Abstract
Limited information exists on the antibody responses elicited against the viral envelope in HIV-1-infected children. In this cross-sectional study, we assessed the antibody responses against three different immunogenic regions of HIV-1 envelope, namely V3 region of gp120, membrane proximal external region (MPER), and immunodominant loop (IDL) of gp41 in HIV-1-infected children from north India. We recruited 75 HIV-1-infected (40 antiretroviral naive and 35 treated) children, with age ranging from 1.5 to 16 y. Antibodies to V3 and the IDL region were found in a majority of the infected children, whereas antibodies to MPER were found in approximately one-third of the children studied. Higher antibody titers to the immunogenic regions corresponded to the symptomatic stages of HIV-1 infection in both naive and antiretroviral therapy (ART)-treated children. High titers of anti-V3C and anti-IDL antibodies were observed in a subset of antiretroviral-naive patients with suppressed viremia (<47 RNA copies/mL), suggesting that antibodies to these immunogenic regions are present regardless of their viremic status. Further, the antibody titers were significantly lower in the plasma of treated patients compared to naive patients, regardless of whether they were virologically suppressed or not. This is the first report on the antibody responses elicited in HIV-1-infected children in India. The study may help to understand the humoral antibody responses directed against viral envelope in HIV-1-infected children.
Introduction
The pandemic of human immunodeficiency virus (HIV) infection continues to affect millions the world over and more so in the developing countries of Africa and Asia. About 370,000 children were newly infected with HIV-1 infection in 2009 worldwide (10). HIV-1 infection in children continues to occur in resource-limited settings. Children account for 3.5% of all HIV-1 infections in India (16).
HIV-1 infection in children leads to rapid disease progression compared to adults (6,19). They are infected at a time when their immune system is still developing. Nevertheless, antibody- and cell-mediated immune responses develop against HIV-1 in infected children. Antibodies against HIV-1 arise very early in the infected children and they continue to evolve. In one of the early studies, Pollack et al. observed that up to 85% of HIV-1-infected infants had detectable antibodies to two or more viral proteins after 6 mo of life. They also noted that antibodies targeting the HIV-1 envelope antigens gp120 and gp41 are among the first to arise (20). Some of the well known immunogenic regions of HIV-1 envelope include the third variable region (V3) of gp120, membrane proximal external region (MPER), and immunodominant loop (IDL) of gp41 (3,5,11,12). We chose the peptides from consensus clade C HIV-1 envelope as, the majority of infections in India are due to clade C (18).
V3 is one of the most immunogenic regions of the HIV-1 envelope. Antibodies to the V3 region are clade-specific and are used for serotyping of HIV-1 infection (24). Antibodies with cross-reactivity start appearing late during the infection. We studied the binding antibody responses to peptides derived from V3 regions of both consensus clade C (V3C) and clade B (V3B) sequences of HIV-1. We wanted to assess the extent of cross-reactivity of the antibodies binding to V3C and V3B peptides in these children, as all of them were chronically infected. A study conducted by De Rossi et al. (1993) revealed that anti-V3 antibodies are elicited as early as 3 mo of age in HIV-1-infected children compared to uninfected children born to HIV-1-infected mothers (5). Previous studies have correlated antibodies to the MPER region with progression of disease in HIV-1-infected children (9,23). However, there is not enough information on the humoral antibody responses in HIV-1-infected children in relation to viremia and antiretroviral therapy (ART).
We recently reported the efficiency of the plasma of HIV-1-infected children from India in neutralizing the primary isolates (21). In this cross-sectional study, we evaluated the binding antibody responses to three immunogenic regions of the viral envelope, namely V3 region of gp120, and MPER and IDL of gp41, in HIV-1-infected children from north India. We then studied the association of different clinical parameters with the binding antibody response to these regions. Study of the antibody responses directed against the viral envelope will lead to clues for vaccine design targeting this population.
Materials and Methods
Patients
Seventy-five HIV-1-infected children (40 antiretroviral naive and 35 ART treated) were recruited for the study. The infected children are managed as per national treatment guidelines (17). Children less than 18 mo of age were excluded, as the presence of maternal antibodies could affect the results (7). We recorded the demographic and clinical data of the patients using a standardized questionnaire. The plasma viral load was determined by real-time PCR (Roche COBAS TaqMan HIV-1 v2.0; Roche Diagnostics, Indianapolis, IN), and CD4 counts were estimated by flow cytometric analysis (BD Biosciences, Sparks, MD). The CD4 counts are routinely used in monitoring these patients. Written informed consent was obtained from the parents or guardians of all the children. The Institutional Ethics Committee approved this study. Blood samples of these children were collected in EDTA Vacutainers. Plasma was separated by centrifugation at 300 g and stored in aliquots at −80°C until use. All the plasma samples were heat inactivated at 56°C for 1 h before using in the assays.
