Abstract
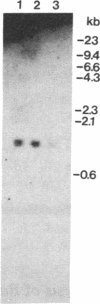
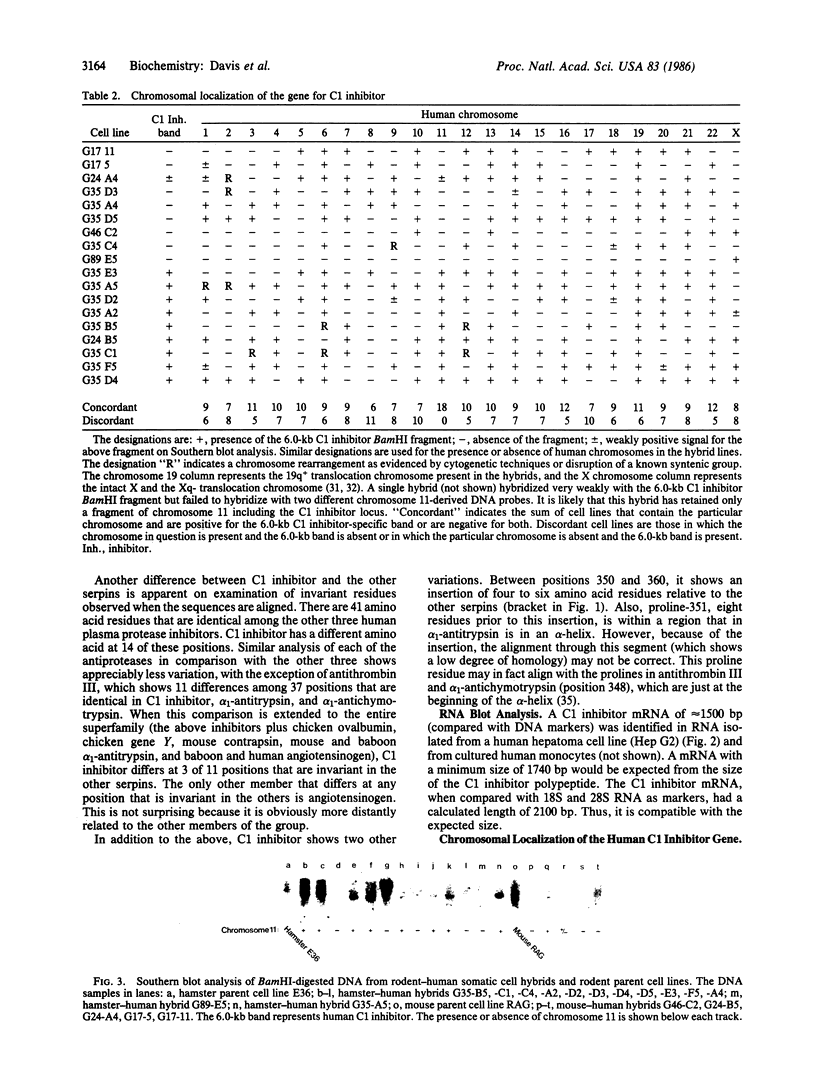
C1 inhibitor is a heavily glycosylated plasma protein that regulates the activity of the first component of complement (C1) by inactivation of the serine protease subcomponents, C1r and C1s. C1 inhibitor cDNA clones have been isolated, and one of these (pC1INH1, 950 base pairs) has been partially sequenced. Sequence analysis demonstrates that the C1 inhibitor is a member of the serpin "superfamily" of protease inhibitors. In the region sequenced, C1 inhibitor has 22% identity with antithrombin III, 26% with alpha 1-antitrypsin and alpha 1-antichymotrypsin, and 18% with human angiotensinogen. C1 inhibitor has a larger amino-terminal extension than do the other plasma protease inhibitors. In addition, inspection of residues that are invariant among the other protease inhibitors shows that C1 inhibitor differs at 14 of 41 of these positions. Thus, it appears that C1 inhibitor diverged from the group relatively early in evolution, although probably after the divergence of angiotensinogen. Southern blot analysis of BamHI-digested DNA from normal individuals and from rodent-human somatic cell hybrid cell lines (that contain a limited but varied human chromosome complement) was used to localize the human C1 inhibitor gene to chromosome 11.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Brook J. D., Shaw D. J., Meredith L., Bruns G. A., Harper P. S. Localisation of genetic markers and orientation of the linkage group on chromosome 19. Hum Genet. 1984;68(4):282–285. doi: 10.1007/BF00292584. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Mintz B. J., Leary A. C., Regina V. M., Gerald P. S. Human lysosomal genes: arylsulfatase A and beta-galactosidase. Biochem Genet. 1979 Dec;17(11-12):1031–1059. doi: 10.1007/BF00504344. [DOI] [PubMed] [Google Scholar]
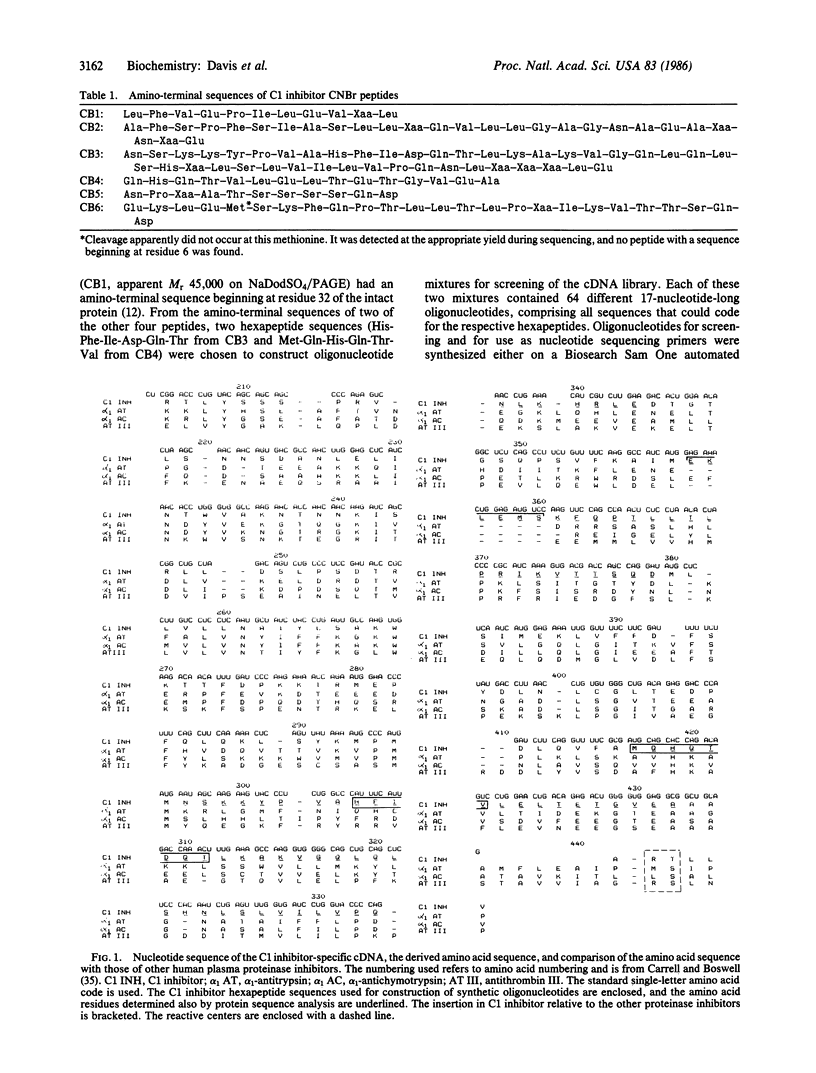
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Chandra T., Stackhouse R., Kidd V. J., Robson K. J., Woo S. L. Sequence homology between human alpha 1-antichymotrypsin, alpha 1-antitrypsin, and antithrombin III. Biochemistry. 1983 Oct 25;22(22):5055–5061. doi: 10.1021/bi00291a001. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Markovic V. D., Teshima I. E. Genes for immunoglobulin heavy chains and for alpha 1-antitrypsin are localized to specific regions of chromosome 14q. Nature. 1982 Jun 3;297(5865):428–430. doi: 10.1038/297428a0. [DOI] [PubMed] [Google Scholar]
- DONALDSON V. H., EVANS R. R. A BIOCHEMICAL ABNORMALITY IN HEREDIATRY ANGIONEUROTIC EDEMA: ABSENCE OF SERUM INHIBITOR OF C' 1-ESTERASE. Am J Med. 1963 Jul;35:37–44. doi: 10.1016/0002-9343(63)90162-1. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Angiotensinogen is related to the antitrypsin-antithrombin-ovalbumin family. Science. 1983 Oct 28;222(4622):417–419. doi: 10.1126/science.6604942. [DOI] [PubMed] [Google Scholar]
