Background: Fascin is a pro-metastasis actin-bundling protein overexpressed in metastatic tumors.
Results: TGFβ induced fascin expression in spindle tumor cells through Smads.
Conclusion: Fascin is a TGFβ target gene essential for the pro-invasion activity of TGFβ.
Significance: Our data shed new light on how TGFβ dysregulates actin cytoskeleton to promote tumor metastasis.
Keywords: Cell Migration, Cytoskeleton, Invasion, Metastasis, Transforming Growth Factor Beta (TGFbeta), Actin Cytoskeleton, Fascin, Filopodia
Abstract
Fascin, an actin-bundling protein overexpressed in all carcinomas, has been associated with poor prognosis, shorter survival, and more metastatic diseases. It is believed that fascin facilitates tumor metastasis by promoting the formation of invasive membrane protrusions. However, the mechanisms by which fascin is overexpressed in tumors are not clear. TGFβ is a cytokine secreted by tumor and mesenchymal cells and promotes metastasis in many late stage tumors. The pro-metastasis mechanisms of TGFβ remain to be fully elucidated. Here we demonstrated that TGFβ induced fascin expression in spindle-shaped tumor cells through the canonical Smad-dependent pathway. Fascin was critical for TGFβ-promoted filopodia formation, migration, and invasion in spindle tumor cells. More importantly, fascin expression significantly correlates with TGFβ1 and TGFβ receptor I levels in a cohort of primary breast tumor samples. Our results indicate that elevated TGFβ level in the tumor microenvironment may be responsible for fascin overexpression in some of the metastatic tumors. Our data also suggest that fascin could play a central role in TGFβ-promoted tumor metastasis.
Introduction
One essential characteristic of metastatic cancer cells is enhanced motility, which facilitates the infiltration of metastatic cells into lymphatic and blood vessels and extravasation out of the circulation (1). The forces that drive tumor cell migration and invasion are provided by the actin cytoskeleton underlying the critical membrane protrusions in migrating tumor cells (2). To efficiently drive the formation of membrane protrusions, it is crucial to cross-link actin filaments into bundles as individual actin filaments are flexible. Cross-linking by bundling protein provides the essential rigidity to counter the compressive forces from the plasma membrane (3).
Fascin is an actin-bundling protein critical for tumor metastasis (4–7). Expression levels of fascin are very low or not detected in normal epithelia, but are highly elevated in malignant tumors (4). Overexpression of fascin protein is associated with poor prognosis in patients (7–12). Knockdown of fascin expression inhibited tumor cell migration and invasion in vitro and decreased tumor metastasis in mouse models. Moreover, ectopic expression of fascin promoted tumor cell invasion and metastasis (5, 6, 13). The causal role of fascin overexpression in tumor metastasis is well established; however, the molecular mechanisms underlying elevated fascin level in metastatic tumors are not clear.
TGFβ is a cytokine in the tumor microenvironment that regulates various tumor progressions in a context-dependent manner (14). In early stage tumors, TGFβ is a potent proliferation inhibitor that deters tumor growth; however, late stage tumors are often able to evade the growth inhibition and secrete elevated levels of TGFβ to promote metastasis (15). The mechanisms by which late stage tumors use TGFβ signaling to promote tumor spreading are largely unknown.
Accompanying the loss of capacity to differentiate during tumor progression, tumor cells undergo a transition in gene expression, reorganization of cytoskeleton, and acquisition of spindle cell morphology (16). Tumors with spindle cell morphology were characterized as highly malignant and invasive (17). In breast cancer, fascin is overexpressed in the estrogen− receptor-negative, basal-like subgroup (11), a highly metastatic group of breast cancers typically with spindle cell morphology (18). In melanoma, elongated, spindle-like tumor cells showed intense fascin staining, whereas rounded, amoeboid-like melanoma cells were generally fascin-negative (19).
Here, we demonstrate that TGFβ elevates fascin protein expression and promotes invasion and filopodia formation in tumor cells with spindle morphology, but not in tumor cells with epithelial-like, polygonal morphology. We also show that transcription of fascin mRNA induced by TGFβ is independent of de novo protein synthesis but relies on the canonical Smad-dependent pathway. Furthermore, fascin is essential for TGFβ to promote invasion and filopodia formation in spindle tumor cells. Therefore, our data suggest that fascin is an immediate TGFβ target gene essential for its pro-invasion activity. Our data also suggest that TGFβ might be responsible for the fascin overexpression in some metastatic tumors.
EXPERIMENTAL PROCEDURES
Cell Culture Media
The cell culture media used were DMEM (for MDA-MB-231 and MCF-7), F-12K (for A549), and RPMI (for CHL-1, WM115, and H1299). All cell culture media were supplemented with 10% fetal bovine serum and penicillin/streptomycin.
Antibodies
The following antibodies were used in this study: fascin, Santa Cruz Biotechnology antibody number sc-21743; phospho-ERK1/2, Cell Signaling antibody number 9101; phospho-c-Jun(Ser-63), Cell Signaling antibody number 9261; Smad3, Cell Signaling antibody number 9523; phospho-Smad3(Ser-423/425), Cell Signaling antibody number 9520; GAPDH, Sigma product number G8795.
RNA Interference
RNAi of Smad2, Smad3, Smad4, and fascin was performed using pSUPER.Retro.puro vector (Oligoengine) encoding small hairpin RNA. The previously reported target sequences were used: GGTGGGCAAAGATGAGCTC (Fascin) (6), GGTGGGCAAAGATGAGCTC (Smad2) (20), GGACGAGGTCTGCGTGAAT (Smad3) (20), and GGTGTGCAGTTGGAATGTA (Smad4) (20).
TGFβ and Inhibitor Treatment
Unless stated otherwise, all cells were treated with 10 ng/ml TGFβ1 (Peprotech, Rocky Hill, NJ) in growth medium for 2 days before being used for assays. Inhibitors, when used, were added together with TGFβ to growth medium.
Transwell Cell Migration Assay
Cells (1 × 105) suspended in starvation medium were added to the upper chamber of an insert (6.5-mm diameter, 8-μm pore size, BD Biosciences), and the insert was placed in a 24-well dish containing starvation medium with or without 10% FBS (21, 22). Migration assays were carried out for 4–6 h for spindle tumor cells and 12–24 h for polygonal tumor cells. Cells were fixed with 3.7% formaldehyde and stained with crystal violet staining solution, and cells on the upper side of the insert were removed with a cotton swab. Three randomly selected fields (10× objectives) on the lower side of the insert were photographed, and the migrated cells were counted. The migration was expressed as the average number of migrated cells in a field.
