Background: The outer membrane multivalent adhesion molecule 7 (MAM7) initiates host cell killing by Gram-negative pathogens.
Results: Biochemical dissection of MAM7 binding to low affinity fibronectin and high affinity phosphatidic acid interactions.
Conclusion: Bacteria initially bind to host cells using a tripartite complex: MAM7, fibronectin, and phosphatidic acid.
Significance: This characterization is essential for developing MAM7-derived tools for the attenuation of Gram-negative bacterial infections.
Keywords: Bacterial Pathogenesis, Ligand Binding Protein, Pathogenesis, Phosphatidic Acid, Receptors, Gram-negative Pathogens, MAM7, Adhesin, Fibronectin
Abstract
The ability of a pathogen to rapidly form a stable interaction with the host cell surface is key to its success. Bacterial pathogens use a repertoire of virulence factors, but their efficient use relies on close contact between the host and the pathogen. We have recently identified a constitutively expressed MAM7 (multivalent adhesion molecule 7), which is widely distributed in Gram-negative pathogens and enables them to establish initial contact with the host cell. Here, we describe the dissection of the MAM7 interaction with the host cell surface into two distinct binding events, involving the host protein fibronectin and the membrane phospholipid phosphatidic acid. We analyzed which domains within MAM7 and fibronectin are necessary for complex formation. We further studied phosphatidic acid binding by MAM7 using site-directed mutagenesis and liposome association assays and demonstrated that a specific distribution of basic charge on MAM7 is required for high affinity binding. Finally, we showed that fibronectin and phosphatidic acid binding to MAM7 are not mutually exclusive and that the three molecules likely assemble into a tripartite complex on the host cell surface.
Introduction
Initial host cell binding is one of the most important steps in bacterial pathogenesis. When the invading pathogen first encounters the host, its ability to rapidly establish a high affinity interaction with eukaryotic cells is decisive for the fate of the infection. Bacterial pathogens possess a large arsenal of virulence factors (toxins and effectors) to deploy against the host upon their first encounter. The high molecular weight toxins are first secreted into the extracellular environment but need to be in the immediate vicinity of the host cell for localized high concentration of the toxins so that they can efficiently dock and translocate into the host cells. Effector proteins require direct contact between pathogen and host to be directly translocated from the bacteria into the host cell cytoplasm via type III and type IV secretion systems (1–4). Therefore, most virulence mechanisms require intimate contact between the host and the pathogen to successfully support infection. We recently identified a novel adhesin, MAM7 (multivalent adhesion molecule 7), which is widely distributed in Gram-negative bacteria including, but not limited to, important pathogens such as Vibrio, Yersinia, Salmonella, and enteropathogenic E. coli (EPEC)2 (5)). MAM7 consists of a hydrophobic stretch of 44 amino acids at its N terminus, which is required for correct localization and outer membrane anchoring of the protein, followed by seven mammalian cell entry (mce) domains. Single mce domain proteins have been described previously to be important for attachment to and invasion of macrophages by mycobacteria, but the structural and molecular details of these processes remain unknown (6, 7). MAM7 is constitutively expressed, enabling Gram-negative pathogens to establish immediate contact with host cells upon their first encounter, which in turn can lead to up-regulation of other, pathogen- and host cell-specific adhesins and virulence factors (5, 8–10). Deletion of MAM7 from Vibrio cholerae, Vibrio parahaemolyticus, and Yersinia pseudotuberculosis lead to a delay in onset of cytotoxicity and overall decrease in cytotoxicity against host cells. Deletion of MAM7 from EPEC delayed the formation of actin pedestals, which are formed as a result of direct host cell contact (11). Our initial characterization of MAM7 from V. parahaemolyticus has revealed two types of host cell ligands: the protein fibronectin and the membrane phospholipid phosphatidic acid.
Fibronectin (Fn) is a high molecular weight glycoprotein consisting of two subunits of ∼220 kDa, although the exact composition and molecular weight varies between different splice variants. In its soluble form, fibronectin is an abundant component of blood plasma (300 μg/ml), but it is also capable of forming fibrils, which can be integrated into the extracellular matrix or incorporated into fibrin clots (12, 13). Fibronectin also functions as a receptor for a variety of bacterial adhesins, both from Gram-positive and Gram-negative pathogens (14, 15). In some cases, Fn binding by bacterial adhesins leads to integrin-mediated uptake of the pathogen, making it an important factor for tissue invasion during bacterial infection (16, 17).
Phosphatidic acids (PAs) are phospholipids consisting of a glycerol backbone linked to a phosphate headgroup via C3 and two fatty acid chains via C1 and C2. Although phosphatidic acids only make up an average of 1–4% of the total phospholipid content of a cell (18), they play an important role as precursors for the biogenesis of other phospholipids in determining membrane curvature and as signaling molecules (19–21). Several PA-binding proteins are known, including Raf-1 kinase, mTOR, and the protein-tyrosine phosphatase SHP-1 (22–24). As such, PA is involved in the regulation of pathways covering a wide variety of cellular functions such as metabolism, trafficking, and proliferation.
