Abstract
Background
Various factors, such as the optimal number of passes, aspiration pressure, and the use of 19-gauge and Trucut biopsy needles, have been studied to improve the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). We retrospectively compared the diagnostic accuracy of EUS-FNA between 25- and 22-gauge needles, which have been widely used recently.
Subjects and Methods
The study group comprised 47 consecutive patients who underwent EUS-FNA with both 22- and 25-gauge needles from October 2007 through March 2010. Their underlying diseases were pancreatic cancer in 24 patients, submucosal tumors in 11, other pancreatic tumors in 4, chronic pancreatitis in 4, enlarged lymph nodes in 3, and gall bladder cancer in 1. Tissue specimens, which were pushed out of the puncture needle, were placed into physiological saline solution. Gray-whitish, worm-like specimens were used for histologic diagnosis. The remaining specimen was centrifuged, and the sediment was plated on slides and examined by a cytopathologist to obtain the cytologic diagnosis.
Results
A total of 75 punctures (mean, 1.6) were performed with 25-gauge needles, and 69 punctures (mean, 1.4) were performed with 22-gauge needles. The overall tissue-sampling rate for cytology was 100% (47/47), which was significantly (p=0.01) superior to 83% (39/47) for histology. The overall diagnostic accuracy on the cytologic and histologic examinations was 79% (37/47) and 85% (33/39) (p=0.48). According to needle type, the tissue-sampling rate for cytology and histology on each puncture was 97% (73/75) and 56% (42/75) with 25-guage needles, and was 97% (67/69) and 58% (40/69) with 22-guage needles, the accuracy of cytologic diagnosis on each puncture was 73% (53/73) with 25-gauge needles and 66% (44/67) with 22-gauge needles (p=0.37); the accuracy of histologic diagnosis on each puncture was 60% (25/42) and 75% (30/40) (p=0.14), respectively. No patient had complications.
Conclusions
The tissue-sampling rate and diagnostic accuracy did not differ significantly between 22- and 25-gauge needles in patients with pancreatic or gastrointestinal diseases who underwent EUS-FNA.
Key words: EUS-FNA, 25-guage needle, 22-guage needle, diagnostic accuracy, pancreatic disease, upper gastroitestinal diseases
Introduction
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was first described by Vilmann et al. in 1992. EUS-FNA is now widely used clinically for a broad range of indications. EUS-FNA has become an essential tool for diagnosis and treatment in clinical practice.1 In general, the diagnostic accuracy of EUS-FNA ranges from 70% to 100%, with a 0% to 3% incidence of complications.2,3 To further improve diagnostic accuracy, devices such as 19-gauge needles and Trucut biopsy needles have been developed, and their clinical usefulness has been evaluated. At present, 19-gauge needles are usually used to improve the diagnostic accuracy of EUS-FNA when tissue specimens cannot be collected with a 22-gauge needle or when large tissue samples are required for the diagnosis of diseases such as autoimmune pancreatitis and malignant lymphoma.4–10 However, puncture may be difficult to perform with even 22-gauge needles, let alone 19-gauge needles. Because of these factors, EUS-FNA has started to be mainly performed with a 25-gauge needle in recent years.10–13 The aim of this study was to compare the tissue-sampling rate and the diagnostic accuracy rate of EUS-FNA between the use of 25- and 22-gauge needles in the same patients.
