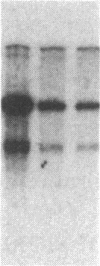
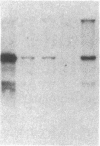
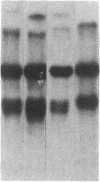
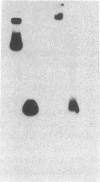
Abstract
The enhancer elements from either simian virus 40 or murine sarcoma virus activate the expression of a transfected rat insulin 1 (rI1) gene when placed within 2.0 kilobases or less of the rI1 gene cap site. Inclusion of 4.0 kilobases of upstream rI1 sequence, however, results in a substantial reduction in the enhancer-dependent insulin gene expression. These observations suggested that a negative transcriptional regulatory element was present between 2.0 and 4.0 kilobases of the rI1 sequence. To test this notion, we employed a heterologous enhancer-dependent transcription assay in which the simian virus 40 72-base-pair repeat is linked to a human beta-globin gene. Addition of the upstream rI1 element to this system decreased the level of enhancer-dependent beta-globin transcription by a factor of 5 to 15. This rI1 "silencer" element functions in a manner relatively independent of position and orientation and requires a cis-dependent relationship to the transcription unit on which it acts. Thus, the silencer sequence seems to have a number of the characteristics of enhancer elements, and we suggest that it may function by the converse of the enhancer mechanism. The rI1 silencer sequence was identified as a member of a long interspersed rat repetitive family. Thus, a potential role for certain repetitive sequences interspersed throughout the eukaryotic genome may be to regulate gene expression by retaining transcriptional activity within defined domains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Cordell B., Bell G., Tischer E., DeNoto F. M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell. 1979 Oct;18(2):533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Fanning T. G., Morris D. W., Cardiff R. D., Bradshaw H. D., Jr Characterization of an endogenous retrovirus-repetitive DNA chimera in the mouse genome. J Virol. 1985 Mar;53(3):998–1000. doi: 10.1128/jvi.53.3.998-1000.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G. Size and structure of the highly repetitive BAM HI element in mice. Nucleic Acids Res. 1983 Aug 11;11(15):5073–5091. doi: 10.1093/nar/11.15.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes D. M., Shenk T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell. 1980 Nov;22(2 Pt 2):405–413. doi: 10.1016/0092-8674(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller D., Jackson M., Leinwand L. Organization and expression of non-Alu family interspersed repetitive DNA sequences in the mouse genome. J Mol Biol. 1984 Mar 15;173(4):419–436. doi: 10.1016/0022-2836(84)90389-9. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikumaran M. S., D'Ambrosio E., Laimins L. A., Lin D. T., Furano A. V. Long interspersed repeated DNA (LINE) causes polymorphism at the rat insulin 1 locus. Mol Cell Biol. 1985 Sep;5(9):2197–2203. doi: 10.1128/mcb.5.9.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Sarver N., Muschel R., Byrne J. C., Khoury G., Howley P. M. Enhancer-dependent expression of the rat preproinsulin gene in bovine papillomavirus type 1 vectors. Mol Cell Biol. 1985 Dec;5(12):3507–3516. doi: 10.1128/mcb.5.12.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Brown F. L., Zavodny P. J., Maio J. J. Transcription of the KpnI families of long interspersed DNAs in human cells. Nature. 1983 Jul 21;304(5923):277–280. doi: 10.1038/304277a0. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Augereau P., Chambon P. The SV40 72 bp repeat preferentially potentiates transcription starting from proximal natural or substitute promoter elements. Cell. 1983 Feb;32(2):503–514. doi: 10.1016/0092-8674(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Witney F. R., Furano A. V. Highly repeated DNA families in the rat. J Biol Chem. 1984 Aug 25;259(16):10481–10492. [PubMed] [Google Scholar]
- de Villiers J., Olson L., Banerji J., Schaffner W. Analysis of the transcriptional enhancer effect. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):911–919. doi: 10.1101/sqb.1983.047.01.105. [DOI] [PubMed] [Google Scholar]