In this study, we dissected the interaction of the adhesin MAM7 with host cells into discrete binding events and analyzed their contribution to overall host cell binding. We analyzed MAM7 for features defining its ability to interact with fibronectin and phosphatidic acid, respectively, and defined the region of fibronectin required for its interaction with MAM7. We also studied whether MAM7 is capable of binding to fibronectin and phosphatidic acid simultaneously and found that the three molecules likely form a tripartite complex.
EXPERIMENTAL PROCEDURES
Construction of Plasmids
Cloning for expression in BL21 of MAM7, MBP-MAM7 (for fluorophore labeling), and GST-MAM7 has been described elsewhere (5). Constructs for GST-MAM6, -mce1–5, -mce2–6, -mce3–7 as well as GST-mce1 to -mce7 were all amplified from V. parahaemolyticus POR1 genomic DNA and cloned into the plasmid pGEX-rTEV using BamHI and NotI sites. GST-mce2 point mutants were generated by whole plasmid mutagenesis using GST-mce2 as template. GST-mce1 concatemers containing three, five, or seven mce1 domains were generated by amplifying mce 1 fragments containing the following restriction sites: BamHI/XbaI, XbaI/HindIII, HindIII/XhoI, XhoI/EcoRI, EcoRI/PstI, PstI/NcoI, and NcoI/NotI for mce1 fragments 1–7, respectively, and cloning into plasmid pGEX-rTEV.
Protein Purification
MBP-His-tagged and GST-tagged proteins were purified using nickel-nitrilotriacetic acid and glutathione-agarose beads, respectively, followed by gel filtration as described previously (5).
Attachment Assays
Attachment assays with live bacteria or purified labeled protein were carried out as described (5). To determine whether attachment was fibronectin- and phosphatidic acid-dependent, tissue culture cells were incubated with anti-Fn antibody (50 μg/ml in PBS, Sigma) or treated with 50 μg/ml phospholipase C (Sigma) in PBS for 15 min prior to infection. For attachment of labeled protein in the absence of fibronectin, we used trypsinized cells as described previously (5). To test whether bacterial attachment could be abolished using heparin, cells were preincubated with heparin at concentrations between 10–500 μm in DMEM for 30 min prior to attachment assays.
Fn Pulldown Assays
A detailed protocol for pulldown assays with GST-MAM constructs and fibronectin can be found elsewhere (5). Variations of this protocol included the use of proteolytic fibronectin fragments (30-kDa N-terminal heparin binding domain and 45-kDa gelatin binding domain, both from Sigma) or an additional incubation step with an equimolar amount of liposomes (30 min at 22 °C). PBS-buffered liposomes were prepared from 1,2-dioleoyl-sn-glycero-3-phosphocholine (PC) or mixtures of PC and 1,2-dioleoyl-sn-glycero-3-phosphate (PA), (both Avanti Polar Lipids, Inc.) as described previously (25).
Quantitation of Phospholipids
Liposomes in load, flowthrough, and eluate fractions were quantified using the method of Worth and Wright (26). Briefly, samples were extracted with a mixture of chloroform and methanol and centrifuged, and molybdophosphoric acid (Sigma) was added to the organic phase. Samples were centrifuged, and the aqueous phase was removed. Metol and sodium bisulfate were added to reduce the organic phase, and the aqueous phase was removed again following centrifugation. The amount of phospholipid was determined by measuring absorbance at 680 nm and expressed as fraction of the amount detected in loaded fractions.
Fn Plate Assay with Labeled Protein
96-well plates coated with 1 μg of fibronectin per well were incubated with MAM constructs prepared in PBS at concentrations between 0.1 and 100 μm at 22 °C for 1 h. Initial fluorescence and fluorescence output were measured on a plate reader (λexcitation, 485 nm; λemission, 520 nm) prior to and following three washes with PBS, respectively. Data were expressed as % bound fluorescence and corrected for values determined for MBP alone. Levels of bound protein were blotted as a function of protein concentration, and data were fit to a single-site binding model using Sigma Plot.
Liposome Association Assays
Binding of GST and GST-mce constructs to liposomes were carried out as described in the literature (5). 300 μg of liposomes containing PC alone or 1–80 mol % PA were incubated with 100 μg GST and GST-mce proteins in PBS for 1 h at 22 °C. Mixtures were centrifuged at 100,000 × g at 4 °C for 1 h, both pellet and supernatant fractions were separated by SDS-PAGE, and proteins were detected by Coomassie staining. Band intensities were determined using the gel analysis software UN-SCAN-IT (Silk Scientific, Inc.), and intensities of pellet samples (% bound) were expressed as fraction of total intensities (supernatant and pellet samples combined).