Subjects and Methods
We investigated retrospectively the subjects of 47 patients (21 men and 26 women) who underwent diagnostic EUS-FNA with the use of both 22- and 25-gauge puncture needles from October 2007 through March 2010 (Table 1). Their mean age was 59.0 years (range, 34 to 86). Informed written consent for the study of EUS-FNA was obtained from all patients. The underlying disease was pancreatic ductal cancer in 24 patients, other pancreatic tumors in 4 (3 endocrine tumors and 1 metastasis of colon cancer), gastrointestinal stromal tumors (GIST) in 6, other gastric submucosal tumors in 5, chronic pancreatitis in 4, enlarged lymph nodes in 3, and gall bladder cancer in 1. Final diagnosis for pancreatic lesions, lymph node, and gall bladder were mainly done by at least 6 months or more clinical follow-up and that for gastrointestinal submucosal lesions were diagnosed by surgical operation. Two convex-array echo endoscopes (GF-UCT240 and GF-2000P, Olympus Co., Tokyo, Japan) were used. Puncture was performed with 22-gauge needles (NA-200H, Olympus Co.) and 25-gauge needles (Echotip, Wilson-Cook, Winston Salem, NC, USA). A 22-gauge needle was used first, followed by a 25-gauge needle randomly, or visa versa by decision of operator. To perform EUS-FNA, the needle was placed in the target site under endoscopic ultrasonographic guidance, and the lesion was punctured. After removing the stylet, a negative pressure of air by 10 or 20 cm syringe was applied in all cases. The needle was passed back and forth in the target lesion 10 to 20 times to obtain a tissue sample. After releasing the negative pressure, the needle was removed. The tissue sample was pushed out of the needle by air delivered with a syringe and placed in physiological saline solution. If the specimen could not be removed, it was flushed out with physiological saline solution delivered with a 2.5-mL syringe. If the specimen still could not be removed, it was pushed out of the needle with a stylet and placed in physiological saline solution. There was no on-site pathogist, then we perfomed EUS-FNA until gray-whitish, worm-like tissue samples were obtained macroscopically. A pathologist prepared cytologic and histologic specimens. Gray-whitish, worm-like tissue samples were used for histologic diagnosis. The physiological saline solution containing the remaining tissue sample was centrifuged, and the sediment was plated on a slide and used for cytological diagnosis. A cytopathologist assessed all specimens to make a diagnosis.
Table 1.
Subjects
| Total Number | 47 | |
| Gender | ||
| Male | 21 | |
| Female | 26 | |
| Averaged age | 59.0 (34–86) | |
| Diseases of subjects | ||
| Pancreatic cancer | 24 (51%) | |
| Submucosal tumor | 11 (23%) | |
| Other pancreatic tumor | 4 (9%) | |
| Chronic pancreatitis | 4 (9%) | |
| Lymph node | 3 (6%) | |
| Gall bladder cancer | 3 (2%) | |
Before EUS-FNA, antiplatelet agents were temporarily discontinued for an appropriate period, and hematologic examinations (including bleeding time) and serum chemical analyses were performed. On the day of the examination, a continuous intravenous infusion line was secured, and a cephem antibiotic was administered 30 minutes before EUS-FNA. Three hours after EUS-FNA and on the following morning, physical examination, hematologic examinations, and serum chemical analyses were performed to confirm the presence or absence of complications.
Statistical analysis
The data collected from the EUS-FNA data sheet were analyzed for overall diagnostic yield for each type of needle (25-gauge and 22-gauge). The comparison of sampling rate, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of 25-gauge and 22-gauge needle was done using Chi-square for independence test. P values less than 0.05 were considered statistically significant.
Results
Overall tissue sampling rate and diagnostic accuracy of EUS-FNA
The mean number of punctures per patient was 3.0 (range, 2 to 4). The overall tissue-sampling rate for cytology was 100% (47/47), which was significantly (p=0.01) superior to 83% (39/47) for histology (Table 2). The overall diagnostic accuracy of cytology and histology was 79% (37/47) and 85% (33/39) (p=0.48). There were no complications such as bleeding, infection, pancreatitis, or perforation.
Table 2.
Diagnostic yield of cytology & histology
| Cytology (n=47) | Histology (n=47) | p | ||