- Forbes C. D., Pensky J., Ratnoff O. D. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J Lab Clin Med. 1970 Nov;76(5):809–815. [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Gigli I., Mason J. W., Colman R. W., Austen K. F. Interaction of plasma kallikrein with the C1 inhibitor. J Immunol. 1970 Mar;104(3):574–581. [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C., Cooper N. R. Studies on human plasma C1 inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. 1975 Mar;55(3):593–604. doi: 10.1172/JCI107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A. Human C1 inhibitor: improved isolation and preliminary structural characterization. Biochemistry. 1983 Oct 11;22(21):5001–5007. doi: 10.1021/bi00290a019. [DOI] [PubMed] [Google Scholar]
- Haupt H., Heimburger N., Kranz T., Schwick H. G. Ein Beitrag zur Isolierung und Charakterisierung des Cl-Inaktivators aus Humanplasma. Eur J Biochem. 1970 Dec;17(2):254–261. doi: 10.1111/j.1432-1033.1970.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Hunt L. T., Dayhoff M. O. A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha 1-proteinase inhibitor. Biochem Biophys Res Commun. 1980 Jul 31;95(2):864–871. doi: 10.1016/0006-291x(80)90867-0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Ohkubo H., Nakanishi S. Primary structure of human preangiotensinogen deduced from the cloned cDNA sequence. Biochemistry. 1984 Jul 31;23(16):3603–3609. doi: 10.1021/bi00311a006. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Morse H. G., Law M. L., Lidsky A., Chandra T., Woo S. L. Genetic mapping of the structural gene for antithrombin III to human chromosome 1. Hum Genet. 1984;67(1):34–36. doi: 10.1007/BF00270555. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- LANDERMAN N. S., WEBSTER M. E., BECKER E. L., RATCLIFFE H. E. Hereditary angioneurotic edema. II. Deficiency of inhibitor for serum globulin permeability factor and/or plasma kallikrein. J Allergy. 1962 Jul-Aug;33:330–341. doi: 10.1016/0021-8707(62)90032-1. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Willard H. F., Gerald P. S. BrdU-33258 Hoechst analysis of DNA replication in human lymphocytes with supernumerary or structurally abnormal X chromosomes. Chromosoma. 1976 Aug 17;57(2):135–153. doi: 10.1007/BF00292912. [DOI] [PubMed] [Google Scholar]
- Laurell A. B., Mårtensson U., Sjöholm A. G. C1 subcomponent conplexes in normal and pathological sera studied by crossed immunoelectrophoresis. Acta Pathol Microbiol Scand C. 1976 Dec;84C(6):455–464. doi: 10.1111/j.1699-0463.1976.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- O'Malley B. W., Roop D. R., Lai E. C., Nordstrom J. L., Catterall J. F., Swaneck G. E., Colbert D. A., Tsai M. J., Dugaiczyk A., Woo S. L. The ovalbumin gene: organization, structure, transcription, and regulation. Recent Prog Horm Res. 1979;35:1–46. doi: 10.1016/b978-0-12-571135-7.50005-9. [DOI] [PubMed] [Google Scholar]