RESULTS
Phosphatidic Acid Is Essential for MAM7 Attachment to Host Cells, whereas Fibronectin Reduces Time Required for Binding
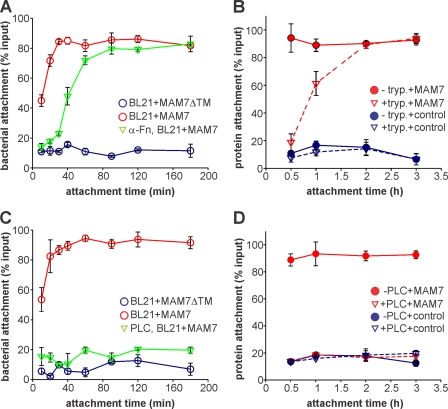
Our previous studies showed that two different types of host receptors, the extracellular matrix protein fibronectin and the membrane phospholipid phosphatidic acid, recognize MAM7 adhesin in vitro and contribute to attachment in vivo (5). When studying MAM7 binding to cells independent of fibronectin, we observed that although no binding was detected without fibronectin after 30 min, attachment to cells would gradually take place if the incubation time was extended. We therefore performed a 3-h time course experiment looking at binding of E. coli BL21 expressing V. parahaemolyticus MAM7 on their surface (BL21-MAM7) to HeLa cells in the presence and absence of fibronectin (Fig. 1A). Attachment was compared with a negative control of BL21 expressing MAM7ΔTM, a version of MAM7 missing the N-terminal 44 amino acids containing the translocation and membrane-anchoring signal (5).
FIGURE 1.
MAM7 binding to fibronectin is required for rapid attachment to host cells. Shown is the attachment of bacteria expressing MAM7 (BL21-MAM7; A and C) or Alexa Fluor 488-labeled MAM7 protein or labeled MBP control (B and D) to host cells treated with α-fibronectin antibody (A), trypsin (B), or phospholipase C (C and D).
In the presence of both host receptors, binding of BL21-MAM7 to host cells was highly efficient, with >40% of bacteria binding within the first 10 min of incubation (Fig. 1A). Full binding capacity was reached after 30 min. When binding to fibronectin was blocked with anti-fibronectin antibodies so that binding could be studied independent of the contribution of fibronectin, no bacterial attachment was observed within the first 30 min of the experiment, and the binding was the same as the negative control (Fig. 1A). However, gradual binding was observed from 40 min onwards, and close to full binding capacity was reached after 60 min. Overall, binding in the absence of fibronectin was delayed by ∼30 min. When we repeated the time course experiment using host cells that were treated with trypsin to degrade fibronectin and purified, fluorophore-labeled MAM7, we obtained similar results showing a delay in host cell binding of ∼1 h (Fig. 1B).
Next, we studied the contribution of phosphatidic acid on host cells to MAM7 attachment. We performed similar time course experiments as described for fibronectin, but instead, phosphatidic acid was eliminated from the host surface by treatment with phospholipase C. In the absence of phosphatidic acid, binding of both BL21-MAM7 (Fig. 1C) and labeled MAM7 protein (Fig. 1D) was reduced to background binding for the duration of the experiment (3 h), similar to that observed with of BL21-MAM7ΔTM and MBP controls, respectively. We conclude that although both fibronectin and phosphatidic acid act as host receptor for MAM7 attachment, the contribution of fibronectin to overall binding is dispensable when more time is allowed for attachment. Although phosphatidic acid is essential for MAM7-mediated binding, fibronectin contributes by accelerating bacterial attachment.
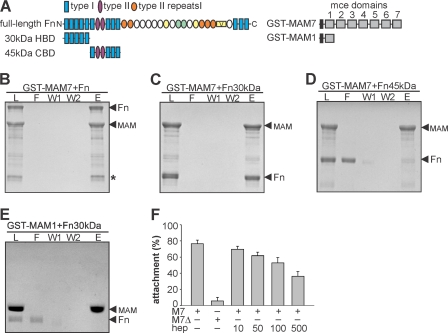
30-kDa N-terminal Fragment of Fibronectin Is Sufficient for Binding to MAM7
Fibronectin is a dimeric 440-kDa glycoprotein involved in many vital processes, including cell adhesion, migration, differentiation, and wound healing (27, 28). Each fibronectin molecule is composed of three types of domains denoted type I, type II, and type III repeats, which vary in terms of structural and functional properties (28) (Fig. 2A). To analyze which region of fibronectin is involved in MAM7 binding, we performed pulldown experiments with GST-MAM7 and either full-length soluble fibronectin, a 30-kDa proteolytic fragment containing the N-terminal five type I repeats (I1–5, heparin binding region I) or a 45-kDa fragment containing repeat I6, II1–2, and I7–9 (gelatin and collagen binding region, Fig. 2A). Both full-length fibronectin and the 30-kDa fragment, but not the 45-kDa fragment, were pulled down by GST-MAM7 (Fig. 2, B–D). As negative control, we used MAM1, which does not bind fibronectin (5), and no interaction with the 30-kDa fragment was observed (Fig. 2E). Because the 30-kDa fragment has been shown to bind heparin (29), we tested whether attachment of BL21-MAM7 to HeLa cells would be inhibited by adding heparin to the attachment assay. Although in the absence of heparin, ∼80% of bacteria attached to the host cells within 30 min, the addition of increasing concentrations (10–500 μm) of heparin from intestinal mucosa gradually blocked attachment, with only ∼40% of BL21-MAM7 remaining attached at 500 μm heparin (Fig. 2F). This demonstrates that the N-terminal region of fibronectin encompassing repeats I1–5 mediates MAM7 attachment and that early binding of bacteria could be blocked by adding the competing fibronectin ligand heparin.
FIGURE 2.