| Pancreas & others (n=36) | ||||
| Sampling rate | 100% (36/36) | 78% (28/36) | 0.009 | |
| Sensitivity | 80% (24/30) | 84% (21/25) | 0.97 | |
| Specificity | 100% (6/6) | 100% (3/3) | - | |
| PPV | 100% (24/24) | 100% (21/21) | ||
| NPV | 50% (6/12) | 43% (3/7) | ||
| Submucosal tumor (n=11) | ||||
| Sampling rate | 100% (11/11) | 100% (11/11) | ns | |
| Sensitivity | 64% (7/11) | 82% (9/11) | 0.63 | |
| Specificity | - | - | ||
| PPV | 100% (7/7) | 100% (9/9) | ||
| NPV | 0% (0/4) | 0% (0/2) | ||
| Total | ||||
| Sampling rate | 100% (47/47) | 83%(39/47) | 0.01 | |
| Sensitivity | 76% (31/41) | 83% (30/36) | 0.4 | |
| Specificity | 100% (6/6) | 100% (3/3) | ns | |
| PPV | 100% (31/31) | 100% (30/30) | ||
| NPV | 38% (6/16) | 33% (3/9) | ||
| Accuracy | 79% (37/47) | 85% (33/39) | 0.48 | |
Comparison of accuracy of EUS-FNA with 22- and 25-gauge needles
The diagnostic accuracy of both cytology and histology was 75% (24/32) and 88% (23/26) in 32 patients in whom puncture was first done with a 22-gauge needle, followed by a 25-gauge needle and 87% (13/15) and 77% (10/13) in 15 patients in whom puncture was first done with a 25-gauge needle, followed by a 22-gauge needles. This difference was not significant (p=0.60, p=0.59). A total of 69 punctures were performed with a 22-gauge needle (mean number of punctures per patient, 1.4 [range, 1 to 2]), and 75 were performed with a 25-gauge needle (mean number of punctures per patient, 1.6 [range, 1 to 2]). The diagnostic accuracy of cytologic examinations on each case did not differ significantly between the use of a 22-gauge needle (70%, 33/47) and the use of a 25-gauge needle (73%, 33/45) (p=0.74) (Table 3). And the diagnostic accuracy of histologic examinations on each case also did not differ significantly between the use of a 22-gauge needle (75%, 24/32) and the use of a 25-gauge needle (70%, 21/30) (p=0.66) (Table 4). According to the number of punctures, the overall tissue-sampling rate of and 25-guage needles for cytology and histology was 97% (67/69) and 58% (40/69) with a 22-guage needle and 97% (73/75) and 56% (42/75) with a 25-guage needle (p=0.67, p=0.81) (Table 5,6), the diagnostic accuracy of cytologic examinations did not differ significantly between the use of a 22-gauge needle (66%, 44/67) and the use of a 25-gauge needle (73%, 53/73) (p=0.37) (Table 5). And the diagnostic accuracy of histologic examinations also did not differ significantly between the use of a 22-gauge needle (75%, 30/40) and the use of a 25-gauge needle (60%, 25/42) (p=0.14) (Table 6, Fig. 1A, Fig. 1B).
Table 3.
Diagnostic yield of 22-guage & 25-guage needle on cytology
| 22G (n=47) | 25G (n=47) | p | ||
| Pancreas & others (n=36) | ||||
| Sampling rate | 100% (36/36) | 94% (34/36) | 0.47 | |
| Sensitivity | 70% (21/30) | 72% (21/29) | 0.84 | |
| Specificity | 100% (6/6) | 100% (5/5) | ns | |
| PPV | 100% (21/21) | 100% (21/21) | ||
| NPV | 67% (2/3) | 38% (5/13) | ||
| Submucosal tumor (n=11) | ||||
| Sampling rate | 100% (11/11) | 100% (11/11) | ns | |
| Sensitivity | 55% (6/11) | 64% (7/11) | 1 | |
| Specificity | - | - | - | |
| PPV | 100% (6/6) | 100% (7/7) | ||
| NPV | 0% (0/5) | 0% (0/4) | ||
| Total (n=47) | ||||
| Sampling rate | 100% (47/47) | 96% (45/47) | 0.47 | |
| Sensitivity | 66% (27/41) | 83% (28/40) | 0.69 | |
| Specificity | 100% (6/6) | 100% (5/5) | ns | |
| PPV | 100% (27/27) | 100% (28/28) | ||
| NPV | 25% (2/8) | 29% (5/17) | ||
| Accuracy | 70% (33/47) | 73% (33/45) | 0.74 | |
Table 4.
Diagnostic yield of 22-guage & 25-guage needle on histology
| 22G (n=47) | 25G (n=47) | p | ||
| Pancreas & others (n=36) | ||||
| Sampling rate | 69% (25/36) | 64% (23/36) | 0.62 | |
| Sensitivity | 64% (14/22) | 67% (14/21) | 0.83 | |
| Specificity | 100% (3/3) | 100% (2/2) | ns | |
| PPV | 100% (14/14) | 100% (14/14) | ||
| NPV | 27% (3/11) | 22% (2/9) | ||
| Submucosal tumor (n=11) | ||||
| Sampling rate | 64% (7/11) | 64% (7/11) | 0.66 | |
| Sensitivity | 64% (7/11) | 71% (5/7) | 0.45 | |
| Specificity | - | - | - | |
| PPV | 100% (7/7) | 100% (5/5) | ||
| NPV | - | 0% (2/2) | ||
| Total (n=47) | ||||
| Sampling rate | 68% (32/47) | 64% (30/47) | 0.66 | |
| Sensitivity | 72% (21/29) | 68% (19/28) | 0.71 | |
| Specificity | 100% (3/3) | 100% (2/2) | ns | |
| PPV | 100% (21/21) | 100% (19/19) | ||
| NPV | 27% (3/11) | 18% (2/11) | ||
| Accuracy | 75% (24/32) | 70% (21/30) | 0.66 | |
Table 5.
