91.2 | 91.0 | 87.0* |
| African-American men (n) | 310 | 415 | 274 | 232 |
| Age (years, mean (s.d.)) | 55.4 (5.5) | 56.5 (6.1)* | 56.6 (5.9)* | 57.4 (5.9)* |
| Education level (% <high school) | 24.5 | 37.6* | 47.1* | 62.1* |
| Smoking status (% current) | 28.4 | 31.3 | 42.7* | 37.5* |
| Drinking status (% current) | 54.2 | 46.8 | 48.2 | 49.6 |
| Marital status (% married) | 82.3 | 74.7* | 78.5 | 68.5* |
Vital exhaustion (VE) score range 0–42—Quartiles: none, ≤3; low, 4–8; moderate, 9–15; high, ≥16.
Significantly different (P < 0.05) than no VE quartile.
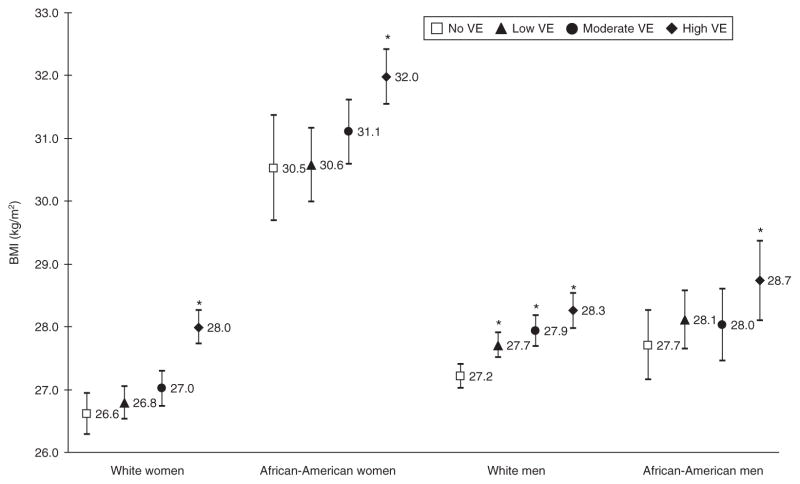
Cross-sectional relationship between VE and BMI
Figure 1 shows the mean BMI of participants within each VE quartile stratified by race–gender group. White women with no VE had significantly lower mean BMI levels than white women with high levels of VE, 26.6 (95% confidence interval (CI): 26.2, 26.9) and 28.0 kg/m2 (95% CI: 27.7, 28.3), respectively. Similar patterns were observed among African-American women. There were no significant differences between no VE and low or moderate VE among white women and African-American women and men. However, the mean BMI in white men with no VE (27.2 kg/m2, 95% CI: 27.0, 27.4) was significantly lower than white men with low (27.7 kg/m2, 95% CI: 27.5, 27.9), moderate (27.9 kg/m2, 95% CI: 27.7, 28.2), and high (28.3 kg/m2, 95% CI: 28.0, 28.5) VE.
Figure 1.
Mean BMI and 95% confidence interval within each vital exhaustion (VE) quartile by race–gender group, the Atherosclerosis Risk in the Communities (ARIC) study, 1990–1998. Models were adjusted for age, field center, level of education, smoking status, drinking status, and marital status. VE score range 0–42—Quartiles: none, ≤3; low, 4–8; moderate, 9–15; high, ≥16. *Significantly different from the no VE quartile (P < 0.05).
Longitudinal relationship between VE quartile and BMI 3 and 6 years later
Mean BMI ~3 and 6 years after assessment of VE are shown in Table 2 by race–gender groups. After adjusting for age, field center, education, smoking, drinking, and marital status (model 1), white and African-American women and men with high VE had a significantly higher mean BMI 3 years later compared to adults with no VE in the same race–gender group. In addition, the mean BMI among white men, with any VE (low, medium, and high) was significantly higher 3 years later compared to white men with no VE. When comparing the relationship between VE and BMI 6 years later, we found similar patterns for all race–gender groups with one exception; white women with moderate VE also had significantly higher mean BMI compared to white women with no VE. However, after also adjusting for baseline BMI (model 2), these trends were no longer apparent. In addition, the magnitude of the 3- and 6-year change in BMI did not differ between VE groups.