Cell Invasion Assay
Cells (1 × 105) suspended in starvation medium were added to the upper chamber of a Matrigel-coated insert (6.5-mm diameter, 8-μm pore size, BD Biosciences), and the insert was placed in a 24-well dish containing medium with or without serum. Invasion assays were carried out for 16 h, and cells were fixed with 3.7% formaldehyde. Cells were stained with crystal violet staining solution, and cells on the upper side of the insert were removed with a cotton swab. Three randomly selected fields (10× objectives) on the lower side of the insert were photographed, and the cells on the lower surface of the insert were counted.
Immunofluorescence Microscopy
A549 cells cultured on collagen-coated glass coverslips were fixed with 3.7% paraformaldehyde in PBS for 10 min at room temperature, permeabilized with 0.1% Triton X-100 for 5 min, and then washed with PBS three times. To block nonspecific binding, the cells were incubated with a solution of PBS containing 1% bovine serum albumin for 30 min and then incubated with Alexa Fluor 594-labeled phalloidin (Invitrogen). The coverslips were then fixed onto slides and imaged using a Zeiss fluorescence microscope.
Live Cell Imaging
A549 cells with or without TGFβ pretreatment were plated on collagen-coated glass-bottomed 35-mm tissue culture dishes (MatTek) overnight. The membrane protrusion dynamics and cell movement were recorded with a differential interference contrast microscopy under a 40× objective using a Zeiss inverted microscope equipped with a live imaging chamber. The temperature and CO2 concentration in the chamber were maintained at 37 °C and 5%, respectively. Time-lapse images were recorded at 10-s intervals.
qRT-PCR
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen), and the reverse transcription was performed using the iScript cDNA synthesis kit (Bio-Rad). The quantitative real-time PCR (qRT-PCR)3 assay was carried out with the Applied Biosystems 7900HT fast real-time PCR system using Applied Biosystems SYBR Green PCR master mix. Primers for qRT-PCR are shown in supplemental Table S1. All reactions were performed in triplicate, and the experiment was repeated three times.
RESULTS
TGFβ Promotes the Migration of Spindle-shaped Tumor Cells
It was recently reported that exposure to TGFβ within the tumor microenvironment might predispose tumor cells for metastasis to distant organs (14). To evaluate the effects of TGFβ exposure on the migration of tumor cells, we treated a panel of tumor cell lines with TGFβ. We used two breast cancer lines (MDA-MB-231, and MCF-7), two non-small cell lung cancer lines (A549 and H1299), and two melanoma lines (CHL-1 and WM115). Three of the six cell lines (MDA-MB-231, A549, and CHL-1) have spindle cell morphology, whereas the other three (MCF-7, H1299, and WM115) have polygonal, epithelial-like morphology (Fig. 1A). After 48 h of treatment, we found that TGFβ induced morphology change in the three spindle-shaped cell lines, with cells becoming more elongated and disperse. Extremely long and finger-like protrusions were also observed in some TGFβ-pretreated cells. However, the effects of TGFβ on the morphology of the three polygonal, epithelial-like tumor cells were much less noticeable (Fig. 1A).
FIGURE 1.
TGFβ promoted cell migration in spindle-shaped tumor cells. A, morphology changes induced by TGFβ. Spindle-shaped tumor cells (MDA-MB-231, A549, and CHL-1) became more elongated and scattered after TGFβ treatment. Tumor cells were cultured in growth medium with or without 10 ng/ml TGFβ. The cell morphologies were recorded with phase contrast microscopy using a 10× objective. Scale bar, 50 μm. B, the effects of TGFβ pretreatment on tumor cell migration. TGFβ pretreatment promoted cell migration in spindle-shaped tumor cells, but not in polygon-shaped tumor cells. The data presented are mean ± S.D. of migrated cells per field from three randomly selected 10× fields. C–F, kymograph analysis of membrane protrusion dynamics in control A549 cell (C and D) and TGFβ-pretreated A549 cells (E and F); C and E, individual frames from time-lapse movies (supplemental Movies 1 and 2) used to generate the kymographs (D and F). Descending and ascending contours in the kymographs indicate membrane protrusion and withdrawal events, respectively. Arrowheads in D and F indicate new membrane protrusions. G, the extension of membrane protrusions in control and TGFβ-treated cells.
Next, we examined the effects of TGFβ pretreatment on cell migration using the Transwell migration assay. Interestingly, TGFβ priming significantly increased the migration of the three spindle-shaped tumor cells by about 3–10-fold (Fig. 1B). In sharp contrast, TGFβ had little effect on (MCF-7 and H1299) or slightly inhibited (WM115) the migration of the three polygon-shaped tumor cell lines (Fig. 1B).
To characterize the functional characteristics of spindle-shaped and polygon-shaped tumor cells in the absence of TGFβ, we used the Transwell assay to evaluate their mobility and invasiveness. Tumor cells were allowed to migration/invade for 6 and 12 h, respectively. As shown in supplemental Fig. S1, the three spindle-shaped tumor cell lines migrate and invade much faster than the three polygonal tumor cells, consistent with the notion that spindle-shaped tumor cells are more invasive and metastatic.
TGFβ Induces Hyperactive Membrane Protrusions
Cell migration is a multistep process involving the formation of lamellipodia, turnover of focal adhesions, contraction of cell body, and retraction of trailing tail (23). To investigate the mechanisms underlying the enhanced cell migration in TGFβ-pretreated spindle tumor cells, we used live cell imaging to study the membrane protrusion dynamics at the leading edge of migrating cells. As shown in supplemental Movies 1 and 2, TGFβ pretreatment induced hyperactive membrane protrusion in A549 cells. The membrane protrusion dynamics in the TGFβ-pretreated cells followed a protrusion-ruffle-protrusion pattern (supplemental Fig. S2). New lamellipodia extended from the edge of membrane ruffles, sometimes expanding in the space between adjacent filopodia. At the end of the lamellipodium expansion, the edge of lamellipodia pulled back and formed membrane ruffles. New filopodia or lamellipodia would extend from the edge of membrane ruffles and begin the next protrusion cycle. In contrast, lamellipodia usually pulled back without membrane ruffle formation in control cells (supplemental Fig. S2 and supplemental Movies 1 and 2).