Diagnostic yield of 22-guage & 25-guage needle puncture on cytology
| 22G (puncture=69) | 25G (puncture=75) | p | ||
| Pancreas & others | ||||
| Sampling rate | 100% (49/49) | 96% (55/57) | 0.54 | |
| Sensitivity | 63% (26/41) | 70% (32/46) | 0.54 | |
| Specificity | 100% (8/8) | 100% (9/9) | ns | |
| PPV | 100% (26/26) | 100% (32/32) | ||
| NPV | 35% (8/23) | 39% (9/23) | ||
| Submucosal tumor (n=11) | ||||
| Sampling rate | 90% (18/20) | 100% (18/18) | 0.52 | |
| Sensitivity | 56% (10/18) | 67% (12/18) | 0.49 | |
| Specificity | - | - | - | |
| PPV | 100% (10/10) | 100% (12/12) | ||
| NPV | 0% (0/8) | 0% (0/6) | ||
| Total (n=47) | ||||
| Sampling rate | 97% (67/69) | 97% (73/75) | 0.67 | |
| Sensitivity | 61% (36/59) | 69% (44/64) | 0.37 | |
| Specificity | 100% (8/8) | 100% (9/9) | ns | |
| PPV | 100% (36/36) | 100% (44/44) | ||
| NPV | 26% (8/31) | 31% (9/29) | ||
| Accuracy | 66% (44/67) | 73% (53/73) | 0.37 | |
Table 6.
Diagnostic yield of 22-guage & 25-guage needle puncture on histology
| 22G (puncture=69) | 25G (puncture=75) | p | ||
| Pancreas & Others | ||||
| Sampling rate | 61% (30/49) | 49% (28/57) | 0.21 | |
| Sensitivity | 67% (18/27) | 54% (14/26) | 0.34 | |
| Specificity | 100% (3/3) | 100% (2/2) | ns | |
| PPV | 100% (18/18) | 100% (14/14) | ||
| NPV | 25% (3/12) | 14% (2/14) | ||
| Submucosal tumor (n=11) | ||||
| Sampling rate | 50% (10/20) | 78% (14/18) | 0.37 | |
| Sensitivity | 90% (9/10) | 64% (9/14) | 0.34 | |
| Specificity | - | - | - | |
| PPV | 100% (9/9) | 100% (9/9) | ||
| NPV | 0% (0/1) | 0% (0/5) | ||
| Total (n=47) | ||||
| Sampling rate | 58% (40/69) | 56%(42/75) | 0.81 | |
| Sensitivity | 73% (27/37) | 58% (23/40) | 0.16 | |
| Specificity | 100% (3/3) | 100% (2/2) | ns | |
| PPV | 100% (27/27) | 100% (23/23) | ||
| NPV | 23% (3/13) | 11% (2/19) | ||
| Accuracy | 75% (30/40) | 60% (25/42) | 0.14 | |
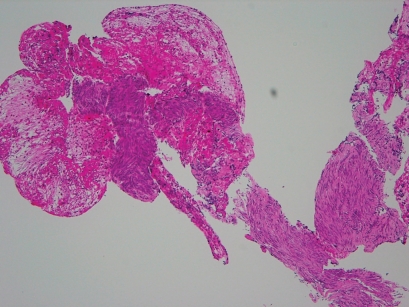
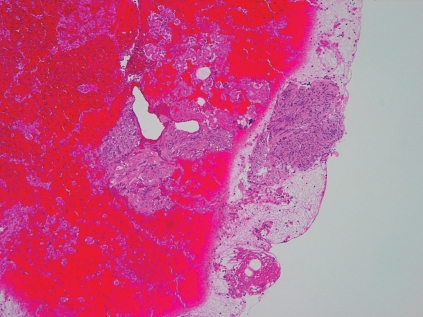
Figure 1.
Histology of GIST of the same patient taken by 25-guage (A) and 22-guage (B) (x10). There is not so difference on sample size and quantity between two needles, if succeeded.