Table 2.
Adjusted mean BMI (95% confidence intervals) after 3 and 6 years follow-up
| VE quartilea | 3 years after VE assessment
|
6 years after VE assessment
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1b |
Model 2c |
Model 1b |
Model 2c |
||||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| White women | None | 27.2 | 26.8, 27.5 | 27.7 | 27.6, 27.8 | 27.4 | 27.0, 27.7 | 27.9 | 27.7, 28.0 |
| Low | 27.4 | 27.1, 27.7 | 27.7 | 25.6, 27.7 | 27.7 | 27.4, 28.0 | 27.9 | 27.8, 28.0 | |
| Moderate | 27.5 | 27.2, 27.8 | 27.7 | 27.6, 27.8 | 27.9* | 27.6, 28.2 | 28.0 | 27.9, 28.1 | |
| High | 28.5* | 28.2, 28.8 | 27.7 | 27.6, 27.8 | 28.7* | 28.4, 29.0 | 28.0 | 27.9, 28.1 | |
| African-American women | None | 31.1 | 30.2, 32.0 | 31.6 | 31.4, 31.9 | 30.9 | 29.9, 31.8 | 31.8 | 31.4, 32.1 |
| Low | 30.6 | 30.0, 31.3 | 31.5 | 31.3, 31.7 | 31.0 | 30.3, 31.7 | 31.6 | 31.3, 31.8 | |
| Moderate | 31.0 | 30.5, 31.6 | 31.3 | 31.1, 31.5 | 31.2 | 30.6, 31.8 | 31.6 | 31.4, 31.8 | |
| High | 32.4* | 31.9, 32.9 | 31.5 | 31.4, 31.7 | 32.9* | 32.3, 33.4 | 31.9 | 31.7, 32.1 | |
| White men | None | 27.5 | 27.3, 27.7 | 28.0 | 27.9, 28.0 | 27.8 | 27.5, 28.0 | 28.1 | 28.1, 28.2 |
| Low | 28.0* | 27.8, 28.2 | 28.0 | 27.9, 28.1 | 28.3* | 28.1, 28.5 | 28.2 | 28.1, 28.3 | |
| Moderate | 28.4* | 28.1, 28.6 | 28.1 | 28.0, 28.2 | 28.6* | 28.3, 28.8 | 28.3 | 28.2, 28.4 | |
| High | 28.7* | 28.3, 29.0 | 28.1 | 28.0, 28.2 | 28.6* | 28.2, 28.9 | 28.2 | 28.0, 28.3 | |
| African-American men | None | 27.8 | 27.2, 28.5 | 28.4 | 28.2, 28.6 | 28.0 | 27.4, 28.6 | 28.6 | 28.3, 28.8 |
| Low | 28.5 | 27.9, 29.0 | 28.4 | 28.3, 28.6 | 28.5 | 27.9, 29.0 | 28.5 | 28.3, 28.8 | |
| Moderate | 28.3 | 27.6, 28.9 | 28.3 | 28.1, 28.5 | 28.8 | 28.1, 29.4 | 28.6 | 28.3, 28.9 | |
| High | 29.1* | 28.3, 29.8 | 28.2 | 28.0, 28.5 | 29.1* | 28.3, 29.9 | 28.2 | 27.9, 28.6 | |
Vital exhaustion (VE) score range 0–42—Quartiles: none, ≤3; low, 4–8; moderate, 9–15; high, ≥16.
Model 1 adjusted for age, field center, level of education, smoking status, drinking status and marital status.
Model 2 also adjusted for BMI at baseline.
Significantly different (P < 0.05) from the no VE quartile at the same assessment.