The N-terminal 30-kDa fragment of fibronectin is sufficient for MAM7 binding. A, fibronectin subunit (220 kDa) consisting of type I, type II, and type III repeats. Each subunit contains an N-terminal region (I1–5, 30-kDa fragment) required for fibrin and heparin binding (HBD), followed by a 45-kDa collagen binding domain (CBD, I6, II1–2, I7–9). MAM7 contains an N-terminal transmembrane region, which was excluded from recombinant proteins, followed by seven consecutive mce domains. GST-MAM7 and MAM1 constructs used for pulldowns are depicted. Pulldown assays with GST-MAM7 and intact Fn (B), 30-kDa Fn subunit (Fn30kDa; C) or 45-kDa Fn subunit (Fn45kDa; C) or GST-MAM1 and 30-kDa Fn subunit (Fn30kDa; E). Heparin (hep) inhibits attachment of BL21-MAM7 to host cells (F). M7, BL21-MAM7; M7Δ, BL21-MAM7ΔTM. L, load; F, flow through; E, eluate; *, Fn30kDa resulting from breakdown of Fn.
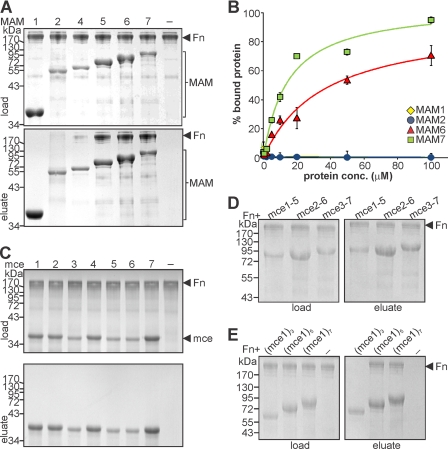
At Least Five Tandem mce Domains Are Required for Stable Binding to Fibronectin
As described previously, full-length MAM7 can stably bind to fibronectin, whereas no binding was detected with a construct containing only the first N-terminal mce domain of MAM7 (MAM1) (5). To further delineate the region of MAM7 required for fibronectin binding, we performed pulldown experiments of fibronectin with GST-tagged proteins containing all seven mce domains (MAM7) or successive truncations of mce domains from the C terminus (designated MAM6 to MAM1). Both load and eluates were analyzed by SDS-PAGE and Coomassie staining. Fibronectin was only pulled down by GST-MAM7, GST-MAM6, and GST-MAM5, whereas no appreciable interaction was observed with GST-MAM1 to -MAM4 (Fig. 3A). Affinity measurements using immobilized fibronectin and fluorophore-labeled MAM constructs showed that MBP-MAM7 bound fibronectin with a KD of 15 ± 3 μm, whereas the affinity was decreased for MBP-MAM6 (KD = 36 ± 9 μm). No interaction could be detected with either MBP-MAM1 or MBP-MAM2 (Fig. 3B). We could not determine affinities for MAM3-MAM5, as MBP-tagged constructs were unstable and selective thiol labeling is unfeasible with GST-tagged proteins (GST itself contains cysteines). To determine whether one mce domain specifically was responsible for mediating the interaction with fibronectin, we used GST constructs of all seven individual mce domains for pulldown experiments. Individual mce domains were folded autonomously into a mixed α/β structure, as determined by NMR.3 This approach showed that none of the single mce domains was bound by fibronectin (Fig. 3C). However, several constructs containing stretches of five mce domains (mce1–5, mce3–6, and mce 2–7) efficiently interacted with fibronectin in pulldown assays (Fig. 3D). These data support the idea that all mce domains contributed to binding, but at least five domains in tandem were required to achieve detectable binding affinity. To further test this, we constructed concatemers containing three, five, or seven identical mce1 domains and analyzed their interaction with fibronectin using pulldowns. Concatemers containing five or seven mce1 domains, but not the concatemer containing three mce1 domains, were able to pull down fibronectin (Fig. 3E). Taken together, these findings are consistent with the hypothesis that, in principle, all mce domains can contribute to fibronectin binding, but at least five domains together are required to achieve a high affinity interaction.
FIGURE 3.
A minimum of five mce domains is required for stable binding of fibronectin to MAM. Pulldown experiments were performed using intact fibronectin and GST-MAM1, -MAM2, -MAM4, -MAM5, -MAM6, or -MAM7. Load and eluate fractions were analyzed by SDS-PAGE and visualized by Coomassie staining (A). Binding of labeled MBP-MAM1, -MAM2, -MAM6, and -MAM7 to Fn-coated plates was determined using fluorescence saturation binding assays (B). Shown are pulldown assays with GST-tagged individual mce domains (mce1 to mce7) and fibronectin. Load and eluate fractions were analyzed by SDS-PAGE and visualized by Coomassie staining (C). Shown are pulldown assays with GST-tagged mce1–5, -2–6, and -3–7 proteins and fibronectin (D), or GST-tagged (mce1)3, (mce1)5, and (mce1)7 concatemers and fibronectin (E). conc., concentration.