Next, we employed kymography to analyze the membrane protrusion kinetics in control cells and TGFβ-pretreated cells. A line was drawn at the leading edge of a migrating cell; intensity values along the defined line region in each image of a time-lapse series were extracted and assembled together side-by-side to generate a kymograph montage (Fig. 1, C–F). As shown in the kymograph in Fig. 1, C and D, transient protrusions frequently extended out from the lamellipodium region of the control A549 cells and quickly withdrew to the starting point, generating spike-like structures on a flat basal line on the kymograph. The leading edge of the control A549 cells barely moved forward during the recording period (Fig. 1, D and G). In contrast, the kymographs of TGFβ-pretreated cells appeared as stair-like structures, indicating that the lamellipodia in these cells extended in “bursts”; in addition, the newly formed protrusionsβ in TGFβ-treated cells were able to hold their positions for a period of time until the next extension, instead of withdrawing to the starting point in the control cell. Consequently, the leading edges moved steadily forward following each extension (Fig. 1, F and G). The hyperactive membrane protrusions may contribute to increased mobility in TGFβ-treated cells.
TGFβ Induces Actin Cytoskeleton Remodeling
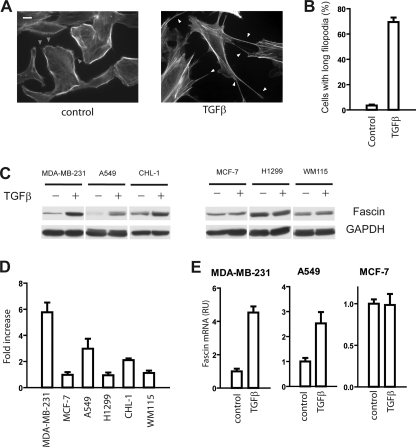
The morphology changes and hyperactive membrane protrusions suggested remodeling of the actin cytoskeleton in TGFβ-treated spindle tumor cells. Therefore, we used fluorescence microscopy to visualize actin filaments in the cells. As shown in Fig. 2A, the phalloidin staining in control A549 cells showed typical cortical actin staining, with the majority of F-actin in the cortical region. On the outer periphery of the strong actin staining, numerous weak and wavy filopodia-like protrusions were also observed in most of the control cells (Fig. 2A, gray arrowheads). In contrast, stress fibers were noted in the majority of the TGFβ-pretreated A549 cells, consistent with previous observations that TGFβ promoted stress fiber formation (24, 25). Most strikingly, TGFβ induced very long and straight filopodia (filopodia protruded more than 20 μm out of the cell boundary) (Fig. 2A). Typically, several such long filopodia extended around the cells, rendering a distinct “spiky” morphology in TGFβ-treated A549 cells. The extremely long filopodia were observed in 70% of TGFβ-treated cells (110 out of a total of 158 cells); in contrast, only less than 5% (8 out of a total of 228 cells) of control cells had such filopodia (Fig. 2B).
FIGURE 2.
TGFβ elevated fascin expression level in spindle tumor cells. A, staining of F-actin in A549 cells with fluorescence-labeled phalloidin. The weak and wavy filopodia in control cells were indicated by the gray arrowheads. The long filopodia in TGFβ-treated cells were indicated by the white arrowheads. The scale bar is 10 μm. B, quantification of cells with long filopodia in A549 cells with TGFβ or control treatment. Results are mean ± S.D. from 3 experiments. C, TGFβ treatment increased the protein expression of fascin in spindle tumor cells (MDA-MB-231, A549, and CHL-1), but not in polygonal tumor cells (MCF-7, H1299, and WM115). The protein levels of fascin in control or TGFβ-treated tumor cells were determined by Western blotting. Results are representatives from 3 independent experiments. D, quantification of the Western blotting results in A by densitometry. Results are mean ± S.D. of 2–5 experiments. E, effects of TGFβ treatment on the mRNA expression of fascin in MDA-MB-231, A549, and MCF-7 cells. Results are mean ± S.D. of triplicates. RU, relative units.
Elevated Fascin Expression in Spindle Tumor Cells after TGFβ Treatment
Filopodia are finger-like protrusions critical for tumor cell invasion and metastasis (26). Cross-linking of parallel actin filaments is considered to be crucial for filopodia formation as individual filaments lack the rigidity required to overcome the compressive forces from plasma membrane (3). Fascin is a key actin-bundling protein in filopodia (27), and fascin overexpression has been reported in all carcinomas examined to date (4). The long filopodia in TGFβ-pretreated cells prompted us to examine the possibility that TGFβ might regulate fascin expression in tumor cells. The effects of TGFβ treatment on fascin protein levels in these tumor cells were evaluated with Western blotting using a fascin-specific antibody. TGFβ treatment elevated fascin protein levels by 2–6-fold in the three spindle tumor cells. In sharp contrast, no TGFβ-induced fascin expression was observed in any of the polygonal tumor cells (Fig. 2, C and D).
To investigate whether TGFβ regulates the degradation of fascin protein, we used cycloheximide (CHX), a protein synthesis inhibitor, to inhibit the new synthesis of fascin in A549 cells and used Western blotting to monitor fascin degradation at different time points after CHX treatment. No degradation of fascin protein in control cells or in TGFβ-treated cells was detected even 24 h after CHX treatment, indicating that fascin is a very stable protein (data not shown). Next, we investigated whether TGFβ regulated fascin expression at the mRNA level in two spindle tumor cell lines (A549 and MDA-MB-231 cells) and one polygonal cell line (MCF-7 cells) using qRT-PCR. Consistent with our Western blot results, we found that TGFβ treatment elevated fascin mRNA levels in A549 and MDA-MB-231 cells, but not in MCF-7 cells, suggesting that TGFβ promoted the transcription of fascin only in spindle tumor cells, but not in polygonal tumor cells (Fig. 2E).
Induction of Fascin Expression by TGFβ Requires Smad3 and Smad4
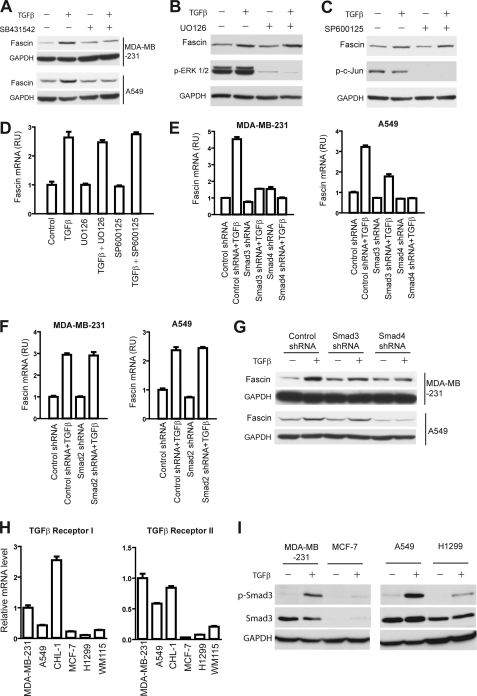
To explore the mechanisms through which TGFβ elevates fascin expression, we first used SB-431542, a TGFβ receptor I (TβRI) kinase inhibitor, to inhibit the activity of the TGFβ receptor complex. Treatment with SB-431542 completely blocked the overexpression of fascin in TGFβ-treated MDA-MB-231 cells and A549 cells, suggesting that TβRI was essential to mediating the TGFβ effects (Fig. 3A).