Influence of VE quartile and excess weight gain over 3 and 6 years
Table 3 shows the adjusted odds ratios of gaining excess weight (≥5%) over ~3 and 6 years after assessment of VE. White women with high VE had 1.3 times the odds of excess weight gain over 3 years compared to white women with no VE. African-American women had a nonsignificant trend of a similar magnitude. Over 6 years, white women with moderate and high VE had increased odds (~30%) of gaining excess weight compared to those with no VE. White men with moderate and high VE were also more likely to have excess weight gain over 3 years but not over 6 years. In contrast, VE did not predict excess weight gain over 3 and 6 years among African-American men. In fact, African-American men with low VE were 37% less likely to experience excess weight gain over 6 years compared to American men with no VE. Similar results were found when models were not adjusted for BMI.
Table 3.
Adjusted odds ratios (95% confidence intervals) for excess weight gain over 3 and 6 years by vital exhaustion quartile
| VE quartilea | Excess weight gainb |
||||||
|---|---|---|---|---|---|---|---|
| Over 3 years
|
Over 6 years
|
||||||
| % | OR | 95% CI | % | OR | 95% CI | ||
| White women | None | 24.4 | 1.00 | 34.5 | 1.00 | ||
| Low | 26.5 | 1.12 | 0.94, 1.38 | 36.3 | 1.11 | 0.93, 1.34 | |
| Moderate | 26.9 | 1.17 | 0.96, 1.43 | 38.4 | 1.24 | 1.03, 1.50 | |
| High | 29.5 | 1.34 | 1.10, 1.63 | 39.6 | 1.29 | 1.07, 1.56 | |
| African-American women | None | 23.9 | 1.00 | 35.7 | 1.00 | ||
| Low | 21.4 | 0.91 | 0.60, 1.39 | 32.8 | 0.97 | 0.65, 1.43 | |
| Moderate | 23.1 | 1.06 | 0.71, 1.58 | 34.4 | 1.12 | 0.76, 1.64 | |
| High | 24.2 | 1.19 | 0.80, 1.75 | 35.6 | 1.30 | 0.89, 1.90 | |
| White men | None | 15.8 | 1.00 | 26.0 | 1.00 | ||
| Low | 17.0 | 1.12 | 0.92, 1.36 | 27.4 | 1.09 | 0.92, 1.29 | |
| Moderate | 20.2 | 1.35 | 1.09, 1.69 | 28.8 | 1.14 | 0.93, 1.39 | |
| High | 20.7 | 1.35 | 1.06, 1.72 | 30.3 | 1.19 | 0.96, 1.49 | |
| African-American men | None | 17.6 | 1.00 | 32.9 | 1.00 | ||
| Low | 18.8 | 1.04 | 0.68, 1.60 | 23.8 | 0.63 | 0.42, 0.93 | |
| Moderate | 15.4 | 0.82 | 0.50, 1.36 | 29.7 | 0.84 | 0.55, 1.30 | |
| High | 17.1 | 0.88 | 0.51, 1.52 | 25.4 | 0.67 | 0.40, 1.10 | |
Models adjusted for age, field center, level of education, smoking status, drinking status, marital status and baseline BMI. Values in boldface represent P ≤ 0.05.
Vital exhaustion (VE) score range 0–42—Quartiles: none, ≤3; low, 4–8; moderate, 9–15; high, ≥16.
Excess weight gain defined as ≥5% between visits.
DISCUSSION
Using cross-sectional data from the ARIC study, we have shown that white and African Americans with high levels of VE have higher mean BMI levels compared to adults in the same race–gender group with no VE. These differences (3–5%) approach clinically significance given that investigators have shown that a 5% weight loss improves cardiovascular risk (16). In addition, we found significant race–gender and VE quartile interactions, with the greatest impact of VE on BMI observed in white men.
Differences in mean BMI levels between VE groups were also observed in longitudinal analysis after 3 and 6 years. However, the strength of the association between VE and future BMI was reduced after adjusting for baseline BMI. This result was anticipated because of the relationship between BMI at all visits. BMI increased over time among all race–gender groups, and it appeared to have gone up by a similar magnitude in each VE group. Hence, it appears that the relationship that was observed between BMI and VE did not change across time and that any relationship between VE and BMI was already established at baseline. It is possible that VE increased across time inline with BMI, but we were unable to examine this because VE was only measured at baseline.