Key Basic Residues Modulate Binding Affinity of mce Domains to Phosphatidic Acid
As described above, phosphatidic acid is essential for stable binding of MAM7 to host cells. Although we had shown previously that MAM1 is sufficient to bind to phosphatidic acid, it was unclear how the other mce domains contributed to phosphatidic acid binding in the absence of fibronectin. We tested all seven individual mce domains (mce1–mce7) for phosphatidic acid binding using liposome association assays. Proteins were incubated with liposomes containing a mixture of PC and increasing amounts (1–80 mol %) of PA, followed by separation of liposome-bound and unbound fractions by ultracentrifugation. All fractions were analyzed by SDS-PAGE (supplemental Fig. S1) followed by densitometry (Fig. 4A). In contrast to GST alone, which did not bind liposomes and was only found in supernatants (supplemental Fig. S1H), all seven mce domains were bound by PA-containing liposomes. However, we observed significant differences in their apparent affinities: although mce1, -2, -3, and -4 bound equally well, binding was decreased ∼6-fold for mce5, ∼20-fold for mce7, and >100-fold for mce6 (Fig. 4A). Although so far only few phosphatidic acid binding proteins have been characterized in detail, it has been demonstrated that basic residues are often key determinants of binding affinity (30, 31). When we analyzed the mce domains of MAM7 for overall charge, we found only minor differences between individual domains. When we looked at charge distribution, however, we observed that several basic residues that are otherwise well conserved between individual domains are mutated in mce6. These include two residues which are lysines in mce2, the strongest binding mce domain, but a serine and glutamine in mce6, respectively, and a well conserved arginine, which is replaced by a histidine in mce6 (Fig. 4D). To test whether these residues contributed to phosphatidic acid binding, we mutated the respective positions in mce6 to the amino acids of the corresponding position in mce2 (mce6 S646K, Q664K, and H703R) and tested the resulting proteins for PA binding in liposome association assays. The mce6 S664K mutant, but not the Q664K or H703R mutants, showed a significant increase in association with liposomes, changing the binding affinity of mce6 relative to high binding mce domains from 100-fold less to ∼10-fold less. (Fig. 4B and supplemental Fig. S1, I–K). We also used liposomes containing 50 mol % PA and 50 mol % PC to perform pulldown experiments, a composition of liposomes where some degree of binding was observed with all mce constructs. GST-mce proteins were immobilized and incubated with liposomes. Bound liposomes were quantified using a molybdophosphoric acid assay. In agreement with liposome assays, these experiments also showed strong binding of liposomes to mce1, -2, -3, -4, and -7, whereas mce5 and -6 showed weaker binding (Fig. 4C). mce6 S646K displayed enhanced affinity compared with wild type mce6, whereas the other mutants had equally low affinities as mce6.
FIGURE 4.
Analysis of phosphatidic acid binding by MAM7 mce domains. Liposome association assays with individual mce domains and liposomes prepared from PC and PA and containing increasing concentrations of PA as indicated (0–80 mol %). Supernatant and pellet fractions were analyzed by SDS-PAGE and visualized by Coomassie staining (supplemental Fig. S1). % Bound protein were determined by densitometry of gels and used to compare the affinities of mce1–7 constructs (A). Shown is the densitometry of mce6 (weakest binding to PA) compared with mce2 (tightest binding) and three mce6 point mutants (B). A pulldown assay of liposomes containing 50 mol % PA and 50 mol % PC on immobilized GST-mce domains is shown (C). A sequence alignment of mce2 and mce6 is shown (D). Positions of point mutations in mce6 are shown in blue (S646K), dark purple (Q664K), and pink (H703R), respectively. aa., amino acids. *, identical aa; :, well conserved aa; ., conserved aa.
MAM7 Forms Tripartite Complex with Fibronectin and Phosphatidic Acid
Although we have dissected the individual interactions between MAM7 and fibronectin and PA, it remains unclear whether MAM7 binds to both types of receptors simultaneously or binding is mutually exclusive. To analyze whether MAM7, fibronectin, and phosphatidic acid is competitive, we performed pulldown assays with equimolar amounts of MAM7, Fn, and PA (in the form of liposomes consisting of a 1:1 mixture of PC and PA or, as negative control, PC only). First, we preincubated GST-MAM7 with liposomes, followed by incubation with fibronectin (Fig. 5, A and C). Next, we preincubated GST-MAM7 with fibronectin first and competed with liposomes (Fig. 5, B and D). For both experiments, we analyzed eluate and flow-through fractions after adding the competing molecule by SDS-PAGE to detect protein (Fig. 5, A and B, and supplemental Fig. S2) and with a molybdophosphoric acid assay to detect liposome-containing fractions (Fig. 5, C and D). Both fibronectin and PA-containing liposomes were pulled down individually by MAM7 (Fig. 5, A–D, lanes 1 and 2, respectively). Addition of an equimolar mix of both ligands also resulted in efficient pulldown of both ligands by MAM7 (Fig. 5, A–D, lane 4). As a control, we performed binding assays with liposomes containing only PC, and we did not observe binding of MAM7 to liposomes but did observe binding to fibronectin (Fig. 5, A–D, lanes 5–7). Furthermore, no binding to MAM1 was observed with fibronectin or liposomes containing PC alone but was observed with liposomes containing a 1:1 mixture of PC:PA (Fig. 5, A–D, lanes 8–10). To test for competition between fibronectin and PA binding, we also performed plate assays using fibronectin-coated plates and fluorophore-labeled MBP-MAM7. MAM7-fluorescence was measured before and after incubation with liposomes containing either PC or a mixture of PC and 80 mol % PA. Incubation with increasing concentrations of liposomes did not replace fibronectin as MAM7 ligand, so that MAM7 remained bound to the plate, and fluorescence levels did not decrease significantly (Fig. 5E). We also analyzed samples from the plate assay after incubation with liposomes containing 100 μm PA or PC only (Fig. 5E, arrow). Using the molybdophosphoric acid assay, we found that PA-containing liposomes remained associated with Fn-bound MAM7 after washing steps, whereas liposomes prepared from PC alone did not (Fig. 5F). In addition, we did not detect any associated liposomes when Fn-coated plates were preincubated with MBP tag alone (Fig. 5F). These results support the hypothesis that MAM7 can interact with both fibronectin and phosphatidic acid ligands simultaneously, forming a tripartite complex.