FIGURE 3.
Fascin is a TGFβ target gene regulated by Smad3 and Smad4. A, inhibition of TGFβ-induced fascin expression by TβRI kinase inhibitor SB431542 in MDA-MB-231 cells and A549 cells. B–D, the effects of MEK inhibitor UO126 (10 μm) and JNK inhibitor SP600125 (20 μm) on TGFβ-induced fascin expression at the protein level (B and C) and the mRNA level (D). MDA-MB-231 cells were treated with TGFβ and the two inhibitors as indicated for 6 h (B and C) or 24 h (D), respectively; the mRNA and protein levels were determined through qRT-PCR and Western blotting. Results± are mean ± S.D. from 3 experiments. p-ERK1/2, phospho-ERK1/2; p-c-Jun, phospho-c-Jun; RU, relative units. E and F, the role of Smads in TGFβ-induced fascin expression. MDA-MB-231 and A549 cells stably expressing Smad3, Smad4, and Smad2 shRNA or control shRNA were treated with 10 ng/ml TGFβ, and the expression of fascin mRNA was determined using qRT-PCR. Smad3 and Smad4 shRNA inhibited TGFβ-induced fascin expression, whereas Smad2 shRNA had no effect. Results are mean ± S.D. of triplicates. G, Western blots showing that Smad3 and Smad4 knockdown inhibited TGFβ-induced expression of fascin protein in MDA-MB-231 cells and A549 cells. Results are representative of 3 independent experiments. H, relative TβRI and TβRII mRNA levels in the six tumor cell lines as determined by qRT-PCR. Results are mean ± S.D. from 3 experiments. I, TGFβ-induced Smad3 phosphorylation in breast cancer and non-small cell lung cancer cells. Cells were treated with 10 ng/ml TGFβ1 for 3 h, and phospho-Smad3 (p-Smad3) levels were detected through Western blotting.
TGFβ could regulate gene transcription either through the Smad-dependent pathways or through the Smad-independent pathways by activation of Erk, JNK, and p38 MAPK (28). We first examined the role of Erk and JNK because it was recently reported that the core promoter region of fascin contains a binding site for AP-1/CREB, which could be activated by Erk and JNK through phosphorylation (29, 30). The addition of the MEK inhibitor UO126 and JNK inhibitor SP600125 significantly reduced the amount of phospho-Erk 1/2 and phospho-c-Jun, respectively, in both control cells and TGFβ-treated cells, indicating the successful inhibition of MAPK and JNK activity (Fig. 3, B and C). However, neither UO126 nor SP600125 had any effect on TGFβ-induced fascin expression (Fig. 3, B–D). We further examined the role of p38 MAPK using p38 kinase inhibitor SB203580 (supplemental Fig. S3). Treatment with p38 inhibitor failed to inhibit TGFβ-induced fascin expression at the mRNA level or the protein level, suggesting that ERK, JNK, and p38 MAPK were not involved (supplemental Fig. S3 and Fig. 3, B–D).
To explore whether TGFβ-induced fascin expression is mediated through the TGFβ-Smad pathway, we employed small hairpin RNA (shRNA) to knock down the expression of Smad4. The knockdown of Smad4 was confirmed with qRT-PCR (supplemental Fig. S4C). MDA-MB-231 and A549 cells stably expressing control shRNA or Smad4 shRNAs were cultured in TGFβ or in control medium, and the expression of fascin was evaluated with qRT-PCR and Western blotting. As shown in Fig. 3, E and G, Smad4 shRNA almost completely inhibited TGFβ-induced fascin expression at both the mRNA and the protein level, arguing that fascin is regulated by TGFβ through the canonical TβRI-Smad pathway. To investigate whether either or both of the two receptor-regulated Smads (R-Smads) are involved, we further used shRNA to decrease the expression of Smad2 and Smad3 (supplemental Fig. S4, A and B). Smad3 shRNA, but not Smad2 shRNA, reduced the TGFβ-induced expression in both MDA-MB-231 cells and A549 cells, suggesting that fascin expression is regulated by TGFβ through the TβRI-Smad3-Smad4 pathway, whereas Smad2 is not required (Fig. 3, E and F).
Characterization of TGFβ Signaling in Spindle-shaped and Polygon-shaped Tumor Cells
To gain insight into the differential regulation of fascin by TGFβ in spindle- and polygon-shaped tumor cells, we used qRT-PCR to examine the expression levels TβRI and TβRII in the six cell lines (Fig. 3H). As shown in Fig. 3H, TGFβ receptor I and receptor II mRNA levels were higher in the spindle-shaped tumor cells (MDA-MB-231, A549, and CHL-1)than in the polygonal cells (MCF7, H1299 and WM115). We further examined the total Smad3 and phoso-Smad3 level in the two breast cancer cell lines (MDA-MB-231 and MCF7) and the two non-small cell lung cancer cell lines (A549 and H1299). As shown in Fig. 3I, both the total Smad3 and the TGFβ-induced phosho-Smad3 levels are higher in the MDA-MB-231 and A549 cells when compared with MCF7 and H1299 cells, respectively. The lower TβRI, TβRII, and phospho-Smad3 levels in the polygonal tumor cells may lead to global inhibition of TGFβ responses. To examine this possibility, we examined the effect of TGFβ treatment on the transcription of several other genes regulated by TGFβ, including p21Cip, p27Kip, N-cadherin, and vimentin, and we noted that TGFβ failed to induce the transcription of any of these genes in MCF-7 cells (supplemental Fig. S5). Therefore, the global inhibition of TGFβ response in some cells might at least partially explain the lack of TGFβ-induced fascin expression.