We examined the prospective association between VE and excess weight gain ~3 and 6 years after assessment of VE. In white women, African-American women and white men, high VE tended to be related to future excess weight gain; however, the opposite relation was observed in African-American men. This ethnic–gender disparity is difficult to explain. Because this area of research is so novel, we could only gather clues from depression research. Although this is scarce in itself, the majority of studies suggest that, while overweight women tend to be more depressed than overweight men (17), ethnic disparities are often not observed (17,27). The mechanism causing this phenomenon is uncertain, but it is possible that the relationship between VE and weight gain is not as strong in African-American men because of a difference in coping strategies and/or the availability of protective social support. This may or may not be relevant to VE, and the lack of understanding in this field of research warrants further investigation. Furthermore, the relatively high baseline BMI in African-American women may have affected our ability to show an association between VE and weight gain in this group.
Many studies have examined the relationship between obesity and psychological state (1,9,17,28,29); however, most of these examined depression using cross-sectional data. Although there are some inconsistencies, studies usually report significant positive associations. Our findings for VE are consistent with these. There are a lack of studies that have examined VE and obesity directly in the literature. VE was linked to BMI in adults who had experienced an MI event in the past (19) and an association has been seen with waist-to-hip ratio, in which greater waist-to-hip ratio was found to relate to increased VE in lean men (30). There has also been some suggestion that the link between VE and CVD (or its mediators) may be influenced by obesity (13).
The relationship between obesity and VE is important because of its association with CVD, which is associated with both conditions. There is consistent evidence from prospective studies that VE is a strong risk indicator for first (12,31–34) and recurrent (19,34–37) cardiac events. Appels and Mulder (12) prospectively examined 3,877 healthy adult men (with no history of CVD) and found that VE predicted angina pectoris (odds ratio 1.86, P < 0.03) and nonfatal MI (odds ratio 2.28, P < 0.001) after 4 years of follow-up. More recently, Schwartz et al. (34) showed an increased risk of stroke in men and women from the ARIC cohort, with VE scores in the upper tertile compared to those with VE scores in the lowest tertile (hazard ratio 2.42, P < 0.001).
The mechanism linking VE to CVD is still not well understood. It is possible that VE may exert its effects through lipid metabolism, by enhancing the insulin and C-peptide response and/or by raising the insulin:glucose ratio (30,38). Janszky et al. (35) examined the association between VE and inflammatory markers and found that VE was related to both C-reactive protein (r = 0.16, P = 0.07) and interleukin-6 (r = 0.21, P = 0.02). Interestingly, abnormal levels of these mediators are also often found in the obese (39), and it has been suggested that adipose tissue is an important determinant of a chronic inflammatory state, as predicted by levels of interleukin-6, C-reactive protein and tumor necrosis factor-α (40). Further research is needed to understand the complex relationship between VE, obesity, and atherosclerosis fully.
To our knowledge, this is the first study to examine the direct association between VE and obesity in a nonclinical sample. We showed that high levels of VE were related to future weight gain in white men and women, but not African-American men and women. We cannot confirm that adults categorized with high VE remained in that state over the next 3 or 6 years. It is possible that the relationship between VE and BMI is bi- directional. For example, it is plausible that being overweight causes distress, which may lead to VE, particularly if it is coupled with stigmatization. We were not able to examine these temporal complexities, because VE was only measured at one clinical visit in the ARIC cohort.
Enhanced understanding of the relationship between VE and obesity could facilitate treatment aimed at reducing VE to prevent future weight gain, improve the success of weight loss interventions and thereby potentially reducing the risk of CVD. Furthermore, weight loss and VE reduction treatment could benefit patients who have already suffered a cardiac event by reducing the risk of subsequent events. Our future studies will focus on understanding the temporal relationships between VE, obesity, and CVD and continue to explore ethnic disparities.