Peptides
Peptides corresponding to the V3 region of consensus clade C (V3C, 35 mer-CTRPNNNTRKSIRIGPGQTFYATGDIIGDIRQAHC), and consensus clade B (V3B, 35 mer-CTRPNNNTRKSIHIGPGRAFYTTGEIIGDIRQAHC) gp120, consensus clade C MPER (25 mer-DLLALDSWKNLWNWFDITNWLWYIK), and consensus clade C IDL (19 mer-LGIWGCSGKLICTTAVPWN) of gp41 were synthesized commercially (Sigma-Aldrich, St. Louis, MO) and were used for the ELISAs.
ELISA
Peptide binding ELISA was performed following the standard protocol. Briefly, 100 μL of each peptide at a concentration of 1 μg/mL was coated in 96-well ELISA plates (Corning Inc., Corning, NY), in sodium bicarbonate buffer (pH 9.6) and kept at 4°C overnight. The non-specific sites were blocked with 100 μL of 15% fetal bovine serum (FBS) in RPMI media and incubated at 37°C for 1.5 h. Plasma samples (100 μL) at different dilutions (from 1:30 to 1:20,000) were added in duplicate in the respective wells and incubated at 37°C for 1.5 h. Next, 100 μL of alkaline phosphatase-conjugated secondary antibody to human IgG Fc (1:2000) was added followed by incubation at the same conditions. The plates were washed thrice using a plate washer with PBS containing 0.2% Tween-20 (Sigma-Aldrich), after each of the above steps. Alkaline phosphatase substrate (1 mg/mL, 100 μL) in 10% DAE buffer was next added and the absorbance was read after half an hour at 405 nm (Bio-Rad Laboratories, Inc., Hercules, CA). Plasma from two healthy children (ages 6 and 7 y) was used as negative control. We substituted plasma with plain media in the background wells. The assays were repeated for each of the plasma samples against the four peptides at least twice. The cut-off was defined as the mean absorbance values +2 SD of the plasma of healthy children. Inhibitory dilution at 50% binding (ID50) of the polyclonal plasma was calculated for each of the patients against the four peptides separately by using non-linear regression by the method of least squares. For each patient's sample against each of the peptides, mean absorbance calculated from all the experiments was used to calculate ID50 titers.
Plasma total IgG levels
Plasma levels of total IgG were estimated for 75 HIV-1-infected children and 2 healthy children using a quantitative ELISA kit (Ray Biotech, Hercules, CA). Sensitivity of the kit was 1 pg/mL.
Statistical analysis
All the statistical analyses were performed with GraphPad Prism version 5.0. Fisher's exact test was used to compare the categorical variables. Wilcoxon's signed-rank test was applied to compare paired data. Correlation tests were performed using the Spearman rank correlation test. The Mann-Whitney U test was used to compare unpaired data. The Kruskal-Wallis test with post-hoc Dunn's multiple comparison test was used to compare more than two groups of patients. A p value of less than 0.05 was considered significant.
Results
Characteristics of HIV-1 infected children
The demographic and clinical data of the HIV-1-infected children are summarized in Table 1. Among the 75 children, 40 were antiretroviral naive and 35 were on ART. The median age was 8 y (range 1.5–16). Boys constituted 78% of the study population (boys:girls 3.68:1). Median age at diagnosis was 3 y (range, at birth–12). Children on ART were on a regimen of two NRTIs and one NNRTI as recommended by NACO (17).
Table 1.