Induction of Fascin Expression by TGFβ Requires No de Novo Protein Synthesis
To explore the kinetics of TGFβ-induced fascin mRNA expression, we used qRT-PCR to determine fascin mRNA levels in MDA-MB-231 cells after treatment with TGFβ for different periods of time. As shown in Fig. 4A (upper panel), there was a 3-h lag in TGFβ-induced fascin transcription. Fascin mRNA levels increased steadily after the lag, reaching the plateau level (∼6.4-fold) after 24 h and remaining at this plateau for at least another 24 h. Next, we further evaluated the effects of TGFβ on the protein expression kinetics of fascin. As shown in Fig. 4A (lower panel), TGFβ significantly elevated the fascin protein level after 24 h, and the fascin protein level remained high for up to at least 72 h. A similar time course was also observed in A549 cells (data not shown), suggesting that TGFβ was able to induce and maintain elevated fascin expression in spindle tumor cells.
FIGURE 4.
TGFβ regulation of fascin transcription requires no de novo protein synthesis. A, upper panel, the kinetics of TGFβ-induced expression of fascin mRNA in MDA-MB-231 cells. MDA-MB-231 cells were treated with TGFβ, and the mRNA levels in the cells at the indicated time points were determined through qRT-PCR. Results are mean ± S.D. of triplicates. Lower panel, the kinetics of TGFβ-induced expression of fascin protein in MDA-MB-231 cells. Cells were treated with TGFβ and lysed at different time points as indicated. The levels of fascin protein were determined through Western blotting. RU, relative units. B, MDA-MB-231 cells were treated with TGFβ for different time points as indicated, and phospho-Smad3 (p-Smad3) levels were determined through Western blotting. C, TGFβ was able to induce fascin mRNA expression in the presence of protein synthesis inhibitor CHX (10 μm). Results± are mean ± S.D. of triplicates. D, CHX (10 μm) inhibited the regulation of E-cadherin and N-cadherin transcription by TGFβ. Results are mean ± S.D. of triplicates. E, treatment with MG132 (10 μm) inhibited TGFβ-induced fascin transcription. Results are mean ± S.D. of triplicates.
We further examined the kinetics of Smad3 phosphorylation in MDA-MB-231 cells. Smad3 phosphorylation was increased by more than 10-fold 3 h after TGFβ treatment. Phospho-Smad3 level decreased gradually after 3 h but remained at a fairly high level for at least 26 h (Fig. 4B).
Next, we investigated whether TGFβ-induced fascin transcription required de novo protein synthesis. TGFβ regulates the expression of a plethora of genes. Some are immediately downstream of the TGFβ signaling cascade, whereas others are indirectly modulated through TGFβ-regulated transcription factors (31, 32). To determine whether the induction of fascin gene transcription by TGFβ was immediately downstream of the TGFβ signaling cascade, we used CHX to block de novo protein synthesis. CHX treatment did not change the TGFβ-induced expression of fascin mRNA in MDA-MB-231 cells and A549 cells, indicating that elevated fascin expression does not require new protein synthesis (Fig. 4C). As controls, CHX treatment successfully blocked the inhibition of E-cadherin transcription and the induction of N-cadherin mRNA expression by TGFβ (Fig. 4D), confirming that gene transcriptions indirectly regulated by TGFβ through other transcription factors required new protein synthesis.
It has been previously reported that TGFβ signaling accelerates the degradation of Smad co-repressors such as Ski and SnoN through the ubiquitin-proteasome pathway (33). To determine whether the ubiquitin-proteasome pathway might be required of TGFβ-induced fascin transcription, we used proteasome inhibitor MG132 to treat MDA-MB-231 cells. As shown in Fig. 4E, treatment with MG132 significantly decreased TGFβ-induced fascin transcription, indicating that degradation of co-suppressors might be involved in the regulation of fascin expression.
Fascin Is Required for TGFβ-induced Invasion and Filopodia Formation
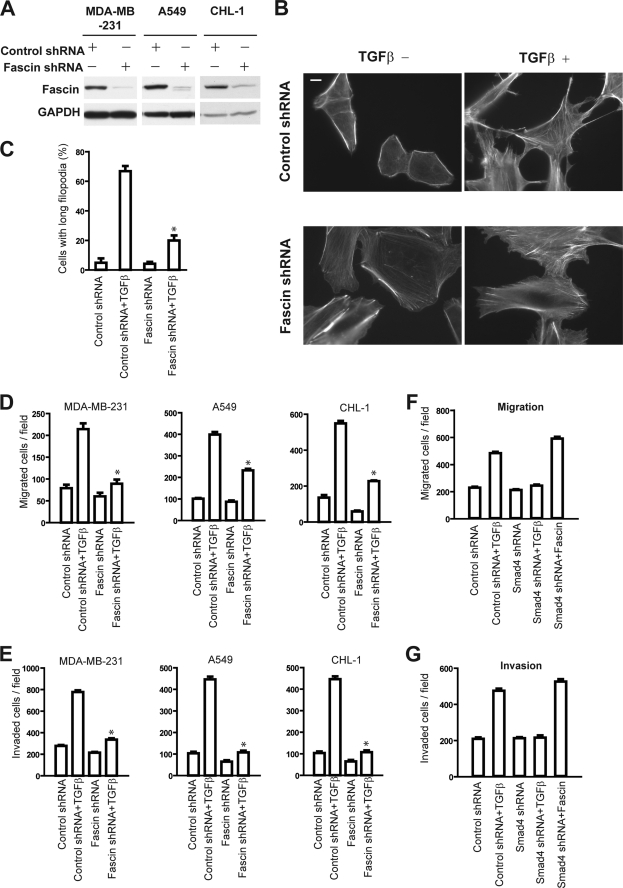
To explore the role of fascin in TGFβ-induced actin cytoskeleton remodeling and cell migration, we employed shRNA to knock down fascin expression in spindle tumor cells. The successful knockdown of fascin protein was confirmed by Western blotting (Fig. 5A). To assess the role of fascin in TGFβ-induced filopodia formation, A549 cells stably expressing control shRNA or fascin shRNA were treated with TGFβ and stained for F-actin. TGFβ induced long filopodia and spiky morphology in control shRNA cells (Fig. 5B). TGFβ-induced long filopodia were noted in about 67% of A549 cells expressing control shRNA (183 out of a total of 274 cells examined) (Fig. 5C). In contrast, TGFβ-induced long filopodia were only observed in roughly 20% of A549 fascin shRNA cells (60 out of a total of 294 cells examined), suggesting that fascin was critical for TGFβ-induced filopodia formation (Fig. 5, B and C).