Acknowledgments
We thank Dr Juhaeri for his help in formulating the ideas for this research. This work was supported in part by Sanofi-Aventis.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Fabricatore AN, Wadden TA. Psychological aspects of obesity. Clin Dermatol. 2004;22:332–337. doi: 10.1016/j.clindermatol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Hainer V, Kunesova M, Bellisle F, et al. Psychobehavioral and nutritional predictors of weight loss in obese women treated with Sibutramine. Int J Obes (London) 2004;29:208–216. doi: 10.1038/sj.ijo.0802850. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira PJ, Going SB, Houtkooper LB, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord. 2004;28:1124–1133. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- 4.Linde JA, Jeffery RW, Levy RL, et al. Binge eating disorder, weight control self-efficacy, and depression in overweight men and women. Int J Obes Relat Metab Disord. 2004;28:418–425. doi: 10.1038/sj.ijo.0802570. [DOI] [PubMed] [Google Scholar]
- 5.Nir Z, Neumann L. Self-esteem and internal-external locus of control, and their relationship to weight reduction. J Clin Persp. 1991;47:568–575. doi: 10.1002/1097-4679(199107)47:4<568::aid-jclp2270470416>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Finnegan DL, Suler JR. Psychological factors associated with maintenance of improved health behaviors in post coronary patients. J Psychol. 1985;119:87–94. doi: 10.1080/00223980.1985.9712610. [DOI] [PubMed] [Google Scholar]
- 7.Stunkard AJ, Myles FS, Allison KC. Depression and obesity. Biol Psychiat. 2003;54:330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 8.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–1147. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 9.Tuthill A, Slawik H, O’Rahilly S, Finer N. Psychiatric co-morbidities in patients attending specialist obesity services in the UK. QJM. 2006;99:317–325. doi: 10.1093/qjmed/hcl041. [DOI] [PubMed] [Google Scholar]
- 10.Becker ES, Margraf J, Turke V, Soeder U, Neumer S. Obesity and mental illness in a representative sample of young women. Int J Obes. 2001;25:S5–S9. doi: 10.1038/sj.ijo.0801688. [DOI] [PubMed] [Google Scholar]
- 11.Siegler IC, Peterson BL, Barefoot JC, Williams RB. Hostility during late adolescence predicts coronary risk factors at mid-life. Am J Epidemiol. 1992;136:146–154. doi: 10.1093/oxfordjournals.aje.a116481. [DOI] [PubMed] [Google Scholar]
- 12.Appels A, Mulder P. Excess fatigue as a precursor of myocardial infarction. Eur Heart J. 1988;9:758–764. doi: 10.1093/eurheartj/9.7.758. [DOI] [PubMed] [Google Scholar]
- 13.Raikkonen K, Lassila R, Keltikangas-Jarvinen L, Hautanen A. Association of chronic stress with plasminogen activator inhibitor–1 in healthy middle-aged men. Arterioscler Thromb Vasc Biol. 1996;16:363–367. doi: 10.1161/01.atv.16.3.363. [DOI] [PubMed] [Google Scholar]
- 14.Van Diest R, Appels A. Vital exhaustion and depression: a conceptual study. J Psychosom Res. 1991;35:535–544. doi: 10.1016/0022-3999(91)90048-s. [DOI] [PubMed] [Google Scholar]
- 15.Appels A. Mental precursors of myocardial infarction. Br J Psychiatry. 1990;156:465–471. doi: 10.1192/bjp.156.4.465. [DOI] [PubMed] [Google Scholar]
- 16.NHLBI. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adult: the Evidence Report. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 17.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV Major depressive disorder, suicide idealtin and suicide atempts: results from a general population study. Am J Public Health. 2000;90:251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord. 1992;16:999–1003. [PubMed] [Google Scholar]
- 19.Huijbrechts IP, Erdman RA, Duivenvoorden HJ, Passchier J. Modification in Quetelet index five months after myocardial infarction: relevance of biographic and personality characteristics. Prev Med. 1999;28:86–91. doi: 10.1006/pmed.1998.0387. [DOI] [PubMed] [Google Scholar]