FIGURE 5.
MAM7, fibronectin, and phosphatidic acid form a tripartite complex. Shown are pulldown assays using GST-MAM7 or -MAM1, fibronectin, and liposomes (with or without PA). GST-MAM proteins were incubated with fibronectin followed by liposomes (A and C) or liposomes followed by fibronectin (B and D). Bound proteins were analyzed by SDS-PAGE and Coomassie staining (A and B). Liposomes in flow-through and eluate fractions were quantitated using a molybdophosphoric acid assay and expressed as fractions of loaded lipid (C and D). Alexa Fluor 488-labeled MAM7 was tested for binding to Fn-coated plates in the presence of increasing concentrations of liposomes prepared from PC (●) or a mixture containing 20:80 mol % PC:PA (○) (E). Samples incubated with the highest concentration (conc.) of liposomes (arrow) were analyzed for bound liposomes using the molybdophosphoric acid assay and compared with plate assays carried out with MBP control (F).
DISCUSSION
Our previous work has identified MAM7 as a factor involved in the initial attachment of bacterial pathogens to host cells. We have also demonstrated that MAM7 expressed on the surface of non-pathogenic bacteria may be utilized as a potent inhibitor of pathogen infection in tissue culture. MAM7 is capable of binding both fibronectin and phosphatidic acid on the host cell surface, and both interactions are required for bacterial adhesion to host cells (5). Upon further dissection of host cell binding into discrete binding events, either by masking fibronectin with an anti-Fn antibody or by degrading phosphatidic acid, we found that in the absence of fibronectin, MAM7 is still able to attach to host cells, but establishment of a stable interaction takes significantly longer than in the presence of fibronectin (Fig. 1). Because fibronectin is an abundant protein in the extracellular matrix surrounding cells in vivo as well as in tissue culture, we speculate that it facilitates rapid initial attachment of bacteria in the vicinity of the host cell membrane, thereby increasing the likelihood for the pathogen to establish a high affinity interaction with the host cell membrane via the second MAM7 ligand, phosphatidic acid.
We found that the MAM7 binding site in fibronectin is located to the 30-kDa N-terminal region (Fig. 2). The same region is also exploited as receptor by other bacterial adhesins, including fibronectin-binding proteins (FnBPs) from the Gram-positive pathogens Staphylococcus aureus and Streptococcus pyogenes (15). Thus, future experiments will be carried out to address whether MAM7 binding to host cells might be able to diminish adhesion and invasion of these pathogens. In contrast to FnBP-expressing pathogens utilizing the 30-kDa fragment for attachment, most of the MAM7-containing pathogens we studied such as V. parahaemolyticus, V. cholerae, Y. pseudotuberculosis, and EPEC remain extracellular during infection and are not internalized by host cells (32–35). Equally, non-pathogenic BL21-MAM7 did not get internalized by host cells. Many factors could account for this difference in bacterial fate following fibronectin binding (36). FnBPs such as S. pyogenes F1 bind to fibronectin in a way that leaves the RGD motif required for integrin recruitment, which is necessary for internalization, exposed (37). It is possible that MAM7 binding renders fibronectin in a different conformation, which does not allow for integrin recruitment. It is also possible that fibronectin does not get recruited by MAM7 at a density sufficient to induce clustering of integrin receptors and thus activation of downstream pathways necessary for cellular uptake (38). Indeed, recent work analyzing the link between repeats within S. aureus FnBPA and cellular invasion has shown that only very high (subnanomolar) affinity interactions between FnBP repeats and fibronectin can lead to sufficient clustering of integrin receptors to promote cellular invasion (39). It is thus possible that the interaction between MAM7 and fibronectin is not of high enough affinity to promote cellular uptake.