Discussion
In 1992, Vilmann et al. first reported the use of EUS-FNA in patients with pancreatic cancer.1 The indications of EUS-FNA have since been broadened to include drainage of pancreatic pseudocysts and abscesses, celiac plexus neurolysis, biliary drainage, pancreatic drainage, immunotherapy, and gene therapy.14–20 Moreover, the clinical usefulness of EUS-FNA, used to obtain tissue specimens for cytologic diagnosis, has been enhanced by combination with immunohistochemical studies and genetic analyses.21,22 Consequently, EUS-FNA is now widely used in clinical practice and has become an essential examination procedure because of its high diagnostic accuracy and acceptable, low incidence of complications. Nonetheless, the most important factor in histological and genetic examinations is to reliably obtain tissue specimens from target lesions for cytologic and histologic diagnoses. Accurate tissue procurement is thus an important issue for EUS-FNA. Previous studies have examined the optimal number of needle passes and found that 5 to 7 passes contributed to increased diagnostic accuracy of EUS-FNA, whereas 8 or more passes did not.23,24 Suction pressure has been also evaluated by various studies, but its impact on diagnostic accuracy remains controversial.25–27 To improve the diagnostic accuracy of EUS-FNA, 19-gauge needles and Trucut biopsy needles have been developed, and their usefulness has been evaluated. Some studies have reported that FNA with 22-gauge needles provides superior or equivalent diagnostic accuracy4,5, whereas others have found that Trucut biopsy needles are superior6. The number of studies showing that a combination of 22-gauge and Trucut needles improves the clinical diagnostic accuracy of EUS-FNA has increased.7–9 At present, 19-gauge and Trucut needles are usually used when tissue specimens cannot be obtained with 22-gauge needles or when large tissue samples are required for the diagnosis of diseases such as autoimmune pancreatitis and malignant lymphoma. This appears to be the general consensus. However, it is usually not possible to puncture lesions situated at the head of the pancreas, particularly those at the uncinate process, with the use of 19-gauge or Trucut needles.10
Diagnostic accuracy has also been attempted to be improved by the development of 25-gauge needles, smaller in diameter than 22-gauge needles. Recent studies have confirmed the clinical usefulness of these smaller needles.10–13 Although these studies were small, currently available evidence suggests that 25-gauge-needles are equivalent to or significantly better than 22-gauge-needles for the diagnosis of pancreatic lesions.10–13 A 25-gauge-needle was also associated with a lower incidence of complications.13 In addition, the puncture of lesions at the head of the pancreas, particularly the uncinate process, has been reported to be significantly easier with a 25-gauge needle.10 This finding is supported by the results of our study. We also found that a 25-gauge needle could easily puncture lesions located at the head of the pancreas, particularly those at the uncinate process, which are considered difficult to puncture, as well as small submucosal tumors. We had the impression that a 25-gauge needle is superior to a 22-gauge needle for the puncture of small, mobile target lesions, such as submucosal tumors, particularly when the deflection of the endoscope makes puncture difficult.
Concerning about the sampling rate, that for cytology is generally superior to that for histology, however the overall diagnostic accuracy did not differ between cytology and histology. As for the diagnostic accuracy of 25-gauge needles, the tissue sampling rate and diagnostic accuracy did not differ significantly between 25- and 22-gauge needles in our study too. Sakamoto et al. and Imazu et al.10,12 have reported that 22-gauge needles can obtain larger specimens than 25-gauge needles. Although the specimen size was sometimes small in our study, diagnostic accuracy on cytologic and histologic examinations did not differ significantly between 22- and 25-gauge needles, confirming that 25-gauge needles are clinically useful.
In conclusion, our results suggest that the diagnostic accuracy of EUS-FNA with a 25-gauge needle is equivalent to that of EUS-FNA with a 22-gauge needle. Randomized studies of larger numbers of patients are needed to confirm our results. However, we believe that 25-gauge needles will be more widely used clinically in the future because they can facilitate the puncture of selected lesions.