Demographic and Clinical Data of HIV-1-Infected Indian Children
| Parameter | Naive (n=40) | Treated (n=35) | p Value |
|---|---|---|---|
| Age (y), median (range) | 6 (1.5–14) | 10 (3–16) | <0.001 |
| Sex (boys:girls) | 4:1 | 3.3:1 | 0.78 |
| Age at diagnosis (y), median (range) | 3 (1–12 mo) | 3.5 (1–11 mo) | 0.68 |
| Duration of antiretroviral therapy (y), median (range) | — | 3 (1–10) | — |
| Clinical stagea (WHO) | |||
| Stage 1 (T1) | 24 | 29 | 0.04b |
| Stage 2 (T2) | 13 | 4 | |
| Stage 3 (T3) | 03 | 2 | |
| CD4 count,c cells/μL, median (range) | 649 (131–2458) | 609 (106–1459) | 0.18 |
| Viral load,d RNA copies/mL, median (range) | 18000 (47–585,000) | 2015 (47–30,300) | 0.03 |
| Plasma total IgG, (g/L) (mean±SD) | 13.07±0.07 | 13.12±0.06 | 0.89 |
T1, T2, T3 represent the clinical stage of the treated children.
p Value represents comparison between stage 1 naive, stage 2 and 3 naive children, T1 treated, and T2 and T3 treated children (Fisher's exact test).
CD4 count data were unavailable for 6 naive and 3 treated children.
Viral load data were unavailable for 10 naive and 11 treated children.
No evidence of hypergammaglobulinemia
We evaluated the plasma total IgG levels in 40 naive, 35 treated patients, and 2 healthy children. All the HIV-1-infected children had similar total IgG levels, regardless of their treatment status (Table 1). We did not observe hypergammaglobulinemia in any of the children studied.
A majority of the children had antibodies to V3 and IDL
Antibody titers to the three regions tested are summarized in Table 2 and discussed in detail below. Anti-V3C antibodies were detectable in 92.5% (37 out of 40) naive children, and in 100% (35 out of 35) of treated children. Anti-V3B antibodies were detectable in 70% (28 out of 40) naive children, and in 40% (14 out of 35) of treated children. Anti-IDL antibodies were present in 95% (38 out of 40) naive and 94.3% (33 out of 35) treated children. Anti-MPER antibodies were present in 35% (14 out of 40) of naïve, and 31.4% (11 out of 35) of treated children. The pattern of immunogenicity between naive and treated patients was similar. Antibody titers against V3C and IDL were significantly higher in naive patients than in the treated patients (V3C naive versus treated p<0.0001, IDL naive versus treated p<0.0001), whereas anti-MPER antibody titers were comparable between the two groups (p=0.64).
Table 2.
Antibody Titers (Median Reciprocal ID50)a with Respect to Different Clinical Parameters
| |
V3C |
IDL |
MPER |
|||
|---|---|---|---|---|---|---|
| Peptide | Naive | Treated | Naive | Treated | Naive | Treated |
| Overall titers (40 N, 35 T)b | 6550 | 1300 | 2050 | 250 | 100 | 100 |
| Clinical stage | ||||||
| Stage 1/T1 (24 N, 29 T) | 7150 | 1150 | 2225 | 250 | 100 | 100 |
| Stage 2,3/T2,3 (16 N, 6 T) | 5713 | 2250 | 1675 | 1275 | 138 | 125 |
| Age | ||||||
| <5 years (13 N, 2 T) | 6825 | 1125c | 2500 | 400 | 100 | 250 |
| ≥5 years (27 N, 33 T) | 5250 | 1300 | 1700 | 250 | 100 | 100 |
| CD4 countd | ||||||
| <350 cells/μL (4 N, 9 T) | 7213 | 2900 | 5250 | 900 | 550 | 100 |
| ≥350 cells/μL (20 N, 22 T) | 6175 | 1100 | 1725 | 225 | 100 | 100 |
| Viral load | ||||||
| <47 RNA copies/mL (9 N, 8 T) | 6175 | 550 | 2300 | 250 | 100 | 100 |
| >47 RNA copies/ml (21 N, 16 T) | 7100 | 2075 | 2150 | 400 | 100 | 100 |
Median reciprocal ID50 titers were calculated from individual ID50 titers of each patient in the respective groups. Individual ID50 titers of the plasma samples whose values were less than 100 (more than three times the lowest dilution tested 1:30) were assigned a value of 100, and then the median reciprocal ID50 titers were calculated for each subgroup.
Numbers in parentheses represent the number of children analyzed in each group (N=naive; T=treated).
Those with italicized and underlined numbers represent mean ID50 titers in the respective groups.
Children <5 y of age and for whom CD4 count was unavailable were excluded from the analysis.
ID50, inhibitory dilution at 50% binding.