The 30-kDa fragment of fibronectin also contains features required for fibronectin cross-linking and fibrin binding (40, 41). Thus, it is possible that binding of MAM7 interferes with these processes, as has been described for other adhesins interacting with the same region (42, 43). We are currently conducting experiments to study the consequences of MAM7 binding on integrin signaling, fibrin-fibronectin cross-linking and fibrillogenesis. We detected no discernible binding with MAM constructs containing one to three mce domains, possibly due to very low affinity, and only weak binding with four mce domains. We found that at least five mce domains are required for stable binding of MAM to fibronectin. The sharp increase in binding affinity between four and five mce domains (Fig. 3A) as well as the non-linear increase in affinity between six and seven mce domains observed in fluorescent saturation binding experiments (Fig. 3B) is in better agreement with a cooperative rather than a linear binding model. However, a clear distinction between the two would require fibronectin binding analyses with MBP proteins containing three to five mce domains, which we currently are unable to produce and purify. Because the 30-kDa region required for MAM binding contains five consecutive type I repeats, it is tempting to speculate that each of these repeats binds to one mce domain. However, determination of the exact stoichiometry of binding will be subject to further studies but might be difficult to determine in the context of lipid binding, which could modulate the affinity and stoichiometry of binding between MAM7 and fibronectin.
In contrast to fibronectin, which seems to contribute to fast binding but is dispensable for high affinity binding, phosphatidic acid is indispensable to establish stable binding of MAM7 to the host surface (Fig. 1, C and D). This underlines earlier findings showing that the relative binding affinity of MAM7 for fibronectin is relatively minor compared with its affinity for intact host cells (apparent KD of 15 μm and 200 nm for fibronectin and intact cells, respectively). No common motif mediating interactions between proteins and phosphatidic acid has been identified to date. However, many PA binding motifs contain a high number of basic residues which are thought to establish electrostatic interactions with the phosphate headgroup (31). Based on alignments of individual mce domains within MAM7 and their differences in affinity for PA, we determined at least one basic residue (mce2 Lys-166), which is crucial for PA binding. The position is well conserved between different mce domains in MAM7 with the exception of mce6 and mce5, both of which bound PA with lower affinity than other mce domains. Mutation of this position to a lysine in mce6 lead to at least 20-fold increased affinity for PA. Mutation of other candidate residues (which were conserved as basic residues in the other mce domains) within mce6, we mutated to basic residues did not seem to have any effect on PA binding. Ultimately, structural studies on mce domains in their free and ligand-bound forms will be required to shed more light on the detailed mechanisms behind host cell binding.
In this study, we dissected the interaction of the adhesin MAM7 with host cells into discrete binding events and analyzed their contribution to overall host cell binding. We analyzed MAM7 for features defining its ability to interact with fibronectin and phosphatidic acid, respectively, and defined the region of fibronectin required for its interaction with MAM7. We also studied whether MAM7 is capable of binding to fibronectin and phosphatidic acid simultaneously and found that the three molecules likely form a tripartite complex. These studies form an important basis for our current and future efforts to develop MAM7-derived tools for the attenuation of Gram-negative bacterial infections.
Supplementary Material
Acknowledgments
We thank the Orth laboratory, especially Dr. L. Zhang and T. J. Calder, for critical reading and comments on the manuscript. We also thank K. H. Gardner for technical assistance with NMR experiments; and E. L. Reddick for help with preparation of liposomes.
This work was supported, in whole or in part, by National Institutes of Health NIAID Grants R01-AI056404 and R01-AI087808. This work was also supported by Welch Foundation Grant I-1561.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
A. M. Krachler, L. Zhang, K. H. Gardner, and K. Orth, unpublished observation.
- EPEC
- enteropathogenic E. coli
- Fn
- fibronectin
- PC
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
- PA
- phosphatidic acid
- FnBP
- fibronectin binding protein
- MBP
- maltose binding protein.
REFERENCES
- 1. Kim Y. R., Lee S. E., Kook H., Yeom J. A., Na H. S., Kim S. Y., Chung S. S., Choy H. E., Rhee J. H. (2008) Cell Microbiol. 10, 848–862 [DOI] [PubMed] [Google Scholar]
- 2. Galán J. E., Wolf-Watz H. (2006) Nature 444, 567–573 [DOI] [PubMed] [Google Scholar]
- 3. Hayes C. S., Aoki S. K., Low D. A. (2010) Annu. Rev. Genet. 44, 71–90 [DOI] [PubMed] [Google Scholar]