FIGURE 5.
Fascin is required for TGFβ-induced filopodia formation, cell migration, and invasion. A, Western blots showing that fascin shRNA decrease the protein level of fascin in MDA-MB-231, A549, and CHL-1 cells. B, F-actin staining showing that TGFβ induced long filopodia in A549 cells expressing control shRNA, but not in cells expressing fascin shRNA. The scale bar is 10 μm. Results are representative of 3 experiments. C, quantification of cells with long filopodia in A549 cells expressing control shRNA or fascin shRNA, with or without TGFβ treatment, respectively. D and E, the effects of TGFβ pretreatment on tumor cell migration (D) and invasion (E) in spindle tumor cells expressing control shRNA or fascin shRNA, respectively. Fascin knockdown with shRNA inhibited TGFβ-promoted tumor cell migration and invasion. F and G, ectopic fascin overexpression restore migration (F) and invasion (G) in MDA-MB-231 stably expressing Smad4 shRNA. The data in C–G are representative of either 3–5 similar experiments or mean ± S.D. of 3 experiments. *, p < 0.05.
Next, we investigated the role of fascin in TGFβ-promoted spindle tumor cell migration. Using the Transwell migration assay, we found that TGFβ-promoted cell migration was significantly lower in fascin shRNA-treated cells when compared with the control shRNA-expressing cells, suggesting that fascin was important for TGFβ-induced cell migration. (Fig. 5D).
To further assess the role of fascin in TGFβ-promoted metastasis in spindle cell tumors, we evaluated the effects of TGFβ on the invasion ability of spindle tumor cells stably expressing fascin or control shRNA. TGFβ pretreatment promoted the invasion of control shRNA-expressing spindle tumor cells by about 3–5-fold, consistent with the pro-metastasis function of TGFβ. Expression of fascin shRNA dramatically inhibited TGFβ-promoted invasion in all three of the spindle tumor cells. In contrast to the 3–5-fold invasion increase in control shRNA cells, TGFβ was only able to promote invasion by about 1.2–1.5-fold in fascin shRNA cells (Fig. 5E).
To further evaluate the hypothesis that fascin is critical for TGFβ-induced tumor cell migration and invasion, we overexpressed fascin in tumor cells expressing Smad4 shRNA. As shown in Fig. 5, F and G, Smad4 knockdown nearly completely abolished TGFβ-induced migration and invasion in MDA-MB-231 cells, consistent with the observation that Smad4 wasβ essential for TGFβ-induced fascin expression; ectopic fascin expression restored shSmad4 cell migration and invasion to levels similar to TGFβ-primed control cells, consistent with the notion that fascin is critical for TGFβ-induced migration and invasion in metastatic tumor cells.
Correlation between TGFβ1, TβRI, and Fascin Expression Levels in Primary Breast Tumor Samples
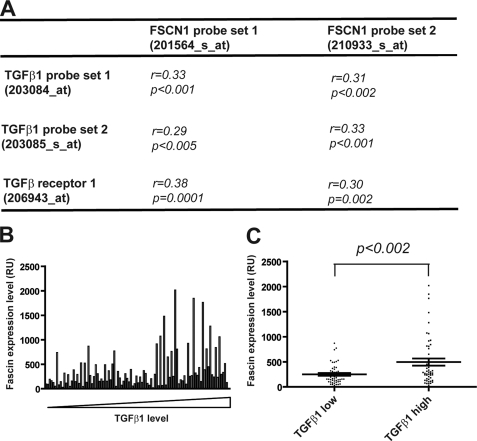
To validate our cell culture-based discoveries in breast cancer patients, we examined the correlation between fascin levels and TGFβ as well as TGFβ receptor levels in a cohort of 99 primary breast tumor samples collected at the Memorial Sloan-Kettering Cancer Center (34). Pearson correlation coefficient (r) and probability (p) values between the two fascin probe sets and each of the probe sets for TGFβ1, TGFβ2, and TGFβ3 and TGFβ receptor I and II were calculated. Fascin expression significantly correlates with TGFβ1 and TGFβ receptor I, but not with TGFβ2 and TGFβ3 or TGFβ receptor II (Fig. 6A and data not shown). Next, tumor samples were sorted according to TGFβ1 expression level (Fig. 6B). The 50 tumor samples with TGFβ1 level at or below median level were assigned to the “TGFβ low” group, and the other 49 samples with TGFβ1 level above the median level were assigned to the “TGFβ1 high” group. Fascin (FSCN1) levels in the TGFβ1 high group were about 2-fold as high as the TGFβ1 low group (p < 0.002) (Fig. 6C), suggesting that elevated TGFβ1 levels in primary breast tumors contributed to fascin overexpression.
FIGURE 6.
Fascin expression levels correlate with TGFβ1 and TGFβ receptor 1 levels in primary breast tumors. A, correlation between fascin (FSCN1), TGFβ1, and TGFβ receptor 1 probe sets in 99 primary tumor samples in the Sloan cohort. Pearson correlation coefficient values (r) and probability values (p) were calculated using Excel. B, fascin expression levels in the 99 breast tumor samples. Tumor samples were sorted according to TGFβ1 levels. RU, relative units. C, fascin expression levels in the TGFβ1 low and TGFβ1 high groups. p value was calculated using two-tailed Student's t test.
DISCUSSION
Fascin Expression Is Regulated by TGFβ
Although there are a plethora of studies about fascin overexpression in various carcinomas (4, 5, 7, 8, 11, 12), the factors contributing to fascin overexpression in metastatic tumors remain largely unknown. Binding sites for several transcription factors, including β-catenin-TCF (T-cell factor), CREB, and AP-1, in the FSCN1 promoter region have been previously reported (5, 29, 30, 35). However, it is not clear whether and how factors in the tumor microenvironment contribute to fascin overexpression in metastatic tumor cells. Our results here demonstrated that TGFβ promoted fascin expression in spindle tumor cells, implicating that cytokines in the tumor microenvironment could affect fascin expression.
The up-regulation of fascin by TGFβ was surprising in a sense because TGFβ target genes have been studied previously in various epithelial and tumor cell lines (including MDA-MB-231), and fascin was not among the TGFβ response genes identified through microarray screening (14, 36). We noticed that previous screenings focused on early response genes and that tumor cells were treated with TGFβ for no more than 3 h (14, 36). Therefore, we explored the kinetics of TGFβ-induced transcription of fascin. Our data revealed a 3-h lag in TGFβ-induced fascin expression (Fig. 3D), which may explain the discrepancy between our data and previous studies. The lag might be due to the requirement for TGFβ-induced degradation of Smad co-suppressors as proteasome inhibitor MG132 inhibited TGFβ-induced fascin transcription. Despite the lag, fascin expression is likely directly downstream of TGFβ signaling because TGFβ was able to promote the expression of fascin mRNA in the presence of CHX, a protein synthesis inhibitor.