Cross-reactive anti-V3 antibodies
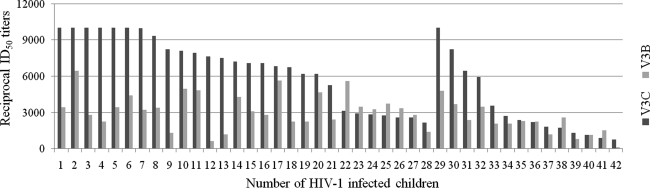
We assessed the antibody binding of the polyclonal plasma to both V3C and V3B peptides to detect cross-reactive anti-V3 antibodies in these infected children. We found that 56% (42 out of 75, 28 naïve, and 14 treated) of the children having anti-V3 antibodies reacting to both V3C and V3B peptides (Fig. 1). Median ID50 titers to V3C peptide (naive 6550, treated 1300; Table 2) were significantly higher (p<0.0001 V3C versus V3B, for both naive and treated children, paired data) than to V3B peptide (naive 2600, treated 100).
FIG. 1.
Antibody titers to V3C and V3B peptides in 42 HIV-1 infected children, which showed cross-reactive anti-V3 antibodies. One to 28 represent naive children and 29–42 represent ART treated children.
Antibody response in relation to clinical stage
We grouped the naive and treated patients according to the clinical stage (WHO) (17) into two groups, stage 1 and stage 2 or 3 (Table 1). Three naive children were of WHO clinical stage 3 who were enrolled just before the initiation of ART. Among the naive children, anti-MPER antibodies were significantly less in stage 1 children compared to stage 2 or 3 children (p=0.02). There was no significant difference in anti-V3C (p=0.35) and anti-IDL (p=0.68) antibodies between the two groups of naive children. Among the treated children, anti-IDL antibodies were significantly less in stage T1 children compared to stage T2 or T3 children (p=0.02), and approached significance for anti-V3C antibodies (p=0.07). The anti-MPER antibody titers were comparable (p=0.32) between the two groups of treated children.
Antibody response in relation to CD4 counts
Absolute CD4 counts vary with age and the variation is less pronounced in children more than 3 y of age (2,22). The cut-off value for immunosuppression for children ≥5 y of age in terms of CD4 counts is same as that of adults per the WHO immunological classification of HIV-1-infected children (17). Hence, we divided the drug-naive children into <5 y (n=13) and ≥5 y of age (n=27). We found that the CD4 counts were significantly higher (p=0.03) in children age <5 y (mean±SD, 1132±715 cells/μL) compared to those ≥5 y of age (mean±SD 648±336 cells/μL). Yet the antibody responses to all the three regions were comparable between the two groups (Table 2; V3C p=0.22; IDL p=0.66; MPER p=0.92). Direct correlation tests in both the groups (<5 y and ≥5 y) of naive children, between CD4 counts and antibody titers against the three regions revealed no significant correlation (data not shown). In ART-treated children ≥5 y of age (n=31), a significant negative correlation was observed between CD4 counts and anti-V3C (r=−0.45, p=0.01), and anti-IDL (r=−0.38, p=0.03) antibodies, but not against anti-MPER antibodies (r=0.05, p=0.8; data not shown). There were only two ART-treated children <5 y of age. We also compared the antibody responses between ART-treated patients with CD4 counts ≥350 cells/μL (n=22) and <350 cells/μL (n=9), leaving out the four children with age <5 y (n=1) for whom CD4 count data were not available (n=3). We found that the anti-IDL antibodies (p=0.002) were significantly lower and approached significance for anti-V3C antibodies (p=0.06) in the patients with CD4 counts ≥350 cells/μL, while for anti-MPER antibodies (p=0.81) there was no significant difference between the two groups. There were only four naive children with CD4 counts <350 cells/μL.
Antibody response in relation to viremia and ART
To assess the correlation of viremia and ART with antibody responses, we categorized the drug-naive and drug-treated patients based on viral load into suppressors (viral load <47 copies/mL), and non-suppressors (viral load >47 copies/mL) [naive suppressors (N-SUP) n=9; naive non-suppressors (N-NSUP) n=21; treated suppressors (T-SUP) n=8; treated non-suppressors (T-NSUP) n=16]. The viral load data for 10 naive and 11 treated children were unavailable. The median viral load in N-SUP, N-NSUP, T-SUP, and T-NSUP were 47, 49,050, 47, and 4755 RNA copies/mL, respectively.