- PENSKY J., LEVY L. R., LEPOW I. H. Partial purification of a serum inhibitor of C'1-esterase. J Biol Chem. 1961 Jun;236:1674–1679. [PubMed] [Google Scholar]
- ROSEN F. S., PENSKY J., DONALDSON V., CHARACHE P. HEREDITARY ANGIONEUROTIC EDEMA: TWO GENETIC VARIANTS. Science. 1965 May 14;148(3672):957–958. doi: 10.1126/science.148.3672.957. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D., Pensky J., Ogston D., Naff G. B. The inhibition of plasmin, plasma kallikrein, plasma permeability factor, and the C'1r subcomponent of the first component of complement by serum C'1 esterase inhibitor. J Exp Med. 1969 Feb 1;129(2):315–331. doi: 10.1084/jem.129.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul A., Arlaud G. J., Sim R. B., Colomb M. G. A simplified procedure for the purification of C1-inactivator from human plasma. Interaction with complement subcomponents C1r and C1s. FEBS Lett. 1977 Jul 1;79(1):45–50. doi: 10.1016/0014-5793(77)80347-5. [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Catanese J. J., Kress L. F., Travis J. Primary structure of the reactive site of human C1-inhibitor. J Biol Chem. 1985 Feb 25;260(4):2432–2436. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. D., Kaplan A. P., Austen K. F. Plasma inhibitors of the components of the fibrinolytic pathway in man. J Clin Invest. 1973 Jun;52(6):1394–1401. doi: 10.1172/JCI107312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Arlaud G. J., Colomb M. G. C1 inhibitor-dependent dissociation of human complement component C1 bound to immune complexes. Biochem J. 1979 Jun 1;179(3):449–457. doi: 10.1042/bj1790449a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Arlaud G. J., Colomb M. G. Kinetics of reaction of human C1-inhibitor with the human complement system proteases C1r and C1s. Biochim Biophys Acta. 1980 Apr 11;612(2):433–449. doi: 10.1016/0005-2744(80)90126-6. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Reboul A., Arlaud G. J., Villiers C. L., Colomb M. G. Interaction of 125I-labelled complement subcomponents C-1r and C-1s with protease inhibitors in plasma. FEBS Lett. 1979 Jan 1;97(1):111–115. doi: 10.1016/0014-5793(79)80063-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ohkubo H., Nakanishi S. Common structural organization of the angiotensinogen and the alpha 1-antitrypsin genes. J Biol Chem. 1984 Jul 10;259(13):8063–8065. [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalut C., Henzel W. J., Harris H. W., Jr Micro quantitative Edman manual sequencing. J Biochem Biophys Methods. 1980 Jul;3(1):11–30. doi: 10.1016/0165-022x(80)90003-2. [DOI] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Active disassembly of the first complement component, C-1, by C-1 inactivator. J Immunol. 1979 Aug;123(2):788–792. [PubMed] [Google Scholar]