- 4. Håkansson S., Schesser K., Persson C., Galyov E. E., Rosqvist R., Homblé F., Wolf-Watz H. (1996) EMBO J. 15, 5812–5823 [PMC free article] [PubMed] [Google Scholar]
- 5. Krachler A. M., Ham H., Orth K. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 11614–11619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chitale S., Ehrt S., Kawamura I., Fujimura T., Shimono N., Anand N., Lu S., Cohen-Gould L., Riley L. W. (2001) Cell Microbiol. 3, 247–254 [DOI] [PubMed] [Google Scholar]
- 7. Saini N. K., Sharma M., Chandolia A., Pasricha R., Brahmachari V., Bose M. (2008) BMC Microbiol. 8, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pettersson J., Nordfelth R., Dubinina E., Bergman T., Gustafsson M., Magnusson K. E., Wolf-Watz H. (1996) Science 273, 1231–1233 [DOI] [PubMed] [Google Scholar]
- 9. Boekema B. K., Van Putten J. P., Stockhofe-Zurwieden N., Smith H. E. (2004) Infect. Immun. 72, 691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couturier M. R., Stein M. (2008) Can J. Microbiol. 54, 537–548 [DOI] [PubMed] [Google Scholar]
- 11. Rosenshine I., Ruschkowski S., Stein M., Reinscheid D. J., Mills S. D., Finlay B. B. (1996) EMBO J. 15, 2613–2624 [PMC free article] [PubMed] [Google Scholar]
- 12. Singh P., Carraher C., Schwarzbauer J. E. (2010) Annu. Rev. Cell Dev. Biol. 26, 397–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mao Y., Schwarzbauer J. E. (2005) Matrix Biol. 24, 389–399 [DOI] [PubMed] [Google Scholar]
- 14. Henderson B., Nair S., Pallas J., Williams M. A. (2011) FEMS Microbiol. Rev. 35, 147–200 [DOI] [PubMed] [Google Scholar]
- 15. Schwarz-Linek U., Höök M., Potts J. R. (2006) Microbes Infect. 8, 2291–2298 [DOI] [PubMed] [Google Scholar]
- 16. Isberg R. R., Tran Van Nhieu G. (1994) Trends Microbiol. 2, 10–14 [DOI] [PubMed] [Google Scholar]
- 17. Hamburger Z. A., Brown M. S., Isberg R. R., Bjorkman P. J. (1999) Science 286, 291–295 [DOI] [PubMed] [Google Scholar]
- 18. Voelker D. R. (1991) Microbiol. Rev. 55, 543–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young B. P., Shin J. J., Orij R., Chao J. T., Li S. C., Guan X. L., Khong A., Jan E., Wenk M. R., Prinz W. A., Smits G. J., Loewen C. J. (2010) Science 329, 1085–1088 [DOI] [PubMed] [Google Scholar]
- 20. Wang X., Devaiah S. P., Zhang W., Welti R. (2006) Prog. Lipid Res. 45, 250–278 [DOI] [PubMed] [Google Scholar]
- 21. Kooijman E. E., Chupin V., de Kruijff B., Burger K. N. (2003) Traffic 4, 162–174 [DOI] [PubMed] [Google Scholar]
- 22. Andresen B. T., Rizzo M. A., Shome K., Romero G. (2002) FEBS Lett. 531, 65–68 [DOI] [PubMed] [Google Scholar]
- 23. Fang Y., Vilella-Bach M., Bachmann R., Flanigan A., Chen J. (2001) Science 294, 1942–1945 [DOI] [PubMed] [Google Scholar]
- 24. Frank C., Keilhack H., Opitz F., Zschörnig O., Böhmer F. D. (1999) Biochemistry 38, 11993–12002 [DOI] [PubMed] [Google Scholar]
- 25. Selyunin A. S., Sutton S. E., Weigele B. A., Reddick L. E., Orchard R. C., Bresson S. M., Tomchick D. R., Alto N. M. (2011) Nature 469, 107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Worth H. G., Wright D. J. (1977) Clin. Chem. 23, 1995–2000 [PubMed] [Google Scholar]
- 27. Grinnell F. (1984) J. Cell Biochem. 26, 107–116 [DOI] [PubMed] [Google Scholar]
- 28. Pankov R., Yamada K. M. (2002) J. Cell Sci. 115, 3861–3863 [DOI] [PubMed] [Google Scholar]
- 29. Ingham K. C., Brew S. A., Atha D. H. (1990) Biochem. J. 272, 605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stace C. L., Ktistakis N. T. (2006) Biochim. Biophys. Acta 1761, 913–926 [DOI] [PubMed] [Google Scholar]
- 31. Lu B., Benning C. (2009) J. Biol. Chem. 284, 17420–17427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burdette D. L., Seemann J., Orth K. (2009) Mol. Microbiol. 73, 639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simonet M., Richard S., Berche P. (1990) Infect. Immun. 58, 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosqvist R., Bölin I., Wolf-Watz H. (1988) Infect. Immun. 56, 2139–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Celli J., Olivier M., Finlay B. B. (2001) EMBO J. 20, 1245–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cossart P., Sansonetti P. J. (2004) Science 304, 242–248 [DOI] [PubMed] [Google Scholar]
- 37. Ensenberger M. G., Annis D. S., Mosher D. F. (2004) Biophys. Chem. 112, 201–207 [DOI] [PubMed] [Google Scholar]
- 38. Tran Van Nhieu G., Isberg R. R. (1993) EMBO J. 12, 1887–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edwards A. M., Potts J. R., Josefsson E., Massey R. C. (2010) PLoS Pathog. 6, e1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hörmann H., Richter H., Jelini V., Wendt C. (1987) Biol. Chem. Hoppe Seyler 368, 669–674 [DOI] [PubMed] [Google Scholar]
- 41. Vakonakis I., Staunton D., Rooney L. M., Campbell I. D. (2007) EMBO J. 26, 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tomasini-Johansson B. R., Kaufman N. R., Ensenberger M. G., Ozeri V., Hanski E., Mosher D. F. (2001) J. Biol. Chem. 276, 23430–23439 [DOI] [PubMed] [Google Scholar]
- 43. Matsuka Y. V., Anderson E. T., Milner-Fish T., Ooi P., Baker S. (2003) Biochemistry 42, 14643–14652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.