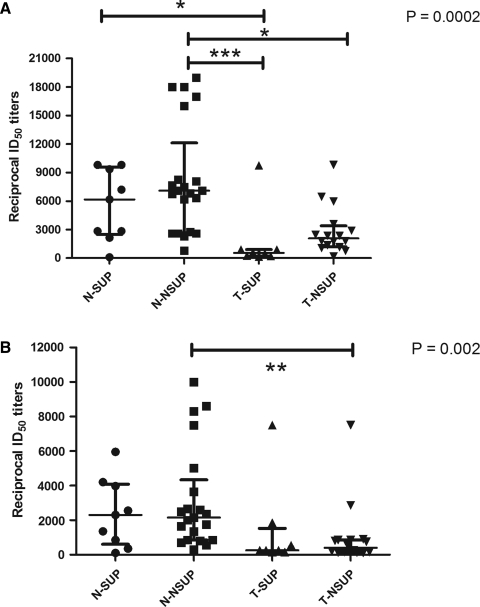
We compared the anti-V3C, anti-IDL, and anti-MPER antibodies in N-SUP, N-NSUP, T-SUP, and T-NSUP groups of infected children. There was no significant difference in the antibody response to V3C and IDL between N-SUP and N-NSUP, suggesting that the polyclonal antibody response elicited by these subgroups of naive patients is similar regardless of their viremic status. Similarly, there was no significant difference in the antibody response between T-SUP and T-NSUP children. The antibody response of N-SUP children tended to be higher though not significant (by Dunn's multiple comparison tests) than T-NSUP children (Fig. 2A and 2B).
FIG. 2.
(A) Antibodies to V3C in HIV-1-infected children. (B) Antibodies to IDL in HIV-1-infected children. Anti-V3C and anti-IDL antibody titers in HIV-1-infected children grouped according to their treatment status and viremia. Patients whose viral loads were undetectable (<47 RNA copies/mL) were categorized as suppressors and the rest as non-suppressors. The bars represent median ID50 titers with interquartile range in the respective patient groups. p Value shows the overall significance comparing all the four groups by the Kruskal-Wallis test. Significance levels shown are by Dunn's multiple comparison test (*p<0.05, **p<0.01, ***p<0.001; N-SUP, naive suppressor with viral load <47 RNA copies/mL [n=9]; N-NSUP, naive non-suppressor with viral load >47 RNA copies/mL [n=21]; T-SUP, treated suppressor with viral load <47 RNA copies/mL [n=8]; T-NSUP, treated non-suppressor with viral load >47 RNA copies/mL [n=16]).
Anti-V3C antibodies were significantly less in T-SUP compared to both N-SUP and N-NSUP patients. T-NSUP children had significantly lower anti-V3C antibody titers compared to N-NSUP children, although both the groups had high levels of viremia (Fig. 2A). Anti-IDL antibody titers were significantly lower in the T-NSUP compared to the N-NSUP children. The titers of anti-IDL antibodies were comparable in all the other subgroups of children (Fig. 2B). There was no significant difference between any of the subgroups in the titers of anti-MPER antibodies (Table 2).
Discussion
The present study evaluated the antibody responses in HIV-1-infected children against three different regions of viral envelope that are known to be immunogenic. Antibodies against V3 and IDL were present in a majority of the HIV-1-infected children at high titers, whereas anti-MPER antibodies were present in low titers. The presence of anti-V3C antibodies and anti-IDL antibodies in most of the infected children confirmed their immunogenic nature, and is in agreement with previous studies in adults (11,25). Cross-reactive anti-V3 antibodies have been shown to be present in HIV-1-infected children (5). We also observed highly cross-reactive anti-V3 antibodies in more than half of the children studied. Previous studies on the antibody reactivity to the MPER region in both adult and pediatric patients have correlated with the disease stage and/or disease progression (9,15,23). We observed that anti-MPER antibodies were significantly higher in the symptomatic (stage 2 and 3) HIV-1-infected antiretroviral-naive children compared to the asymptomatic children. This was in contrast to previously reported studies in adults and children, which showed a decrease in the antibody reactivity to the MPER region in stage C patients according to the CDC classification (9,15). The anti-IDL antibody titers in HIV-1-infected children were significantly higher in treated children who were symptomatic. In general, higher antibody titers to the immunogenic regions corresponded to the symptomatic stages of HIV-1 infection in both naive and ART-treated children. The inverse correlation of the antibody titers with CD4 counts in the treated children is also corroborative with the above finding.