Fascin Is Regulated by TGFβ through the Canonical TβRI-Smad Pathway
Our data further demonstrated that fascin expression was regulated through the canonical TβRI-Smad pathway. Smads in the TGFβ pathway recognized the consensus DNA sequence CAGAC (37). To determine whether the human fascin 1 gene contains Smad-binding sites, we examined the promoter region of FSCN1 and discovered that there were two CAGAC sequences at −381 and −1225 positions, respectively. It is possible that activated Smad3 and Smad4 induced fascin expression by binding to one or both of the Smad-binding sites.
It is intriguing to note that TGFβ only induced fascin expression in spindle tumor cells, but not in polygonal cells. One way for tumor cells to overcome growth inhibition effects exerted by TGFβ is through loss of expression or functional inactivation of TβRI and TβRII (38). We noted that that TβRI and TβRII levels in the three spindle tumor cells were higher than the three polygonal cells. The reduced expression of TGFβ receptors might partially explain the lack of TGFβ-induced fascin expression in polygonal cells.
The Smad co-activator context in cells may also contribute to the regulation of fascin by TGFβ. Although Smad3 and Smad4 can directly bind to DNA to mediate gene transcription, interaction between Smads and their target sequence is of low affinity, and DNA-binding co-factors are required to provide specific regulation of gene transcriptions (37). Consequently, the transcriptomic output of activated Smads is highly dependent on the cellular context of Smad co-factors (15). The conversion from polygonal morphology to spindle morphology during tumor progression is a recessive event resulting from the change of gene expressions that control epithelial differentiation (16). It is possible that the transition from epithelial-like to mesenchymal-like tumor cells provides the cellular co-factor context required for the induction of fascin expression by activated Smads. We noted that some polygonal tumor cells (e.g. H1299 and WM115, data not shown) have fairly high fascin levels when compared with epithelial cells, suggesting that factors other than TGFβ also contribute to fascin overexpression in tumors.
Role of Fascin in TGFβ-promoted Tumor Metastasis
The presence of TGFβ in the tumor microenvironment has profound impacts on tumor progression and metastasis (14, 15, 17). Activated TGFβ signaling in primary tumor was linked to lung metastasis in estrogen receptor-negative breast tumors (14). Our data demonstrated that TGFβ induced fascin expression and promoted migration/invasion in MDA-MB-231 breast tumor cells. More importantly, TGFβ1 and TGFβ receptor I significantly correlate with fascin (FSCN1) level levels in a cohort of primary breast tumors, suggesting that activation of TGFβ signaling may contribute to fascin overexpression in primary tumors. Interestingly, fascin overexpression in breast tumor has been associated with lung metastasis, and fascin is one of the top performing lung metastasis signature genes (34). It is possible that elevated fascin expression is critical for TGFβ to promote tumor cell migration, invasion, and lung metastasis in estrogen receptor-negative breast tumors. Indeed, TGFβ promoted migration and invasion in all three spindle tumor cell lines with elevated fascin expression, but not in the three polygonal tumor cells, in which TGFβ-induced fascin expression was absent.
More importantly, knockdown of fascin expression with shRNA significantly inhibited migration and invasion in TGFβ-treated spindle tumor cells. The near complete inhibition of TGFβ-promoted invasion by fascin shRNA was quite remarkable considering that TGFβ also up-regulates the expression of other pro-invasion genes such as matrix metalloproteases 2 and 9 (17, 39). During invasion, tumor cells use protrusion structures termed invadopodia to secrete matrix metalloproteases and to coordinate the degradation of extracellular matrix (40). The formation and elongation of invadopodia requires filopodia-associated protein components (41). Fascin is the major actin-bundling protein in filopodia protrusions (27). It was recently reported that fascin was critical to stabilize actin in invadopodia (26, 41, 42). Depletion of fascin with shRNA destabilized invadopodia and impaired extracellular matrix degradation (42). Given the similar components and regulatory mechanism between invadopodia and filopodia, it has been suggested that invadopodia are “invasive filopodia” (26, 42). Our data showed that fascin was required for long filopodia induced by TGFβ. It is possible that fascin played a central role in the invasive machinery mobilized by TGFβ in spindle tumor cells. By stabilizing actin cytoskeleton within invadopodia and filopodia, fascin coordinates the invasion and metastasis promoted by TGFβ.
Supplementary Material
Acknowledgments
We thank Drs. Srikumar Chellappan and Xin-Yun Huang for critically reading the manuscript, Dr. Steven Enkeman for consultation on microarray data mining, the Analytic Microscopy Core at Moffitt Cancer center for image acquisition assistance, and Rasa Hamilton for editorial assistance.
This work was supported in part by an Institutional Research Grant from the American Cancer Society (IRG-93-032-16), a Career Development Award from the Donald A. Adams Comprehensive Melanoma Research Center, and a Milestone Award from Miles for Moffitt Foundation Center (to S. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental supplemental Movies 1 and 2 and Figs. S1–S5.
- qRT-PCR
- quantitative real-time PCR
- CHX
- cycloheximide
- TβRI
- TGFβ receptor I
- TβRII
- TGFβ receptor II
- CREB
- cAMP-response element-binding protein.