- 20.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 21.Jackson R, Chambless LE, Yang K. Differences between respondents and non-respondents in a multicenter community based study vary by gender and ethnicity. J Clin Epidemiol. 1996;49:1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 22.Appels A, Falger PR, Schouten EG. Vital exhaustion as risk indicator for myocardial infarction in women. J Psychosom Res. 1993;37:881–890. doi: 10.1016/0022-3999(93)90177-h. [DOI] [PubMed] [Google Scholar]
- 23.Kop WJ. Acute and chronic psychological risk factors for coronary syndromes: Moderating effects of coronary artery disease severity. J Psychosom Res. 1997;43:167–181. doi: 10.1016/s0022-3999(97)80002-5. [DOI] [PubMed] [Google Scholar]
- 24.Mendes de Leon CF, Kop WJ, Se Swart HB, Bar FW, Appels A. Psychosocial characteristics and recurrent events after precutaneous transluminal coronary angioplasty. Am J Cardiol. 1996;77:252–255. doi: 10.1016/s0002-9149(97)89388-5. [DOI] [PubMed] [Google Scholar]
- 25.WHO. WHO Technical Report Series 894. Geneva: 2000. Obesity. preventing and managing the global epidemic. Report of a WHO Consultation., World Health Organization. [PubMed] [Google Scholar]
- 26.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes. 2006;30:391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 27.Laferrère B, Zhu S, Clarkson JR, et al. Race, menopause, health-related quality of life, and psychological well-being in obese women. Obes Res. 2002;10:1270–1275. doi: 10.1038/oby.2002.172. [DOI] [PubMed] [Google Scholar]
- 28.Friedman MA, Brownell KD. Psychological correlates of obesity; moving to the next research generation. Psychol Bull. 1995;117:3–20. doi: 10.1037/0033-2909.117.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Van Hout GCM, Van Oudheusden I, Van Heck GL. Psychological Profile of the Morbidly Obese. Obes Surg. 2004;14:579–588. doi: 10.1381/096089204323093336. [DOI] [PubMed] [Google Scholar]
- 30.Raikkonen K, Hautanen A, Keltikangas-Jarvinen L. Association of stress and depression with regional fat distribution in healthy middle-aged men. J Behav Med. 1994;17:605–616. doi: 10.1007/BF01857600. [DOI] [PubMed] [Google Scholar]
- 31.Keltikangas-Jarvinen L, Raikkonen K, Hautanen A, Adlercreutz H. Vital exhaustion, anger expression, and pituitary and adrenocortical hormones: implications for the insulin resistance syndrome. Arterioscler Thromb Vasc Biol. 1996;16:275–280. doi: 10.1161/01.atv.16.2.275. [DOI] [PubMed] [Google Scholar]
- 32.Appels A, Otten A. Exhaustion as precursor of cardiac death. Br J Clin Psychol. 1992;31:351–356. doi: 10.1111/j.2044-8260.1992.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 33.Kopp M, Falger P, Appels A, Szedmak S. Depressive symptomatology and vital exhaustion are differentially related to behavioral risk factors for coronary artery disease. Psychos Med. 1998;60:752–758. doi: 10.1097/00006842-199811000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz SW, Carlucci C, Chambless LE, Rosamond W. Synergism between smoking and vital exhaustion in the risk of Ischemic Stroke: evidence from the ARIC study. Ann Epidemiol. 2004;14:416–424. doi: 10.1016/j.annepidem.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain Behav Immun. 2005;19:555–563. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 36.McGowan L, Dickens C, Percival C, et al. The relationship between vital exhaustion, depression and comorbid illnesses in patients following first myocardinal infarction. J Psychosom Res. 2004;57:183–188. doi: 10.1016/S0022-3999(03)00610-X. [DOI] [PubMed] [Google Scholar]
- 37.Kopp M. PhD Thesis. University of Maastricht; 1994. The Predictive Value of Vital Exhaustion in the Clinical Course After Coronary Angioplasty. [Google Scholar]
- 38.Raikkonen K. [Accessed 2005];Vital exhaustion: a syndrome of psychological distress. 1997 < http://www.macses.ucsf.edu/Research/Allostatic/notebok/vital.html>.
- 39.Möhlig M, Boeing H, Spranger J, et al. Body mass index and C-174G interleukin-6 promoter polymorphism interact in predicting type 2 diabetes. J Clin Endocrinol Metab. 2004;89:1885–1890. doi: 10.1210/jc.2003-031101. [DOI] [PubMed] [Google Scholar]
- 40.Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]