Antiretroviral naive HIV-1-infected children had higher antibody titers to the immunogenic regions compared to the ART-treated children. The presence of high antibody titers in a subset of antiretroviral-naive patients with suppressed viremia implies that there may be a continuous antigenic stimulation, even in virologically-suppressed children (Fig. 2A and B). There is a significant difference in the viral load (Table 1) in the treated patients compared to naive patients, showing the effectiveness of ART in lowering viremia in these children. Despite this, 45% (16 out of 35) of the treated children had high viremia, with some children having very high viral load (>10,000 RNA copies/mL), suggesting virological failure in them (1). Antibody titers have been shown to be decreased after the initiation of ART, largely owing to the reduction in viremia (4,8,13,14), as has also been observed by us. Interestingly, we found significantly lower antibody titers (against V3C and IDL), even in those treated patients without virological suppression, compared to naive non-suppressors (Fig. 2A and B). This observation suggests that ART leads to decreased antibody titers regardless of their viremic status. The findings reported here, however, need to be confirmed by studying a larger number of patients.
We did not determine the clade of the viral isolates infecting these children, which is one of the major limitations of this study. A lack of CD4 count data and viral load data in some of our patients constitute another limitation. Information regarding these would have further strengthened our findings. Our study, for the first time, has characterized the envelope-specific antibody responses in HIV-1-infected Indian children, and correlated them with different clinical, immunological, and virological parameters. A follow-up study may shed more light on the kinetics of anti-HIV-1 envelope antibody responses in these children, which could help in developing envelope-based vaccines against HIV-1.
Acknowledgments
We thank the study participants. This work was supported by a grant from the Indian Council of Medical Research (grant no. HIV/50/137/2010-ECD-II).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2006 revision. http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [Mar 1;2011 >]. http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf [PubMed]
- 2.Bunders M. Cortina-Borja M. Newell ML. European Collaborative Study: Age-related standards for total lymphocyte, CD4+ and CD8+ T cell counts in children born in Europe. Pediatr Infect Dis J. 2005;24:595–600. doi: 10.1097/01.inf.0000168835.01233.64. [DOI] [PubMed] [Google Scholar]
- 3.Davis KL. Gray ES. Moore PL, et al. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology. 2009;387:414–426. doi: 10.1016/j.virol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deayton JR. Sabin CA. Britt WB, et al. Rapid reconstitution of humoral immunity against cytomegalovirus but not HIV following highly active antiretroviral therapy. AIDS. 2002;16:2129–2135. doi: 10.1097/00002030-200211080-00004. [DOI] [PubMed] [Google Scholar]
- 5.De Rossi A. Zanotto C. Mammano F. Ometto L. Del Mistro A. Chieco-Bianchi L. Pattern of antibody response against the V3 loop in children with vertically acquired immunodeficiency virus type 1 (HIV-1) infection. AIDS Res Hum Retroviruses. 1993;9:221–228. doi: 10.1089/aid.1993.9.221. [DOI] [PubMed] [Google Scholar]
- 6.Devi NP. Shenbagavalli R. Ramesh K. Rathinam SN. Swaminathan S. Rapid progression of HIV infection in infancy. Indian Pediatr. 2009;46:53–56. [PubMed] [Google Scholar]
- 7.European collaborative study: Children born to women with HIV-1 infection: natural history and risk of transmission. Lancet. 1991;337:253–260. [PubMed] [Google Scholar]
- 8.Falkensammer B. Freissmuth D. Hubner L. Speth C. Dierich MP. Stoiber H. Changes in HIV-specific antibody responses and neutralization titers in patients under ART. Front Biosci. 2007;12:2148–2158. doi: 10.2741/2218. [DOI] [PubMed] [Google Scholar]
- 9.Geffin RB. Scott GB. Melenwick M, et al. Association of antibody reactivity to ELDKWA, a glycoprotein 41 neutralization epitope, with disease progression in children perinatally infected with HIV type 1. AIDS Res Hum Retroviruses. 1998;14:579–590. doi: 10.1089/aid.1998.14.579. [DOI] [PubMed] [Google Scholar]