REFERENCES
- 1. Mareel M., Leroy A. (2003) Physiol. Rev. 83, 337–376 [DOI] [PubMed] [Google Scholar]
- 2. Webb D. J., Parsons J. T., Horwitz A. F. (2002) Nat. Cell Biol. 4, E97–100 [DOI] [PubMed] [Google Scholar]
- 3. Mogilner A., Rubinstein B. (2005) Biophys. J. 89, 782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hashimoto Y., Skacel M., Adams J. C. (2005) Int. J. Biochem. Cell Biol. 37, 1787–1804 [DOI] [PubMed] [Google Scholar]
- 5. Vignjevic D., Schoumacher M., Gavert N., Janssen K. P., Jih G., Laé M., Louvard D., Ben-Ze'ev A., Robine S. (2007) Cancer Res. 67, 6844–6853 [DOI] [PubMed] [Google Scholar]
- 6. Chen L., Yang S., Jakoncic J., Zhang J. J., Huang X. Y. (2010) Nature 464, 1062–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pelosi G., Pasini F., Fraggetta F., Pastorino U., Iannucci A., Maisonneuve P., Arrigoni G., De Manzoni G., Bresaola E., Viale G. (2003) Lung Cancer 42, 203–213 [DOI] [PubMed] [Google Scholar]
- 8. Hashimoto Y., Skacel M., Lavery I. C., Mukherjee A. L., Casey G., Adams J. C. (2006) BMC Cancer 6, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hashimoto Y., Shimada Y., Kawamura J., Yamasaki S., Imamura M. (2004) Oncology 67, 262–270 [DOI] [PubMed] [Google Scholar]
- 10. Hashimoto Y., Ito T., Inoue H., Okumura T., Tanaka E., Tsunoda S., Higashiyama M., Watanabe G., Imamura M., Shimada Y. (2005) Clin. Cancer Res. 11, 2597–2605 [DOI] [PubMed] [Google Scholar]
- 11. Yoder B. J., Tso E., Skacel M., Pettay J., Tarr S., Budd T., Tubbs R. R., Adams J. C., Hicks D. G. (2005) Clin. Cancer Res. 11, 186–192 [PubMed] [Google Scholar]
- 12. Puppa G., Maisonneuve P., Sonzogni A., Masullo M., Chiappa A., Valerio M., Zampino M. G., Franceschetti I., Capelli P., Chilosi M., Menestrina F., Viale G., Pelosi G. (2007) Br. J. Cancer 96, 1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hashimoto Y., Parsons M., Adams J. C. (2007) Mol. Biol. Cell 18, 4591–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Padua D., Zhang X. H., Wang Q., Nadal C., Gerald W. L., Gomis R. R., Massagué J. (2008) Cell 133, 66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang Y. (2006) J. Cell Biochem. 98, 1380–1390 [DOI] [PubMed] [Google Scholar]
- 16. Stoler A. B., Stenback F., Balmain A. (1993) J. Cell Biol. 122, 1103–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Derynck R., Akhurst R. J., Balmain A. (2001) Nat. Genet. 29, 117–129 [DOI] [PubMed] [Google Scholar]
- 18. Neve R. M., Chin K., Fridlyand J., Yeh J., Baehner F. L., Fevr T., Clark L., Bayani N., Coppe J. P., Tong F., Speed T., Spellman P. T., DeVries S., Lapuk A., Wang N. J., Kuo W. L., Stilwell J. L., Pinkel D., Albertson D. G., Waldman F. M., McCormick F., Dickson R. B., Johnson M. D., Lippman M., Ethier S., Gazdar A., Gray J. W. (2006) Cancer Cell 10, 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goncharuk V. N., Ross J. S., Carlson J. A. (2002) J. Cutan Pathol. 29, 430–438 [DOI] [PubMed] [Google Scholar]
- 20. He W., Dorn D. C., Erdjument-Bromage H., Tempst P., Moore M. A., Massagué J. (2006) Cell 125, 929–941 [DOI] [PubMed] [Google Scholar]
- 21. Yang S., Huang X. Y. (2005) J. Biol. Chem. 280, 27130–27137 [DOI] [PubMed] [Google Scholar]
- 22. Yang S., Zhang J. J., Huang X. Y. (2009) Cancer Cell 15, 124–134 [DOI] [PubMed] [Google Scholar]
- 23. Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 24. Moustakas A., Stournaras C. (1999) J. Cell Sci. 112, 1169–1179 [DOI] [PubMed] [Google Scholar]
- 25. Bakin A. V., Safina A., Rinehart C., Daroqui C., Darbary H., Helfman D. M. (2004) Mol. Biol. Cell 15, 4682–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machesky L. M., Li A. (2010) Commun. Integr. Biol. 3, 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vignjevic D., Kojima S., Aratyn Y., Danciu O., Svitkina T., Borisy G. G. (2006) J. Cell Biol. 174, 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Derynck R., Zhang Y. E. (2003) Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto Y., Loftis D. W., Adams J. C. (2009) PLoS One 4, e5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bros M., Ross X. L., Pautz A., Reske-Kunz A. B., Ross R. (2003) J. Immunol. 171, 1825–1834 [DOI] [PubMed] [Google Scholar]
- 31. Feng X. H., Lin X., Derynck R. (2000) EMBO J. 19, 5178–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romano L. A., Runyan R. B. (2000) Dev. Biol. 223, 91–102 [DOI] [PubMed] [Google Scholar]
- 33. Liu X., Sun Y., Weinberg R. A., Lodish H. F. (2001) Cytokine Growth Factor Rev. 12, 1–8 [DOI] [PubMed] [Google Scholar]
- 34. Minn A. J., Gupta G. P., Siegel P. M., Bos P. D., Shu W., Giri D. D., Viale A., Olshen A. B., Gerald W. L., Massagué J. (2005) Nature 436, 518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grothey A., Hashizume R., Ji H., Tubb B. E., Patrick C. W., Jr., Yu D., Mooney E. E., McCrea P. D. (2000) Oncogene 19, 4864–4875 [DOI] [PubMed] [Google Scholar]
- 36. Adorno M., Cordenonsi M., Montagner M., Dupont S., Wong C., Hann B., Solari A., Bobisse S., Rondina M. B., Guzzardo V., Parenti A. R., Rosato A., Bicciato S., Balmain A., Piccolo S. (2009) Cell 137, 87–98 [DOI] [PubMed] [Google Scholar]
- 37. Massagué J., Chen Y. G. (2000) Genes Dev. 14, 627–644 [PubMed] [Google Scholar]
- 38. Buck M. B., Fritz P., Dippon J., Zugmaier G., Knabbe C. (2004) Clin. Cancer Res. 10, 491–498 [DOI] [PubMed] [Google Scholar]
- 39. Hagedorn H. G., Bachmeier B. E., Nerlich A. G. (2001) Int. J. Oncol. 18, 669–681 [DOI] [PubMed] [Google Scholar]
- 40. Yamaguchi H., Lorenz M., Kempiak S., Sarmiento C., Coniglio S., Symons M., Segall J., Eddy R., Miki H., Takenawa T., Condeelis J. (2005) J. Cell Biol. 168, 441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schoumacher M., Goldman R. D., Louvard D., Vignjevic D. M. (2010) J. Cell Biol. 189, 541–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li A., Dawson J. C., Forero-Vargas M., Spence H. J., Yu X., König I., Anderson K., Machesky L. M. (2010) Curr. Biol. 20, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.