Abbreviations
- EUS-FNA
endoscopic ultrasound-guided fine-needle aspiration
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
References
- 1.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–173. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 2.O'Toole D, Palazzo L, Arotçarena R, Dancour A, Aubert A, Hammel P, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–474. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 3.Kida M. Pancreatic masses. Gastrointest Endosc. 2009;69:S102–S109. doi: 10.1016/j.gie.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Wiersema MJ, Jondal ML, Schwartz DA, Clain JE, Levy MJ, Vazquez-Sequeiros E, et al. Prospective comparison of a 19- versus 22-gauge needle for performing EUS-FNA of pancreas mass lesions: assessment of accuracy and factors influencing safety. Gastrointest Endosc. 2002;55:AB240. [Google Scholar]
- 5.Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, et al. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 6.Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–106. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 7.Storch I, Jorda M, Thurer R, Raez L, Rocha-Lima C, Vernon S, et al. Advantage of EUS Trucut biopsy combined with fine-needle aspiration without immediate on-site cytopathologic examination. Gastrointest Endosc. 2006;64:505–511. doi: 10.1016/j.gie.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 8.Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and Trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 9.Shah SM, Ribeiro A, Levi J, Jorda M, Rocha-Lima C, Sleeman D, et al. EUS-guided fine needle aspiration with and without trucut biopsy of pancreatic masses. JOP. 2008;9:422–430. [PubMed] [Google Scholar]
- 10.Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointestinal Endosc. 2009;70:1093–1097. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Imazu H, Uchiyama Y, Kakutani H, Ikeda K, Sumiyama K, Kaise M, et al. A prospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol Research and Practice. 2009;2009:546390. doi: 10.1155/2009/546390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yusuf TE, Ho S, Pavey DA, Michael H, Gress FG. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy. 2009;41:445–448. doi: 10.1055/s-0029-1214643. [DOI] [PubMed] [Google Scholar]
- 14.Kida M, Itoi T. Current status and future perspective of interventional endoscopic ultrasound in Japan. Digest Endosc. 2009;21:S50–S52. doi: 10.1111/j.1443-1661.2009.00862.x. [DOI] [PubMed] [Google Scholar]
- 15.Grimm H, Binmoeller K, Soehendra N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc. 1992;38:170–171. doi: 10.1016/s0016-5107(92)70384-8. [DOI] [PubMed] [Google Scholar]
- 16.Seewald S, Ang TL, Kida M, Teng KY, Soehendra N. EUS 2008 Working Group. EUS 2008 Working Group document: evaluation of EUS-guided drainage of pancreatic-fluid collections (with video) Gastrointest Endosc. 2009;69:S13–S21. doi: 10.1016/j.gie.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 17.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–662. doi: 10.1016/s0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 18.Irisawa A, Takagi T, Kanazawa M, Ogata T, Sato Y, Takenoshita S, et al. Endoscopic ultrasound-guided fine-needle injection of immature dendritic cells into advanced pancreatic cancer refractory to gemcitabine: a pilot study. Pancreas. 2007;35:189–190. doi: 10.1097/01.mpa.0000250141.25639.e9. [DOI] [PubMed] [Google Scholar]
- 19.Chang KJ. EUS-guided fine needle injection (FNI) and anti-tumor therapy. Endoscopy. 2006;38:S88–S93. doi: 10.1055/s-2006-946664. [DOI] [PubMed] [Google Scholar]
- 20.Posner M, Chang K, Rosemurgy A, Stephenson J, Khan M, Reid T, et al. Multi-center phase2/3 Randomized controlled trial using TNFerade TM gene delivery combined with chemoradiation in patients with locally advanced pancreatic cancer. ASCO annual meeting. 2007 [Google Scholar]
- 21.Khalid A, Nodit L, Zahid M, Bauer K, Brody D, Finkelstein SD, et al. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493–2500. doi: 10.1111/j.1572-0241.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 22.Ashida R, Nakata B, Shigekawa M, Mizuno N, Sawaki A, Hirakawa K, et al. Gemcitabine sensitivity-related mRNA expression in endoscopic ultrasound-guided fine-needle aspiration biopsy of unresectable pancreatic cancer. J Exp Clin Cacer Res. 2009;28:83. doi: 10.1186/1756-9966-28-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson RA, Sayage-Rabie L, Beissner S. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 24.LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, et al. Optimal number of EUS-guided fine needle passes needed to obtaine a correct diagnosis. Gastrointest Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 25.Wallace MB, Kennedy T, Durkalski V, Eloubeidi MA, Etamad R, Matsuda K, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441–447. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]
- 26.Larghi A, Noffsinger A, Dye CE, Hart J, Waxman I. EUS-guided fine needle tissue acquisition by using high negative pressure suction for the evaluation of solid masses: a pilot study. Gastrointest Endosc. 2005;62:768–774. doi: 10.1016/j.gie.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Puri R, Vilmann P, Sãftoiu A, Skov BG, Linnemann D, Hassan H, et al. Randomized controlled trial of enoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]