- 10.Global report: UNAIDS report on the global AIDS epidemic 2010. http://www.unaids.org/documents/20101123_GlobalReport_em.pdf. [Mar 1;2011 ]. http://www.unaids.org/documents/20101123_GlobalReport_em.pdf
- 11.Gnann JW. Nelson JA. Oldstone MB. Fine mapping of an immunodominant domain in the transmembrane glycoprotein of human immunodeficiency virus. J Virol. 1987;61:2639–2641. doi: 10.1128/jvi.61.8.2639-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montero M. van Houten NE. Wang X. Scott JK. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope dominant site of antibody neutralization and target for vaccine design. Microbiol Mol Biol Rev. 2008;72:54–84. doi: 10.1128/MMBR.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris L. Binley JM. Clas BA, et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris MK. Katzenstein DA. Israelski D. Zolopa A. Hendry RM. Hanson CV. Characterization of the HIV-1 specific humoral immune response during highly active antiretroviral therapy (HAART) J Acquir Immune Defic Syndr. 2001;28:405–415. doi: 10.1097/00042560-200112150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Muhlbacher M. Spruth M. Siegel F. Zangerle R. Dierich MP. Longitudinal study of antibody reactivity against HIV-1 envelope and a peptide representing a conserved site on Gp41 in HIV-1 infected patients. Immunobiology. 1999;200:295–305. doi: 10.1016/s0171-2985(99)80078-3. [DOI] [PubMed] [Google Scholar]
- 16.NACO Annual Report 2009-10. http://www.nacoonline.org/upload/AR%202009-10/NACO_AR_English%20corrected.pdf. [Mar 1;2011 ]. http://www.nacoonline.org/upload/AR%202009-10/NACO_AR_English%20corrected.pdf
- 17.NACO guidelines for HIV care and treatment in infants and children. Nov, 2006. http://www.whoindia.org/LinkFiles/HIV-AIDS_NACO_guidelines_on_ART_for_paediatric_HIV_AIDS.pdf. [Mar 1;2011 ]. http://www.whoindia.org/LinkFiles/HIV-AIDS_NACO_guidelines_on_ART_for_paediatric_HIV_AIDS.pdf
- 18.Neogi U. Sood V. Banerjee S, et al. Global HIV-1 molecular epidemiology with special reference to genetic analysis of HIV-1 subtypes circulating in North India: functional and pathogenic implications of genetic variation. Indian J Exp Biol. 2009;47:424–431. [PubMed] [Google Scholar]
- 19.Palumbo PE. Raskino C. Fiscus S, et al. Disease progression in HIV infected infants and children: Predictive value of HIV RNA and CD4 lymphocyte count. JAMA. 1998;279:756–761. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- 20.Pollack H. Zhan MX. Ilmet-Moore T. Ajuang-Simbiri K. Krasinski K. Borkowsky W. Ontogeny of anti-human immunodeficiency virus (HIV) antibody production in HIV-1 infected infants. Proc Natl Acad Sci USA. 1993;90:2340–2344. doi: 10.1073/pnas.90.6.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakash SS. Chaudhary AK. Lodha R, et al. Efficient neutralization of primary isolates by the plasma from HIV-1 infected Indian children. Viral Immunol. 2011;24(5):409–413. doi: 10.1089/vim.2011.0028. [DOI] [PubMed] [Google Scholar]
- 22.Shah I. Correlation of CD4 count, CD4% and HIV viral load with clinical manifestations of HIV in infected Indian children. Ann Trop Paediatr. 2006;26:115–119. doi: 10.1179/146532806X107458. [DOI] [PubMed] [Google Scholar]
- 23.Srisurapanon S. Louisirirotchanakul S. Sumransurp K. Ratanasrithong M. Chuenchitra T. Jintakatkorn S. Wasi C. Binding antibody to neutralizing epitope gp41 in HIV-1 subtype CRF 01_AE infection related to stage of disease. Southeast Asian J Trop Med Public Health. 2005;36:221–227. [PubMed] [Google Scholar]
- 24.Walker PR. Cilliers T. Choge IA. Taylor N. Cohen SS. Morris L. High specificity of V3 serotyping among human immunodeficiency virus type-1 subtype C infected patients with varying disease status and viral phenotype. J Med Virol. 2006;78:1262–1278. doi: 10.1002/jmv.20690. [DOI] [PubMed] [Google Scholar]
- 25.Warren RQ. Wolf H. Zajac RA. Boswell RN. Kanda P. Kennedy RC. Patterns of antibody reactivity to selected human immunodeficiency virus type 1 (HIV-1) gp160 epitopes infected individuals grouped according to CD4+ cell levels. J Clin Immunol. 1991;11:13–21. doi: 10.1007/BF00918790. [DOI] [PubMed] [Google Scholar]